Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 13
Protein-Energy Wasting and Associated Factors Among Chronic Kidney Disease Patients at St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia
Authors Merga C, Girma M, Teshome MS
Received 25 July 2020
Accepted for publication 6 October 2020
Published 2 November 2020 Volume 2020:13 Pages 307—318
DOI https://doi.org/10.2147/IJNRD.S273874
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Pravin Singhal
Chaltu Merga,1 Meron Girma,2 Melese Sinaga Teshome2
1Department of Nutrition, Ambo University, Ambo, Ethiopia; 2Department of Nutrition and Dietetics, Faculty of Public Health, Health Institute, Jimma University, Jimma, Ethiopia
Correspondence: Melese Sinaga Teshome Email [email protected]
Background: Protein-energy wasting is a major issue in patients with chronic kidney disease (CKD), adversely affecting morbidity, mortality, functional activity, and quality of life. Assessment of nutritional status in CKD patients is important to ensure patient’s normal protein stores and avoid metabolic complications. However, in Ethiopia, there were very few studies done to assess the prevalence of protein-energy wasting (PEW) and its associated factors among CKD patients.
Objective: To assess protein-energy wasting and associated factors among chronic kidney disease patients at adult OPD St. Paulo’s Hospital Millennium Medical College, Addis Ababa Ethiopia.
Materials and Methods: An institution-based cross-sectional study was conducted from March to April 2019. Data were collected using a structured interviewer-administered questionnaire and anthropometric measurements, and laboratory data were collected from patient files. Data were entered into Epi-data version 3.1 and analyzed using SPSS version 20. Both bivariate and multiple logistic regression analyses were performed to identify associated factors. P values < 0.05 were considered to declare statistical significance.
Results: In this study, 274 respondents were interviewed with a response rate of 100%. The prevalence of protein-energy wasting in CKD patients was 23.4%. Chronic kidney disease patients at age ≥ 65 years were seven times more likely to have protein-energy wasting as compared to patients aged 18– 34 years [AOR=7.0, 95% (CI: 2.4, 20.5)]. Patients who had a history of anorexia were 5.2 times more likely to have protein-energy wasting as compared to those who had no history of anorexia [(AOR=5.2, 95% (CI: 2.7, 10.3)] and were significantly associated with the risk of having CKD.
Conclusion: The prevalence of protein-energy wasting among CKD patients was high and associated with age, physical activity, history of anorexia, and CKD stages. Interventions that address nutritional problems and lifestyle factors in CKD patients need to be implemented together with medical treatment.
Keywords: chronic kidney disease, protein-energy wasting, body mass index, serum albumin
Introduction
Chronic kidney disease (CKD) is defined as a reduction of kidney function that an estimated Glomerular filtration rate (eGFR) <60 mL/min/1.73m2, increased urinary albumin excretion (albuminuria defined as > 30 mg of urine albumin per gram of urine creatinine) or both for >3 months.1 National Kidney Disease Education Program defines kidney as it regulates the composition and volume of blood, removes metabolic wastes in the urine, and helps control the acid/base balance in the body.2
Chronic kidney disease (CKD) is a worldwide public health problem associated with adverse outcomes of kidney failure, cardiovascular disease (CVD), and premature death.3 There are an increasing incidence and prevalence of patients with kidney failure requiring replacement therapy, with poor outcomes and high cost.4 The risk of all-cause, and cardiovascular mortality, kidney failure, cardiovascular disease and hospitalizations is higher among diabetic patients with CKD.5 Obesity, hypertension, and diabetes resulted due to changes in lifestyle and urbanizations have led to an increased risk of CKD. Years of high blood pressure can damage the delicate filters in the kidney, leading to less efficient removal of waste products from the kidney.6 In the United States, the prevalence of CKD is approximately 30% among adults with hypertension and 17% among obese adults.7
The International Society of Renal Nutrition and Metabolism (ISRNM) panel has described PEW as a state of decreased body stores of protein and energy fuels. This deterioration of the clinical nutritional status is characterized by low visceral proteins’ levels (serum albumin, prealbumin), cholesterol, and decreased anthropometric measurements.8 Protein-energy wasting (PEW) is common in patients with chronic kidney disease (CKD) and is associated with adverse clinical outcomes, especially in individuals receiving maintenance dialysis therapy.9 There is a high prevalence of protein-energy malnutrition in both non-dialyzed patients with advanced chronic renal failure and those individuals receiving dialysis therapy. Approximately one-third of maintenance dialysis patients have mild to moderate protein-energy malnutrition, and about 6% to 8% of these individuals have severe malnutrition. These statistics are of major concern because markers of protein-energy malnutrition are strong predictors of morbidity and mortality.10
Its Complications include increased all-cause and cardiovascular mortality, kidney-disease progression, acute kidney injury, cognitive decline, anemia, protein-energy wasting, mineral and bone disorders, and fractures which have a huge impact on global economic growth.11 Adverse changes in nutrition are prevalent with decreasing renal function and are a strong indicator of adverse outcomes in patients with CKD.12 By 2020, the burden of diabetes and cardiovascular diseases would have increased by 130% in Africa, with concomitant increases in the incidence of CKD and ESRD which contribute to increasing adverse changes in their nutritional status.13
Globally, meta-analysis observational studies (published during 2000–2014) from the International Society of Renal Nutrition and Metabolism (ISRNM) conducted five studies, patients with CKD stages 3–5 reported PEW prevalence ranging from 11% to 54%. The large variation in PEW prevalence across studies remained even when accounting for moderators.14 A study conducted in Europe reported that 26% had moderate PEW (SGA 3–5), and less than 1% had severe PEW (SGA 1–2).15 A study conducted among patients with CKD at Mulago hospital, Kampala-Uganda: shows that the prevalence of PEW among CKD patients was 47.3% compared to 21.3% among the non-CKD participants.16
In Africa, a study conducted at HKM teaching hospital in Cotonou in Sub Saharan based on different tools assessment Mini Nutritional Assessment (MNA) score, Subjective Global Assessment (SGA) score, and ISRNM criteria reported that 42.75%, 36.24%, and 14.09% of CKD patients were experienced protein-energy wasting, respectively.17
In Ethiopia, there are no national-level data but studies from Addis Ababa, at Black Lion Hospital revealed a significant prevalence of protein-energy wasting (PEW) (20%) in chronic kidney disease patients. There was a significant lowering of serum albumin, and BMI among all groups of CKD patients when compared with controls p<0.05. The prevalence of protein-energy wasting increased with the advancing stage of CKD. Sixty-three point two percent (63.2%) of patients with GFR <15 mL/min/1.73m2 had lowest Body mass index (BMI) values <18.5 kg/m2.18
The clinical consequence of PEW may be severe and requires rapid and effective treatment since it is associated with increased hospitalization rates, poor wound healing, increased susceptibility to infection, increased overall, and cardiovascular mortality rate.19
However, limited studies in Ethiopia were conducted to estimate the magnitude of PEW and its associated factors among CKD patients which is an important step towards planning effective intervention strategies. Therefore, the aim of this study was conducted to determine the prevalence of protein-energy wasting and associated factors among chronic kidney disease patients and provide the finding to improve the patients’ treatment outcome. This study will also useful to provide baseline information and directions for further research activities on similar problems.
Materials and Methods
Study Setting, Design and Participants
An institutional-based cross-sectional study was conducted for a period of 6 weeks from March 1 to April 30, 2019, at St. Paul’s Hospital Millennium Medical College which is a referral hospital in Addis Ababa under the guidance of the Ethiopian Federal Ministry of Health (FMOH). St. Paul’s Hospital Millennium Medical College currently has 392 beds with an annual average of 200,000 patients and a catchment population of more than 5 million. There is over 1300 clinical and non-clinical staff in over 15 departments, most recently launching its new hemodialysis unit. Adult renal unit averagely treats 30–40 patients per day by 3 nephrology students (residents), around seven nurses, and 3 porters.
All chronic kidney disease patients who have a follow-up at the adult outpatient department (OPD) St. Paulo’s medical hospital was source of population, while all randomly selected individuals enrolled in the study were the study population. All chronic kidney disease patients who have a follow-up at adult OPD St. Paulo’s hospital at least for the past 3 months were included in the study. Exclusion criteria were the presence of edema, body deformity, pregnancy, newly diagnosed, and inability to respond to questions.
Sample Size
The sample size was determined by considering a 95% confidence level (CL) for both objectives separately. For the first objective, the sample size was calculated using a single population proportion formula with the following assumptions: by using the prevalence of PEW among CKD patents, the study was done in Addis Ababa at Black Lion Hospital 20%,18 95% confidence level (Z=1.96), and 5% margin of error. The calculated sample size was n=246.
For the second objective sample size was calculated by using the prevalence of different associated factors that get from the literature the prevalence of CKD with HTN (hypertension) was 58.9%.5 Then, the larger sample size (372) was taken to address both dependent and independent variables. The expected number of source population in the study period (N), Based on the Health Management Information System reports of St. Paulo’s hospital the average number of CKD patients coming to the hospital with a total of 6 weeks was 750. Since the source population is less than 10,000, the correction formula is used.
The sample size was determined by using the following formula
n = 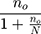 , where: N= Population size
, where: N= Population size
n=249
Non-respondents 10%=249× 10%=24.9
By considering a 10% non-response rate, thus, the total sample size was n=274.
A systematic random sampling method was employed to select individuals during their follow-up period. To select individual participants, the K value was determined (interval). So, k=750/274 =2.7~3, the starting participant was randomly selected from 1 to 3 by using the lottery method. Data were collected from every 3rd patient.
Data Collection and Measurements
A structured questionnaire developed in English after reviewing different literature was used for data collection. Data were collected face to face by using structured interviewer-administered questionnaires and anthropometric measurements. A structured questionnaire was used to collect socio-economic and demographic data, family history of chronic disease, presence of co-morbidities, risky Lifestyle behavior, and dietary practice was assessed using a semi-quantitative dietary history method. The anthropometric measures of weight and height were measured using standardized techniques.
The body weight was measured in a digital anthropometric weighing scale. The scale was placed on a hard flat floor surface. Weighing scales were validated with known weight object every morning and checked against zero reading after weighing every participant. The patient was positioned in the center of the equipment, with light clothing, no shoes, erect, with feet together and arms extended along the body. Weight was measured in all patients in this way and was taken to the nearest 0.1 kg.
Height was measured, using a portable stadiometer to the nearest 0.1 cm. Height was measured in all participants, with the weight distributed between the feet, heels together, in an upright posture, with the head free of props and looking straight ahead at a fixed point at eye level. Based on weight and height and Body mass index was calculated.
The most recent data on laboratory markers, serum albumin, serum total cholesterol, serum creatinine, and Blood Urea Nitrogen (BUN) were taken from the patient’s file. Estimation of Glomerular Filtration Rate (GFR) based on age, sex, and creatinine was calculated separately for men and women by using the Modification of Diet in Renal Disease (MDRD) formula.
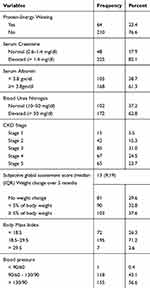
The subjective global assessment tool was used to assess the nutritional status of the study participants. The SGA is widely used in clinical settings to get a quick assessment of nutritional status. The SGA tool used in this study has seven components (weight change, dietary intake, gastrointestinal symptoms, co-morbidities, a physical change which contains fat loss and muscle wasting). A patient is rated on a scale of 1 to 5 on each component. The total score ranges between 7 and 35, 7 being normal and 35 indicating the most severe malnutrition.24
PEW was defined according to the International Society of Renal Nutrition and Metabolism (ISRNM) expert panel criteria as.8
Serum albumin <3.8 g/100mL,
BMI < 18.5 kg/m2and
Weight loss ≥5% of body weight in the past 3 months
The questionnaire prepared in English was translated into the local language Amharic for fieldwork purposes and back to English for rechecking language consistency. Data qualities were ensured during tool development, data collection, entry, cleaning, and analysis. The training was given for 1 day for the three Bsc Nurses data collectors and Supervisors including the procedure.
A pre-test was done among 14 (5%) of the total sample size at RDDMH before the actual data collection period and proper modifications were done based on the feedback. Weighing scales were checked against zero reading after weighing every participant and validated with known weight object every morning.
Statistical Analysis
The collected data were checked for completeness and consistency manually. Then, data were entered into Epi-data version 3.1 and exported to SPSS software program version 20 for analysis. Data were checked for missing values and outliers before analysis. A one-sample Kolmogorov–Smirnov test was used to assess the normality of the data. Descriptive statistics were used to examine the frequency distributions of study variables. Mean, median, frequency, and percentiles were done. To allow for comparisons with other studies, PEW was analyzed using ISRNM criteria. The cross-tab test for independence was used to determine the association between PEW and certain nominal variables. The correlations between quantitative variables using the Pearson correlation coefficient were done. A bi-variable binary logistic regression model was used to check the association between each independent and dependent variable by estimating the crude odds ratio (COR) with 95% CI. All independent variables which had an association with the outcome variable at a p-value of less than 0.25 were chosen for multivariate analysis. Then, multivariate analysis using the backward method was done to determine the presence of statistically significant associations between independent variables and the outcome variables at p-values less than or equal to 0.05 and adjusted odds ratio (AOR) with 95% CI. Statistical significance was declared at a p-value of less than 0.05 with a 95% confidence interval. The goodness of fit of the model was assessed using the Hosmer and Lemeshow test, which indicated the non-significant chi-square (p-value of 0.602). This indicates that the model was appropriate for data analysis. Finally, the results are presented in the form of tables, figures, and text using frequencies and summary statistics.
Results
Description of Study Participants
In this study, 274 respondents were interviewed with a response rate of 100%. Of those respondents, 164 (59.9%) were males, 110 (40.1%) of the females. The mean and standard deviation of the age was 45.0 (SD±14.6), with a range of 18–81 years. Concerning educational status, 60 (21.9%) of respondents did not attend formal education, whereas 74 (27%) attended primary school followed by 50 (18.2%) who attended secondary, 90 (32.8%) who had a diploma and above. The majority of the respondents were married 160 (58.4%), and 54 (19.7%) were single. The majority of the study subjects were Oromo in ethnicity 96 (35%) followed by Amara 81 (29.6%), and Gurage 53 (19.3%). On the other hand, 82 (29.9%) were government employees and 39 (14.2%) of the respondents were merchants (Table 1).
 |
Table 1 Socio-Economic and Demographic Characteristics of CKD Patients Attending Adult OPD at St Paulo’s Hospital Millennium Medical College, Addis Ababa, Ethiopia, March 1 to 30 April, 2019 (n=274) |
Disease and Risky Life Style Related Characteristics of the Respondents
Out of 274 patients, 104 (38.0%) had a family history of chronic disease with most reporting a history of hypertension 44 (42.3%) and diabetes mellitus 34 (32.7%). Close to half, 69 (41.6%) and 42 (25.3%) of the respondents had a history of HTN and Diabetes Mellitus (DM) respectively, before they become sick chronic kidney disease. However, currently, the majority of the respondents 235 (85.8%) had co-morbidity conditions. Of those, Hypertension 116 (42.3%), Diabetes Mellitus 36 (13.3%), both Hypertension and Diabetes Mellitus 49 (17.9%), other (CVD, Anemia) 34 (12.4%). The majority of the patients 146 (53.3%) had 1–5 years durations of CKD follow up at the renal unit while only 70 (25.5%) had >5 years duration of follow up. On the other hand, 32 (11.7%) of the respondents had a history of smoking tobacco and the rest were non-smokers. From those smokers, 87.5% had a history of smoking tobacco for more than 10 years and nobody currently smokes tobacco. Furthermore, 85 (31.0%) of respondents had a history of alcohol consumption, and the majority of the 96.5% reported consuming alcohol for more than ten years. Currently, 19 (23.8%) drink at least more than two alcoholic drinks rarely (Table 2).
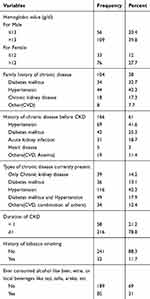 |
Table 2 Disease and Health-Related Characteristics of CKD Patients Attending Renal Unit at St Paulo’s Hospital Millennium Medical College, Addis Ababa, Ethiopia, March 1 to 30 April, 2019 (n=274) |
Dietary Practice and Physical Activity Characteristics of the Respondents
Regarding dietary practice, 77 (28.1%) of the respondents were having a history of eating foods from restaurants, and of those 42 (54.5%) used most of the time from restaurants. The majority of the respondents 146 (53.3%) used solid oil for meal preparation in their household while 73 (26.6%) used liquid oil. Out of the total, 234 (85.4%) of respondents had a history of eating fruit and vegetables. However, 88 (32.1%) of the respondents experienced a loss of appetite. Among those 61 (69.3%) of the respondents experienced a loss of appetite most of the time while 19 (21.6%), 8(9.1%) rarely, and always experienced loss of appetite, respectively.
Fourteen percent of 39 (14.2%) of the respondents reported that they engaged in moderate to vigorous scheduled exercise before they become sick. However, 59 (21.5%) currently had an exercise plan they set with their doctors and almost all of them 57 (98.3%) reported that they adhere to this plan. The majority of the patients 139 (50.7%) reported they currently have an active activity level, while 19 (6.9%) were sedentary (Table 3).
 |
Table 3 Dietary Practice and Physical Activity Characteristics of CKD Patients Attending Adult OPD at St Paulo’s Hospital Millennium Medical College, AA, Ethiopia, March 1 to 30 April, 2019 (n=274) |
Anthropometric Status and Biochemical Markers
Anthropometric and biochemical parameters were used to assess protein-energy wasting among chronic kidney disease patients. In Body mass category, body mass index and unintentional weight loss were assessed. Regarding body mass index, 72 (26.3%) had BMI <18.5 kg/m2 and about 103 (37.6%) of patients lost ≥5% of their body weight in the past 3 months. In the biochemical category, s-albumin, s-Creatinine, and Blood Urea Nitrogen were analyzed. About 105 (38.7%) patients had s-albumin below the reference value (<3.8 g/dl). Moreover, the majority of the patients 225 (82.1%) had elevated serum Creatinine or above the normal range (0.6–1.4 mg/dl). The interquartile range (IQR) of subjective global assessment scores is 13 (9, 19) median, 25th, 75th percentiles, respectively. These suppose that 50% of the subjective global assessment score of the CKD patients found between 9 and 19 (Table 4).
The prevalence of protein-energy wasting in CKD patients according to ISRNM criteria was 23.4%. The percentage was substantially higher in stage 4, 18 (28.1%) and stage 5, 25 (39.1%), when compared to stage 3, 16 (25.0%), stage 2, 4(6.2%) and 1(1.6%) in stage 1 (Figure 1).
Correlation Between SGA and Some Biochemical Variables
SGA score had a moderate association and was significantly correlated with kidney function biomarkers. Pearson correlation analysis showed that there was a significant positive correlation between SGA with BUN (r=0.36. P < 0.001) and serum creatinine (r= 0.33, P < 0.001). On the other, as is expected SGA scores were negatively correlated with serum albumin (r= −0.44, P < 0.001) (Table 5).
Factors Associated with PEW Among CKD Patients
In bivariate analysis age, educational status, presence of any chronic disease before the development of CKD, currently presence of any chronic disease, family monthly income, duration of CKD follow-up, presence of an exercise plan set prescribed by a doctor, history of anorexia, and stage of chronic Kidney disease were identified as candidate variables (p-value <0.25) and were considered for multivariate analysis.
In the multivariate analysis, age, presence of an exercise plan set prescribed by a doctor, history of anorexia, and stage of chronic Kidney disease were independent predictors of protein-energy wasting among CKD patients.
Chronic Kidney disease patients at age ≥65 years were seven times more likely to have protein-energy wasting as compared to patients aged 18–34 years [AOR=7.0, 95% (CI: 2.4, 20.5)]. Patients who had any chronic disease before CKD were 1.8 times more likely to have protein-energy wasting [AOR=1.8, 95% (CI: 0.9, 3.6)] as compared to patients who had not any chronic disease before CKD. Odds of PEW among chronic kidney disease patients who had exercise plans were 82% less as compared to patients who had not to exercise plan set with their doctor [(AOR=0.18, 95% CI: (0.057, 0.55)].
Patients who had a history of anorexia were 5.2 times more likely to have protein-energy wasting as compared to those who had no history of anorexia [(AOR=5.2, 95% (CI: 2.7, 10.3)]. Furthermore, odds of PEW among chronic kidney disease patients at stages 4 and 5 were 3.6 times higher than odds of PEW among patients at stage 2, and stage one [AOR=3.6, 95% CI: (1.3, 10.3)] (Table 6).
Discussion
This study aimed to assess the level of protein-energy wasting and its associated factors among CKD patients. The prevalence of protein-energy wasting in CKD patients was 23.4%; age, presence of exercise plan set prescribed by a doctor, history of anorexia, and stage of chronic Kidney disease were significantly associated with protein-energy wasting after controlling for educational status, family monthly income, presence of any chronic disease before the development of CKD, duration of CKD follow-up, and the current presence of any co-morbidity.
There is only one study that has assessed protein-energy wasting (PEW) among Ethiopian CKD patients. The prevalence of PEW seen in this study was comparable to what was reported for CKD patients in Addis Ababa at Black Lion Hospital 20%.18 However, the prevalence of PEW reported in both studies for Ethiopian CKD patients is much lower than what was reported from Mulago hospital, Kampala-Uganda which shows that the prevalence of PEW among CKD patients was 44%.16 This difference might be due to geographical and lifestyle variations among populations included in the study.
The finding of this study showed a significant association between age and protein-energy wasting with a high prevalence of protein-energy wasting in elder CKD patients. Chronic kidney disease patients at age ≥65 years were seven times more likely to have protein-energy wasting as compared to patients age 18–34 years [AOR=7.0, 95% (CI: 2.4, 20.5)] with p=0.000. This finding was similar to the Southern Nigeria study which reported that malnutrition was significantly commoner in elderly patients (p=0.047).20
The finding of this study showed a significant association between physical activity (exercise) and protein-energy wasting with a high prevalence of protein-energy wasting in physically inactive CKD patients. Odds of PEW among chronic kidney disease patients who had exercise plans were 82% less as compared to patients who had not to exercise plan set with their doctor [(AOR=0.18, 95% CI: (0.057, 0.55)]. A study conducted in Brazil reported that exercise is important to reduce inflammatory cytokines which often caused anorexia that reduced food intake of CKD patients and improving protein utilization, serum albumin levels, muscle strength even in CKD patients on a low-protein diet.23
The finding of this study shows that 32.1% of the respondents had a history of anorexia which is closer to a study conducted in Niger in Sub-Saharan Africa, reported that 27.7% of CKD patients had a history of anorexia.22 But these both findings are lower than the finding of a study done among 40 hospitalized children from 2013 to 2014 which shows that 33 (82.5%) of them experienced anorexia.21 This discrepancy is due to the socio-demography of the study subjects especially age. This study also showed that patients who had a history of anorexia were 5.2 times more likely to have protein-energy wasting as compared to those who had no history of anorexia [(AOR=5.2, 95% (CI: 2.7, 10.3)].
The finding of this study showed a significant association between the stage of chronic kidney disease and protein-energy wasting with a high prevalence of protein-energy wasting in advanced CKD stage. Odds of PEW among chronic kidney disease patients at stages 4 and 5 were 3.6 times higher than odds of PEW among patients at stage 2, and stage one [AOR=3.6, 95% CI: (1.3, 10.3)]. This finding is similar to the Ugandan study which reported that the stage of CKD was associated with PEW; especially CKD patients with stage 4 were 6.4 times more likely to develop PEW compared to stage 1 CKD.16
As renal function decrease with progression in the CKD stage, inflammatory cytokines increase due to uremic toxin which often causes anorexia that reduces food intake of CKD patients thus predisposing patients to PEW.25 Furthermore, this study showed a significant positive correlation of SGA score with BUN (r=0.36. P < 0.001) and serum creatinine (r= 0.33, P < 0.001) and was negatively correlated with serum albumin (r= −0.44, P < 0.001). A study conducted by the National Kidney Foundation reported that a moderate to a good level of agreement was found between the anthropometric parameters and presence of PEW evaluated by SGA.26 SGA is a tool used by health care providers as a reliable, quick, and easy method for protein-energy wasting (PEW) evaluation in chronic kidney disease (CKD) patients and aid in the prediction of nutrition-associated clinical outcomes.27
Moreover, SGA has been recommended by the National Kidney Foundation (NKF) Kidney Disease/Dialysis Outcomes and Quality Initiative (K/DOQI) for use in nutritional assessment in both the adult non-dialysis and dialysis population.28 The strong association seen between PEW and SGA score in this study indicates that SGA can be a valid tool in the assessment of nutritional status in Ethiopian CKD patients.
Even though this study addressed very important issues, it should be highlighted with the following limitations. A cross-sectional design does not allow inferring cause and effect relationships. Due to different constraints’ laboratory data were taken from patient’s file and since the lifestyle and dietary habits were self-reported, there might be over- or under-reporting.
Conclusion
A different study showed that the prevalence of protein-energy wasting among CKD patients was considerably high. This study also showed that the prevalence of protein-energy wasting in CKD patients was 23.4%. Even if factors of protein-energy wasting are complex and are not limited to these, the present study isolated certain socio-demographic, co-morbidity, dietary, lifestyle, and CKD stage factors that are linked with greater risk of protein-energy wasting among this population.
Accordingly, age, presence of an exercise plan set prescribed by a doctor, history of anorexia, and stage of chronic Kidney disease were significantly associated with protein-energy wasting. However, the strong association seen between PEW and SGA score in this study indicates that SGA can be a valid tool in the assessment of nutritional status in Ethiopian CKD patients. Interventions that address nutritional problems and lifestyle factors in CKD patients need to be implemented together with medical treatment.
Data Sharing Statement
The dataset used for this study cannot be shared, and in the future, interested parties may request the approval to access the data by writing to Jimma University Institutional Review Board.
Ethical Consideration
Ethical clearance was obtained from the Ethical Review Board of Jimma University and submitted to St. Paulo’s Hospital Millennium Medical College research director office with a Support letter. Permission letters were obtained from St. Paulo’s Hospital Millennium Medical College research director office to communicate with relevant bodies in the nephrology clinic. Finally, after explaining the study procedures for the study subjects, verbal as well as written informed consents were obtained from each patient during the data collection period. This study was conducted in accordance with the Declaration of Helsinki.
Consent for Publication
Not applicable.
Acknowledgments
The authors would like to acknowledge the research office of Jimma University Institute of Health, Faculty of Public Health, Department of nutrition and dietetics forgiving as the opportunity to conduct this research. My appreciation also goes to the study participants, data collectors, and all staff of renal units of St. Paulo’s Hospitals for their full cooperation during the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Melese Sinaga Teshome; the Corresponding author had the responsibility to submit the manuscript for publication.
Funding
The study was fully funded by Jimma University, Institute of Health, Faculty of Public Health, Department of Nutrition and dietetics.
Disclosure
We confirm that this research is our original paper and that there is no conflict of interest in this work.
References
1. Wang AY The impact of CKD identification in large countries: the burden of illness global kidney disease 3 chronic kidney disease: global dimension and perspectives. Lancet. http://dx.doi.10.1016/S0140-6736(13)60687-X
2. Kidney N, Education D. Making sense of CKD A concise guide for managing chronic. 2014;
3. Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney international. 2005;67(6):2089–2100.
4. Eknoyan G, Lameire N, Barsoum R, et al. The burden of kidney disease: improving global outcomes. Kidney Int. 2004;66(4):1310–1314. doi:10.1111/j.1523-1755.2004.00894.x
5. Fiseha T, Kassim M, Yemane T. Prevalence of chronic kidney disease and associated risk factors among diabetic patients in southern Ethiopia. Am J Health Res. 2014;2(4):216–221.
6. Mathew AB, Ravi K, Gopalan TR. Impact of diabetes on the nutritional status of CKD patients. Heighpubs Otolaryngol Rhinol. 2017;1. doi:001-004.10.29328/journal.hor.1001001.
7. Luyckx VA, Tuttle KR, Garcia-Garcia G, et al. Reducing major risk factors for chronic kidney disease. Kidney International Supplements. 2017;7(2):71–87. doi:10.1016/j.kisu.2017.07.003
8. Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein–energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391–398.
9. Ikizler TA, Cano NJ, Franch H, et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the international society of renal nutrition and metabolism. Kidney Int. 2013;84(6):1096–1107. doi:10.1038/ki.2013.147
10. Kopple JD. Pathophysiology of protein-energy wasting in chronic renal failure. J Nutr. 1999;129(1):247S51S. doi:10.1093/jn/129.1.247S
11. Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. doi:10.1016/S0140-6736(13)60687-X
12. Fouque D, Pelletier S, Mafra D, Chauveau P. Nutrition and chronic kidney disease. Kidney Int. 2011;80(4):348–357. doi:10.1038/ki.2011.118
13. Krzesinski JM, Sumaili KE, Cohen E. How to tackle the avalanche of chronic kidney disease in sub-saharan africa: the situation in the democratic republic of congo as an example. 2007;22(2):332–335. doi:10.1093/ndt/gfl494
14. Carrero JJ, Thomas F, Nagy K, et al. Global prevalence of protein-energy wasting in kidney disease: a meta-analysis of contemporary observational studies from the international society of renal nutrition and metabolism. J Renal Nutrition. 2018;28(6):380–392. doi:10.1053/j.jrn.2018.08.006
15. Windahl K, Irving GF, Almquist T, et al. Prevalence and risk of protein-energy wasting assessed by subjective global assessment in older adults with advanced chronic kidney disease: results from the EQUAL study. J Renal Nutrition. 2018;28(3):165–174. doi:10.1053/j.jrn.2017.11.002
16. Namuyimbwa L, Atuheire C, Okullo J, Kalyesubula R. Prevalence and associated factors of protein-energy wasting among patients with chronic kidney disease at Mulago hospital, Kampala-Uganda: a cross-sectional study. BMC Nephrol. 2018;19(1):139. doi:10.1186/s12882-018-0920-7
17. Agboton BL, Agueh VD, Vigan J, et al. Assessing the nutritional status of hemodialysis patients in a Sub-Saharan Country. J Kidney. 2017;3(145):12202472.
18. Mamuye BI Assessment of Protein Energy Wasting in Patients with Chronic Kidney Disease Attending Renal Unit at Tikuranbesa Specialised Hospital. Addis Ababa University. 2016 Apr. p. 18–28.
19. Thompson K. Assessing nutrition in patients with chronic kidney disease. Today’s Dietitian, the Magazine for Nutrition Professionals. 2012;23.
20. Oluseyi A, Enajite O. Malnutrition in pre-dialysis chronic kidney disease patients in a teaching hospital in Southern Nigeria. Afr Health Sci. 2016;16(1):234–241. doi:10.4314/ahs.v16i1.31
21. Evenepoel P, Meijers BK. Dietary fiber and protein: nutritional therapy in chronic kidney disease and beyond. Kidney Int. 2012;81(3):227–229. doi:10.1038/ki.2011.394
22. Sharifian M, Shiva MR, Sepahi MA, Shohadaee S, Esfandiar N, Fallah F. Urinary ghrelin concentration in children with urinary tract infections before and after treatment. Arch Pediatr Infect Dis. 2016;4(2):e34096. doi:10.5812/pedinfect.34096
23. Canaud B, Kooman J, Selby NM, et al. Sodium and water handling during hemodialysis: new pathophysiologic insights and management approaches for improving outcomes in end-stage kidney disease. Kidney Int. 2019;95(2):296–309. doi:10.1016/j.kint.2018.09.024
24. Steiber AL, Kalantar-Zadeh K, Secker D, McCarthy M, Sehgal A, McCann L. Subjective Global Assessment in chronic kidney disease: a review. J Renal Nutrition. 2004;14(4):191–200. doi:10.1016/S1051-2276(04)00139-6
25. Ikizler TA. Nutrition and kidney disease in humans content presented at the 2017 hill ’ s global symposium. 2017;1–10.
26. Cuppari L, Meireles MS, Ramos CI, Kamimura MA. Subjective global assessment for the diagnosis of protein–energy wasting in nondialysis-dependent chronic kidney disease patients. J Renal Nutrition. 2014;24(6):385–389. doi:10.1053/j.jrn.2014.05.004
27. Dai L, Mukai H, Lindholm B, et al. Clinical global assessment of nutritional status as predictor of mortality in chronic kidney disease patients. PLoS One. 2017;12(12):e0186659. doi:10.1371/journal.pone.0186659
28. Foundation NK. K/DOQI, national kidney foundation: clinical practice guidelines for nutrition in chronic renal failure.AJKD off J Natl Kidney Found Am J Kidney Dis. 2000;35(6):19.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.