Back to Journals » OncoTargets and Therapy » Volume 15
Protein Expression of Folate Receptor Alpha in Adenoid Cystic Carcinoma of the Head and Neck
Authors Schnoell J , Jank BJ, Kadletz-Wanke L, Stoiber S, Gurnhofer E, Schlederer M, Heiduschka G, Kenner L
Received 20 December 2021
Accepted for publication 10 March 2022
Published 16 May 2022 Volume 2022:15 Pages 531—538
DOI https://doi.org/10.2147/OTT.S351500
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev K. Srivastava
Julia Schnoell,1 Bernhard J Jank,1 Lorenz Kadletz-Wanke,1 Stefan Stoiber,2,3 Elisabeth Gurnhofer,2 Michaela Schlederer,2 Gregor Heiduschka,1,* Lukas Kenner2– 5,*
1Department of Otorhinolaryngology, Head and Neck Surgery, Medical University of Vienna, Vienna, 1090, Austria; 2Department of Pathology, Medical University of Vienna, Vienna, Austria; 3Christian Doppler Laboratory for Applied Metabolomics, Vienna, Austria; 4Unit of Laboratory Animal Pathology, University of Veterinary Medicine, Vienna, Austria; 5CBmed GmbH - Center for Biomarker Research in Medicine, Graz, Styria, Austria
*These authors contributed equally to this work
Correspondence: Lukas Kenner, Department of Experimental Pathology and Laboratory Animal Pathology, Medical University and University of Veterinary Medicine, Vienna Waehringer Guertel 18-20, Vienna, A-1090, Austria, Tel +43 1 40400 51720, Email [email protected]
Purpose: Folate receptor alpha (FRα) is overexpressed in various cancer entities while expression in normal tissue is limited. Thus, FRα is an attractive target in cancer therapy. Currently, various therapeutic and diagnostic approaches are under investigation in clinical trials. The aim of this study was to assess the expression and clinical relevance of FRα in adenoid cystic carcinoma of the head and neck.
Patients and Methods: In this retrospective cohort study, 43 patients with adenoid cystic carcinoma (ACC) of the head and neck were included. FRα expression was analyzed in tumor tissue and tumor-free margin in a tissue microarray using immunohistochemical staining. Protein levels were correlated with clinical parameters.
Results: FRα staining was positive in 47% of ACC patients. The tumor-free margin was positive in 22%. Patients with positive tumor tissue showed positive margin staining in 55%. FRα expression was not associated with the clinical parameters (sex, age, staging, grading, perineural invasion, lymphovascular invasion).
Conclusion: FRα expression is common in ACC of the head and neck. Therefore, FRα should be further evaluated as a therapeutic target in ACC.
Keywords: folate receptor alpha, adenoid cystic carcinoma, prognosis, targeted therapy, head and neck
Introduction
Adenoid cystic carcinoma (ACC) of the head and neck accounts for 1% of all head and neck carcinomas and 20% of salivary gland malignancies. It annually affects up to 4.5 new patients per million. While the short-term outcome is favorable, the disease is characterized by late recurrences and distant metastasis. Mainstay of treatment is surgical resection and postoperative radiotherapy. Treatment options of recurrent and metastatic disease are limited as response rates to chemotherapy are poor. Therefore, chemotherapy is reserved for rapid progression or symptomatic disease. The rare nature of the disease makes the investigation of new therapeutic targets difficult. Among various investigated therapies, only targeting MYB and NOTCH1 seem promising. Therefore, investigation of new therapeutic targets is crucial to overcome therapeutic limitations of recurrent and progressive ACC of the head and neck.1–4
Folate is essential for DNA synthesis, repair and methylation. The uptake into cells is mediated by three transport mechanisms: The main transport is via the reduced folate carrier (RFC), followed by the proton-coupled folate transporter (PCFT) and the folate receptors. While RFC is ubiquitously expressed, PCFT is mainly responsible for the intestinal folate uptake. Among the folate receptors, the folate receptor alpha (FRα) is the most interesting for cancer treatment. FRα is a membranous glycoprotein and facilitates folate uptake via endocytosis of the folate-bound receptor.5–7 While expression in normal tissue is rare, FRα is commonly found in cancers, such as lung adenocarcinoma, breast and ovarian cancer.8–12 The prognostic role of FRα in cancer is controversial since it is linked to a better and worse prognosis depending on the cancer type.10,11,13 Currently, a plethora of targeted therapies against FRα are under evaluation in clinical trials in various cancers (phase I–III).7,14–18 Additionally, different approaches to use FRα as a diagnostic imaging tool are being investigated.5–7
The aim of this study was to evaluate FRα protein expression in ACC of the head and neck. Furthermore, the association with clinicopathologic parameters and outcome was investigated.
Patients and Methods
Patients and Study Design
Forty-three patients with ACC of the head and neck were included in this retrospective study. They were treated at the Department of Otorhinolaryngology, Head and Neck Surgery of the Medical University of Vienna between 1996 and 2016. Exclusion criteria were diagnosis of a second carcinoma, age under 18 years or incomplete patient records. Data were obtained by review of medical patient records. The study was approved by the institution’s ethics committee (EK 1517/2018) and complies with the Declaration of Helsinki. All patients who are treated at the Department of Otorhinolaryngology of Medical University of Vienna comply with scientific use of their data. Therefore, no additional written consent had to be obtained for this retrospective study.
Patient and Public Involvement
It was not appropriate or possible to involve patients or the public in the design, or conduct, or reporting or dissemination plans of our research.
Tissue Microarray and Immunohistochemistry
A tissue microarray with three tumor cores and two tumor-free margin cores per patient was analyzed for FRα expression using immunohistochemistry. In short, the tissue was deparaffinized and rehydrated. FRα was stained using the Folate Receptor alpha IHC Assay Kit (Biocare Medical, CA, USA). The staining was visualized using AEC Substrate Kit (BD Biosciences, NJ, USA) and counterstaining was performed with Mayer’s hemalum solution. Tissue slides were scanned using PANNORAMIC Digital Slide Scanner. Analysis of staining intensity was performed using QuPath (Version 0.2.3) under the visual control of the researcher J.S. Tissue was analyzed for the intensity of staining (negative, low positive, moderately positive, high positive) and the percentage (0–100%) of stained cells. To stratify protein levels, a score was calculated (3 x % of cells with high positive staining + 2 x % of cells with moderately positive staining + 1 x % of cells with low positive staining) ranging from 0 to 300. Protein expression was considered positive when the calculated score was >5, indicating positive staining at any intensity in more than 5% of cells. Positive protein levels were further stratified into low positive (5–15), moderately positive (15–50) and high positive (>50).
Statistical Analysis
Statistical analysis was calculated with Stata (Stata Corp., TX, USA) and visualized using Prism GraphPad software (GraphPad Software, CA, USA). FRα was correlated with clinicopathological parameters (sex, age, staging, grading, perineural invasion, lymphovascular invasion) using Fisher’s exact or chi-squared test. The median survival was calculated using the method by Schemper et al.19 Overall survival (OS), cause-specific survival (CSS) or disease-free survival (DFS) were calculated from the date of surgery to the date of death, disease-specific death or recurrence, respectively. Survival curves were visualized using Kaplan–Meier curves and analyzed for significance using Log rank test. The cox proportional hazard model was used to calculate uni- and multivariable analysis. The multivariable model was corrected for staging and perineural invasion. Continuous data are reported as median and 25th and 75th percentiles and categorical values as absolute frequencies.
Results
Baseline Analysis
Forty-three patients with ACC of the head and neck were included in this study (Table 1). Fifty-three percent (n = 23) of patients were female. Most patients presented with an advanced stage (II–IV in 72%, n = 31) without lymph node metastasis (N0 in 77%, n = 33) or distant metastasis (M0 in 93%, n = 40). Perineural invasion was present in 51% (n = 22). Patients were treated with primary resection in 88% (n = 36) and postoperative radiotherapy in 46% (n = 19) and/or chemotherapy in 20% (n = 8). The median observation period was 77 months (49–173). The median OS was 121 months (76–173), the median CSS was 121 months (49-not reached) and the median DFS was 52 months (25-not reached). Fifty-two percent (n = 22) of patients developed a recurrence.
 |
Table 1 Baseline patient characteristics of adenoid cystic carcinoma patients |
Expression of FRα and Correlation with Clinicopathological Features
FRα expression was investigated using immunohistochemistry and was considered positive if more that 5% of cells expressed FRα. ACC FRα staining was positive in 47% (n = 20) and was generally localized in the membrane and the cytoplasm (Figure 1). In detail, FRα staining was low positive in 21% (n = 9), moderately positive in 19% (n = 8) and high positive in 5% (n = 2). The median FRα expression score was 2.6 (0.1–17.7). All FRα positive tissues showed cytoplasmic staining of granules. Tumor-free margin tissue was available in 74% (n = 32) and was FRα-positive in 22% (n = 7) of patients. The median FRα expression score in the tumor-free margin was 0.8 (0.2–4.7). In detail, margin tissue was low positive in 13% (n = 4), moderately positive in 6% (n = 2) and high positive in 3% (n = 1). 55% (n = 6) of patients with positive tumor staining showed positive margin staining (fisher’s exact p = 0.003). Association with clinicopathological parameters (sex, age, staging, grading, perineural invasion, lymphovascular invasion) was investigated using Fisher’s exact and chi-squared test (Table 2). However, no association was found.
 |
Table 2 Correlation analysis for associations between expression of FRα and clinicopathological features |
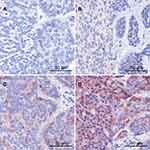 |
Figure 1 (A) Negative, (B) low positive, (C) moderately positive and (D) high positive staining of FRα in ACC. |
Analysis of Cause-Specific Survival and Disease-Free Survival
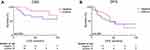
The association of FRα expression was investigated for the association with CSS and DFS (Figure 2). Patients with positive FRα levels showed a longer CSS (121 months vs not reached, p = 0.093) and DFS (48 vs 51 months, p = 0.826); however, the difference was not significant. Next, uni- and multivariable analysis, corrected for staging and perineural invasion, was performed. There was no significant change in risk for death or disease recurrence (all p > 0.05, Table 3).
 |
Table 3 Univariable and multivariable analysis of cause-specific survival (CSS) and disease-free survival (DFS) and FRα expression |
Discussion
ACC of the head and neck is one of the most common salivary gland malignancies. Long-term survival rates decrease steadily over time since the disease is characterized by late recurrences and distant metastasis. The preferred therapeutic options are surgery and radiotherapy. Due to the slow-growing characteristics of the disease the effect of chemotherapy is dismal. Therefore, investigation of new therapeutic options and biomarkers is required.3,4
In this study, FRα protein expression was evaluated in 43 patients with ACC of the head and neck using immunohistochemistry. FRα levels were positive in 47% of patients with ACC of the head and neck. The intensity of staining intensity was mostly low (21%) and moderate (19%), and only 5% showed high FRα staining. The staining was generally localized in the cytoplasm and membrane, with stained granules in all positive cells. This staining pattern is consistent with the transport mechanism of FRα. Folate is bound by membranous FRα. Subsequently, the folate-bound receptor is invaginated and transported into the cytoplasm as an endosome. Here, the pH in the endosomes acidifies and causes the release of folate from FRα, which further allows the transport of folate into the cytoplasm.20 Therefore, we hypothesize that the granules found in the immunohistochemical staining may represent the formed endosomes with folate-bound FRα. In conjunction with the membranous FRα staining, this indicates active FRα folate transport in ACC. However, the granules were not analyzed for endosomal markers.
Different therapeutic approaches targeting FRα, such as small molecules, antibodies and drug conjugates, are currently under investigation.5 The observed FRα staining in ACC of the head and neck indicates a mostly weak to moderate heterogeneous staining pattern. Patient outcome was not associated with FRα levels. In ovarian cancer and lung adenocarcinoma, FRα expression is mostly moderate or strong.8,10 While FRα is linked to a worse prognosis in ovarian cancer, high expression of FRα was associated with a better prognosis in lung cancer.11,21 In ovarian cancer, patients who received additional Vintafolide, a folate-conjugate, showed a better progression-free survival. When analyzed for FRα expression with Etarfolatide (a folate-technetium conjugate imaging agent), patients with 100% FRα expression showed the highest benefit.21 In contrast, there are also treatment options which may tolerate heterogeneous FRα expression. For example, Mirvetuximab, an antibody–drug conjugate, showed a cytotoxic effect in FRα-negative cells adjacent to FRα-positive cells.22 However, in a Phase III trial, ovarian cancer patients with high levels of FRα showed a better response regarding secondary endpoints but no difference in progression-free survival.18
Next, the tumor-free margin was analyzed. Here, the tumor-free margin tissue showed (mostly low) positive FRα staining in 22%. Interestingly, patients with positive FRα levels in the tumor showed positive margin staining in 55%. However, margin tissue was only available for 74% of ACC patients. In normal tissue FRα generally shows limited expression. Positive FRα expression is reported in the salivary glands, pancreas, thyroid, kidney, lung, placenta and breast.11,23 In contrast to our results, O’Shannessy et al observed strong staining of all investigated salivary gland samples (n = 3).11 When the radiolabeled folate [18F]AzaFol was investigated as a PET radiotracer for FRα-imaging, the uptake in salivary glands was generally low.24 Due to the limited expression of FRα in normal tissue which is mainly present at the apical surface, adverse effects of FRα targeted therapies may be less than those of chemotherapy.7,18 However, based on the available data, no clear conclusion can be drawn regarding the physiological expression of FRα in salivary glands.
Limitations, of this study are the retrospective setting and the small study cohort. FRα staining was investigated on a tissue microarray with three representative cores per tumor and two cores per tumor-free margin. However, distribution of staining may vary when examined on whole tissue slides. Therefore, external validation on whole tissue slides or using FRα specific imaging is recommended to further evaluate the feasibility of FRα targeted therapy in ACC of the head and neck. Furthermore, the margin samples are microscopically tumor-free, but may be altered at the molecular level.
In conclusion, FRα protein is present in 47% of ACC of the head and neck and shows no association with patient outcome. Based on the observed staining pattern which indicates active FRα transport mechanism, further evaluation of FRα as a targeted therapy in ACC of the head and neck is warranted. Altogether, a more detailed analysis is needed to further evaluate the distribution of FRα expression in ACC using either FRα specific imaging or whole tumor analysis.
Abbreviations
ACC, adenoid cystic carcinoma; CSS, cause-specific survival; DFS, disease-free survival; FRα, folate receptor alpha; OS, overall survival; PCFT, proton-coupled folate transporter; RFC, reduced folate carrier.
Data Sharing Statement
The datasets of this study are available from the corresponding author on reasonable request.
Ethics Approval
This study was approved by the ethics committee of the Medical University of Vienna (EK 1517/2018).
Funding
There is no funding to report.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Bradley PJ. Adenoid cystic carcinoma evaluation and management: progress with optimism! Curr Opin Otolaryngol Head Neck Surg. 2017;25(2):147–153. doi:10.1097/MOO.0000000000000347
2. Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck - an update. Oral Oncol. 2015;51(7):652–661. doi:10.1016/j.oraloncology.2015.04.005
3. Lorini L, Ardighieri L, Bozzola A, et al. Prognosis and management of recurrent and/or metastatic head and neck adenoid cystic carcinoma. Oral Oncol. 2021;115:105213. doi:10.1016/j.oraloncology.2021.105213
4. Dillon PM, Chakraborty S, Moskaluk CA, Joshi PJ, Thomas CY. Adenoid cystic carcinoma: a review of recent advances, molecular targets, and clinical trials. Head Neck. 2016;38(4):620–627. doi:10.1002/hed.23925
5. Scaranti M, Cojocaru E, Banerjee S, Banerji U. Exploiting the folate receptor α in oncology. Nat Rev Clin Oncol. 2020;17(6):349–359. doi:10.1038/s41571-020-0339-5
6. Cheung A, Bax HJ, Josephs DH, et al. Targeting folate receptor alpha for cancer treatment. Oncotarget. 2016;7(32):52553–52574. doi:10.18632/oncotarget.9651
7. Birrer MJ, Betella I, Martin LP, Moore KN. Is targeting the folate receptor in ovarian cancer coming of age? Oncologist. 2019;24(4):425–429. doi:10.1634/theoncologist.2018-0459
8. Markert S, Lassmann S, Gabriel B, et al. Alpha-folate receptor expression in epithelial ovarian carcinoma and non-neoplastic ovarian tissue. Anticancer Res. 2008;28(6A):3567–3572.
9. Köbel M, Madore J, Ramus SJ, et al. Evidence for a time-dependent association between FOLR1 expression and survival from ovarian carcinoma: implications for clinical testing. An ovarian tumour tissue analysis consortium study. Br J Cancer. 2014;111(12):2297–2307. doi:10.1038/bjc.2014.567
10. Kalli KR, Oberg AL, Keeney GL, et al. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol Oncol. 2008;108(3):619–626. doi:10.1016/j.ygyno.2007.11.020
11. O’Shannessy DJ, Yu G, Smale R, et al. Folate receptor alpha expression in lung cancer: diagnostic and prognostic significance. Oncotarget. 2012;3(4):414–425. doi:10.18632/oncotarget.519
12. Bremer RE, Scoggin TS, Somers EB, O’Shannessy DJ, Tacha DE. Interobserver agreement and assay reproducibility of folate receptor α expression in lung adenocarcinoma: a prognostic marker and potential therapeutic target. Arch Pathol Lab Med. 2013;137(12):1747–1752. doi:10.5858/arpa.2013-0039-OA
13. Zagorac I, Lončar B, Dmitrović B, Kralik K, Kovačević A. Correlation of folate receptor alpha expression with clinicopathological parameters and outcome in triple negative breast cancer. Ann Diagn Pathol. 2020;48:151596. doi:10.1016/j.anndiagpath.2020.151596
14. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/results?term=folate+receptor+alpha&Search=Apply&age_v=&gndr=&type=Intr&rslt=.
15. O’Malley DM, Matulonis UA, Birrer MJ, et al. Phase Ib study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol Oncol. 2020;157(2):379–385. doi:10.1016/j.ygyno.2020.01.037
16. Shimizu T, Fujiwara Y, Yonemori K, et al. First-in-human Phase 1 study of MORAb-202, an antibody–Drug conjugate comprising farletuzumab linked to Eribulin Mesylate, in patients with folate receptor-a–Positive advanced solid tumors. Clin Cancer Res. 2021;27(14):3905–3915. doi:10.1158/1078-0432.CCR-20-4740
17. Moore KN, Vergote I, Oaknin A, et al. FORWARD I: a Phase III study of mirvetuximab soravtansine versus chemotherapy in platinum-resistant ovarian cancer. Futur Oncol. 2018;14(17):1669–1678. doi:10.2217/fon-2017-0646
18. Moore KN, Oza AM, Colombo N, et al. Phase III, randomized trial of mirvetuximab soravtansine versus chemotherapy in patients with platinum-resistant ovarian cancer: primary analysis of FORWARD I. Ann Oncol. 2021;32(6):757–765. doi:10.1016/j.annonc.2021.02.017
19. Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–346. doi:10.1016/0197-2456(96)00075-X
20. Desai A, Sequeira JM, Quadros EV. The metabolic basis for developmental disorders due to defective folate transport. Biochimie. 2016;126:31–42. doi:10.1016/j.biochi.2016.02.012
21. Naumann RW, Coleman RL, Burger RA, et al. PRECEDENT: a randomized Phase II trial comparing vintafolide (EC145) and pegylated liposomal doxorubicin (PLD) in combination versus PLD alone in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2013;31(35):4400–4406. doi:10.1200/JCO.2013.49.7685
22. Ab O, Whiteman KR, Bartle LM, et al. IMGN853, a folate receptor-α (FRα)-targeting antibody-drug conjugate, exhibits potent targeted antitumor activity against FRα-expressing tumors. Mol Cancer Ther. 2015;14(7):1605–1613. doi:10.1158/1535-7163.MCT-14-1095
23. Weitman SD, Lark RH, Coney LR, et al. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992;52(12):3396–3401.
24. Gnesin S, Müller J, Burger IA, et al. Radiation dosimetry of 18F-AzaFol: a first in-human use of a folate receptor PET tracer. EJNMMI Res. 2020;10(1). doi:10.1186/s13550-020-00624-2
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.