Back to Journals » Medical Devices: Evidence and Research » Volume 15
Protective Mechanisms of Liquid Formulations for Gastro-Oesophageal Reflux Disease in a Human Reconstructed Oesophageal Epithelium Model
Authors Ceriotti L , Buratti P, Corazziari ES, Meloni M
Received 3 March 2022
Accepted for publication 27 April 2022
Published 18 May 2022 Volume 2022:15 Pages 143—152
DOI https://doi.org/10.2147/MDER.S363616
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Laura Ceriotti,1 Paolo Buratti,1 Enrico Stefano Corazziari,2 Marisa Meloni1
1In vitro Innovation Center, VitroScreen srl, Milan, Italy; 2Department of Gastroenterology, IRCCS Humanitas Research Hospital, Rozzano (Milan), Italy
Correspondence: Laura Ceriotti, VitroScreen, in vitro Innovation Center, Via Mosè Bianchi, 103, Milan, 20149, Italy, Email [email protected]
Purpose: A novel experimental design based on a human-reconstructed oesophageal epithelium (HO2E) model has been applied to quantitively assess the properties of a set of liquid formulations, Device A (Gerdoff® Protection), Device B (Esoxx® One), and Device C (Marial® gel) developed to form a temporary physical barrier on the oesophageal epithelium and modify epithelial permeability so to protect the oesophageal mucosa from refluxate components.
Methods: The formulations were applied to a prewetted HO2E model for 15 min. Then, a 0.5% caffeine solution was applied, and its penetration kinetics was assessed at 1 h and 2 h in acidic environments (pH= 3.3) to mirror exposure of the oesophageal mucosa to acidic reflux in GORD patients. Caffeine permeated into the basolateral compartment (evaluated by HPLC-UV) and Lucifer yellow (LY) permeability were quantified 15 min after application of the caffeine in acidic environments.
Results: At the 15 min timepoint, Device A reduced caffeine permeation by 77.2% and LY flux by 30.4% compared to the untreated control and with a faster mode of action than that of the other liquid formulations. Transepithelial caffeine flux was reduced, albeit with different timing and efficiency, by all three compounds up to the end of the 2 hour experiment. At 1 h, Device A reduced the caffeine flux by 79.2%; Device B, by 67.2%; and Device C, by 37%.
Conclusion: These results confirm the ability of the medical devices tested to interact with the oesophageal epithelium and create a temporary physical protective film for up to 2 hours after their application. The results underline differences in the mechanism of action of the three medical devices, with Device A performing faster than the other formulations. The overall results support the relevance of the reconstructed mucosal model to investigate oesophageal epithelium–product interactions and precisely differentiate liquid formulation performance.
Keywords: gastro-oesophageal reflux disease, film-forming properties, caffeine permeation, epithelial permeability, epithelial protection, Lucifer yellow assay
Introduction
Heartburn is the most frequent typical symptom of gastro-oesophageal reflux disease (GORD or GERD) and is experienced by 14% of the global population, reaching 20% in North America and 22% in Turkey.1,2 GORD is a chronic disease that affects quality of life and, if not properly managed, can lead to well-known complications (eg, erosive oesophagitis (EO), oesophageal stenosis, Barrett’s oesophagus and adenocarcinoma). Many patients self-medicate with OTC medications such as antacids and low-dose histamine H2-receptor antagonists (H2RAs) to relieve episodic or food-related symptoms of GORD without seeking medical advice unless symptoms worsen. Proton pump inhibitors (PPIs) are commonly used to treat GORD; however, not all patients benefit from them, as such agents are ineffective in 30% and 40% of GORD patients with Erosive Reflux Disease (ERD) and with Non-Erosive Reflux Disease (NERD), respectively.3–10
In recent years, attention has been focused on the mechanism that impairs oesophageal barrier function,11–14 and the development of new products that adhere to the oesophageal mucosa to form a stable film with a barrier effect. Such an effect aims to protect the oesophageal mucosa, enhancing its physiological defence mechanisms against the damaging action of gastro-oesophageal reflux (GOR) and thus preventing the GORD-related symptoms of heartburn and retrosternal and epigastric pain.2,11 It is, however, still challenging to reproduce the GORD model mirroring the anatomic system constituted by the oesophageal mucosa in the presence of GOR, and many preclinical approaches have been proposed adopting ex vivo explants of human or animal origins.11–15 These models were mainly used to demonstrate the adhesiveness of the tested substances to the oesophageal and intestinal epithelium. In these investigations, the barrier effect in reducing the transmucosal permeability of the tested substances was assessed in experiments in which noxious agents previously caused epithelial lesions. Despite their scientific rationale and robustness, these approaches suffer from the limited availability and reproducibility of ex vivo animal explants. Furthermore, they raise ethical concerns for their sustainability with respect to the European Directive n. 2010/63 regarding animal use for scientific purposes. In the same direction, EMA guidelines encourage stakeholders and authorities to support the development and use of 3R (reduction, replacement, refinement) testing approaches (EMA/CHMP/CVMP/JEG-3Rs/450091/2012)16, and Medical Device Regulation 2017/745 requires the observation of the 3R principles and the avoidance of test duplication.
As an alternative to ex vivo oesophageal models, in a previous study, we validated the feasibility of a 3D reconstructed human oesophageal epithelium to assess transepithelial permeability.17 In experiments performed with such a model, we have shown the barrier protective effect of solid formulations combining hyaluronic acid (HA) and chondroitin sulfate (CS) to reinforce the oesophageal epithelium and oppose the spontaneous and intrinsic transepithelial permeation of small intraluminal toxic/acid molecules. We have also shown that HA-CS compounds, alone or in the presence of antacid components (ie, aluminium hydroxide or magnesium trisilicate) have the same barrier protective effect in the presence of both neutral and acidic luminal contents.
Several medical devices are commercially available and are widely employed in clinical practice, either alone or as add-on therapy, to obtain prompt relief from painful GOR symptoms such as heartburn, epigastric and retrosternal acidity, and pain. The beneficial effect of these therapies usually involves rapid, within-minutes relief after their administration and differs from the delayed symptomatic effect of PPIs. Anecdotal observations and clinical studies have reported the symptomatic efficacy of several mucosal protective products, but little is known about how efficiently they reinforce the oesophageal epithelial barrier by opposing to transmembrane permeability and protecting the epithelium from luminal noxious agents and how fast they start acting after their assumption.
The aim of this study was to comparatively assess the efficiency and timing of the protective barrier effect of three different medical device liquid formulations in a previously validated oesophageal epithelial HO2E model.17,18
Materials and Methods
Test System
The 3D reconstructed human oesophageal epithelium (HO2E/S/5) is produced by Episkin (Lyon, France): it is an epithelium formed after 5 days of airlift culture of the K510 cell line (derived from squamous cell carcinoma) on inert polycarbonate filters in a chemically defined medium that reproduces the human oesophageal epithelium morphology. The epithelium is 0.5 cm2 in size and has a standard thickness of approximately 60 µm. The batch was tested for the absence of hepatitis B, hepatitis C and mycoplasma, and the maintenance medium was tested for sterility. The inserts containing the tissues at day 5 were placed at room temperature in a multiwell plate filled with an agarose nutrient solution in which they were embedded for shipment.
After arrival, the HO2E tissues were removed from the agarose nutrient solution under a sterile airflow cabin. The inserts were rapidly transferred to 4-well plates previously filled with maintenance medium (1 mL/well) at room temperature and incubated at 37°C, 5% CO2 and saturated humidity.
The test was performed 2 days after reception as HO2E/S/7.
Test Items
The liquid medical devices and their qualitative formulas (main active ingredients) are reported in Table 1. The untreated control was net caffeine passage on HO2E treated with saline solution according to the prewetting procedure (15 μL of saline).17
 |
Table 1 Characterization of the Liquid Medical Devices Investigated in This Study |
Oesophageal Epithelium Treatment
The culture inserts were placed in 6-well plates previously filled with 1 mL/well saline solution (basolateral compartment, receptor fluid). All the series were evaluated in triplicate. HO2E/S/7 tissues were prewetted with 15 μL of saline for 15 min at room temperature to recapitulate the epithelium conditions and to achieve a homogeneous distribution of the formulations. Thirty microlitres of liquid products as such and 30 μL of negative control (saline solution) were applied directly on the prewetted epithelium surface for an exposure of 15 min.
Trans-Epithelial Probes and Their Kinetics
After treatment with test items, 100 μL of 0.5% w/v acidic caffeine solution (1 mg caffeine/cm2 at pH 3.3) was applied to the apical compartment: this volume of liquid (100 μL) at pH 3.3 was relatively high for the 0.5 cm2 surface and allowed to better mimic the microenvironment of the oesophageal lumen in the presence of gastric fluid refluxate.
Caffeine penetration was monitored in 2 parallel experimental settings:
- in a dynamic procedure on the same tissue series by collecting the receptor fluids (1 mL) from the basolateral compartment at 1 h and 2 h of exposure
- in a static procedure by collecting the receptor fluid (1 mL) at 15 min. The LY permeability assay was performed at the same 15 min timepoint.
Caffeine Quantification: Analytical Method
Receptor fluid samples were stored at 4℃ before UPLC/MS analysis. The caffeine concentration was determined by using a 1290 Infinity II LC System (AGILENT Santa Clara, Ca. USA) equipped with a C18 reversed-phase column (ACQUITY UPLC BEH-C18, 1.7 μm, 100×2.1 mm, WATERS CORPORATION, Ma. USA) set at 25℃. A 5 μL sample was injected for isocratic elution at 0.25 mL/min. The composition of the eluent was 80% water/20% methanol. The wavelength was set at 273 nm. Standard calibration curves for caffeine (0.1 and 1000 mg/L) were used.
Lucifer Yellow (LY) Assay
LY is a fluorescent dye that is impermeable to the cell membrane and is used to study the paracellular permeability. When the cell junctions are unbroken, LY has a very low permeability; if the junctions are damaged, the LY flow will be much higher. Therefore, this assay is used to verify the integrity of cell junctions in the presence of the substance that needs to be evaluated.
The LY flux was evaluated 15 min after caffeine application in parallel to the caffeine permeability assay: products were removed from the tissue surface, 0.5 mL of LY (500 μM in saline solution) was applied to the epithelial surface, and 1 mL of saline solution was added to the basolateral compartment. After incubation at 37℃, the relative flux of LY from the apical (AP) to the basolateral (BL) compartment was quantified.
The measurement of fluorescence (RFU) was performed in a spectrofluorometer (TECAN INFINITE M200) with 428 nm excitation and 535 nm emission. For each tissue, the measurement was performed at the basolateral level, and flux was calculated with the following formula: LY Flux % = (RFU BL/RFU AP t=0) × 100 where BL: basolateral, AP: apical and APt=0: mean of the RFU of LY 500 μM solution in the apical compartment at starting time (t=0).
Statistical Analysis
One-way ANOVA followed by a post hoc Tukey HSD test was performed using GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, Ca. USA). The products were compared with the untreated control and with each other, and statistically significant results were reported as p-values.
Results
Dynamic Measure of Caffeine Permeation and Epithelial Permeability (Multiple Measures on the Same Tissue Series)
In Figure 1, the results of caffeine quantification in the receptor compartment at 1 h and 2 h are presented. The results are expressed as the caffeine percentage compared to the amount of caffeine applied (490 μg) as a function of time.
At 1h from caffeine application, there was a significant decrease in caffeine permeation (p< 0.0001) compared to that in the untreated control, for which a permeation of 54.3% was quantified. The percentage of permeated caffeine was found to be 11.3% for Device A (Gerdoff® Protection), 17.8% for Device B (Esoxx® One), and 34.2% for Device C (Marial® Gel), showing a reduction in caffeine penetration of 79.2% for Device A, 67.2% for Device B, and 37.0% for Device C compared to the untreated control. At 2 h, the percentage of permeated caffeine was found to be 30.2% for Device A, 35.0% for Device B, and 52.0% for Device C.
Although a similar behaviour was reported for Device A and Device B, a significantly lower amount of permeated caffeine was measured for Device A compared to Device B at both timepoints (p<0.0001 at 1 h and p<0.05 at 2 h). A statistically significant reduction of caffeine passage was evident between device A and device C, at 1 h (p< 0.0001) and 2 h (p<0.0001) and between device B and device C, at 1 h (p<0.0001) and 2 h (p<0.0001).
The caffeine permeability rates at 1 h and 2 h are reported in Figure 2A and B, respectively. Compared to the negative control, all tested products showed a significant decrease in the caffeine permeation rate at both timepoints (p< 0.0001). This rate, used to assess the barrier protective effect of the medical devices, was significantly lower (p< 0.0001) at 1 h with Device A (0.19% caffeine/min) than with Device B and Device C (0.30% and 0.57% caffeine/min, respectively) (Figure 2A). After 2 h from caffeine application, the permeation rates were comparable between the three devices (Figure 2B). In particular, no significant difference was found between Device A and Device B, while a significant difference (p<0.05) has been measured between Device A and Device C as well as Device B and Device C.
Caffeine Permeation and Epithelial Permeability 15 Min After Caffeine Application
The protective effect of the medical devices was evaluated at a short timepoint (15 min) after caffeine application.
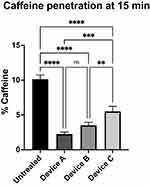
In Figure 3, the results of caffeine quantification in the receptor compartment at 15 min with the relative statistical analysis are presented. The results are expressed as the caffeine percentage compared to the amount of caffeine applied (510 μg).
At 15 min, compared to the untreated control (10.1%), Device A showed a significantly higher capacity to block caffeine flux (2.3%, p<0.0001), followed by Device B (3.5%, p<0.0001) and Device C (5.5%, p<0.0001). These data correspond to a reduction in caffeine permeation of 77.2% for Device A, 65.3% for Device B and 45.5% for Device C compared to control tissues. The difference between Device A and Device B was not significant (ns), while it was significant between Device A and Device C (p< 0.001) and between Device B and Device C (p< 0.01).
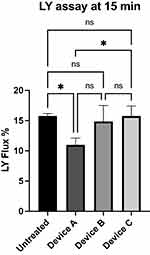
The LY flux data (Figure 4) after 15 min are in agreement with the caffeine permeation analysis. Compared to the untreated control (LY Flux= 15.8%), Device A (11.0%, p<0.05), but not Device B (14.9%, ns) and Device C (15.7%, ns), significantly reduced LY permeation in this short time after product treatment. Even in this experiment, a significant difference was observed between Device A and Device C (p< 0.05) but not with Device B. These data are in agreement with the results obtained in the caffeine permeation experiment.
Discussion
The clinical relevance of mucosal protective agents, now marketed in the European Union as medical devices, is their use as a therapeutic aid for 40% of NERD patients and 30% of ERD patients not responding to PPI treatment and for the many other conditions in which PPIs are not indicated (Table 2). These formulations are classified as medical devices, and they have been developed to quickly counteract the typical GOR symptoms of heartburn, retrosternal and epigastric pain, and sensation of acidity.
 |
Table 2 Clinical Indications of Mucosal Protective Agents When PPIs are Not Indicated or Not Sufficiently Efficacious |
The potential barrier effect of Device B on an ex vivo swine oesophageal mucosa model previously treated with an acidic solution without or with pepsin to induce damage has been investigated.11 In that study, the permeability of Evans blue (EB) was prevented by perfusing Device B onto the damaged mucosa for 10 min, followed by a brief washing with saline and then perfusion with EB. More recently, the protective effects of a novel medical device against oesophageal mucosal damage have been assessed in vitro on an oesophageal cell monolayer and ex vivo in an excised rat oesophagus model.19 In those studies, the barrier effect of mucosal protective agents was qualitatively assessed by measuring the transmural permeation of probes, eg, EB in ex vivo specimens of oesophageal mucosa with the epithelium lining disrupted by noxious agents.
In the present study, an innovative experimental protocol developed on a novel 3D human reconstructed oesophageal epithelium was used to assess the film-forming properties of liquid medical devices. These reconstructed models are produced under standardized conditions and have well characterized and quantifiable barrier properties that mimic the structural, biochemical and physiological properties of human tissues. The presence of organized tissue with different living cell layers allows the formulations to be applied at realistic clinical doses and exposure conditions. For these reasons, such models are gaining relevance and predictivity vs human tissue explants and, in our opinion, today represent the most promising alternative (to either human or animal explants) for assessing, as a first instance, the mechanism of action and performance of active molecules and formulations in the food, pharma, medical device and cosmetic industries.
The “film-forming” protocol used in the present investigation overcomes the limitations of previously cited studies that, by using damaged mucosa, could not assess the permeability of an intact epithelium and the role of a tested substance in protecting the oesophageal epithelium from acid injury. The present protocol is based on a quantitative approach that uses caffeine as a probe to assess epithelial permeability for up to 2 h. The caffeine molecule is a small molecule that has fast kinetics (MW = 194.2, logKo/w = −0.07); thus, it is useful to study the modification of epithelial permeability vs time.20,21 The reduction in caffeine passage after application of the product was used as an index of the efficacy to reduce transepithelial passage.22 Similar experimental approaches have been applied to different mucosae, such as oral reconstructed epithelium, showing that caffeine penetration kinetics are completed within 2 h.23 In the present study, the application of a relatively high volume of caffeine solution (100 μL) at pH 3.3 to the oesophageal epithelium allowed us to better mimic the acidic environment of the oesophagus of GORD patients.
In this study, the same test system described in our previous publications17,18 was used to characterize a set of commercial liquid medical devices developed to temporarily protect the oesophageal mucosa from acid refluxate. Of the many products available in the Italian market, we have chosen three that are widely used and, although differing somewhat in their complex composition, share some basic ingredients that have been shown in previous studies to have protective effects on the oesophageal mucosa,11 as well as against GORD symptoms,24,25 HA is present in all three products, and CS is present in both Device A and Device B.
LY is a low molecular weight (MW = 457.25), hydrophilic, green fluorescent dye that does not specifically bind to cell components and is unable to penetrate intact cell membranes or to cross the stratum corneum of skin models.26,27 It is commonly used to measure paracellular transport in in vitro systems and to assess barrier integrity associated with the presence of effective tight junction structures.28–30 The LY probe therefore appears suitable to assess the protective effect of the tested products on the oesophageal epithelium. Such an effect would be maximally required in the presence of dilated epithelial intercellular spaces, a condition that, by allowing the transepithelial passage of offensive luminal contents, sensitizes the subepithelial peripheral nerve endings and gives rise to the painful symptomatology of NERD and oesophageal hypersensitivity.31 It is also of clinical relevance that the protective effect of the compounds takes place within a short period to prevent the damaging effect of the offensive luminal contents on the epithelium.
The novel protocol based on static investigation at 15 min has been defined to better address the oesophageal epithelium–product interactions within a short period from product application mirroring the real in-use persistence of the formulations on the oesophageal epithelium. Therefore, we assessed the protective effect of the tested products with the LY probe as early as 15 min after treatment with the liquid medical devices.
All three tested mucosal protective devices behaved similarly, but their efficiency and activity time after application varied markedly. Device A was more efficient than the other two products in reducing the transepithelial permeation rate at any timepoint tested, up to 2 hours after application. Indeed, the permeation rate of Device A at 1 h was significantly lower than the values obtained in the samples treated with the other products. This prompt effect of Device A on epithelial permeability was paralleled by an epithelial protection rate that was faster, with the LY transepithelial flux being lower than that of Device B and Device C 15 min after application. It would therefore appear that the protective effect of this compound on the epithelium is the first mechanism of effectiveness, followed by reduced transepithelial permeability.
The substantial and partial protective effect of the three tested medical devices is likely due to HA, which is present in all of them. Xanthan gum, which is present in both Device A and Device C, may add some adhesiveness to the formulations by modulating the compound viscosity but is unlikely by itself to add significant barrier protection, as shown by the lower effectiveness of the latter product. The combined presence of HA and CS might explain the enhanced protective effect of Device A and Device B but is not sufficient to explain the more efficient and rapid epithelial protective effect of the former that is likely due to the synergistic effects of the combination of HA and CS with the other ingredients, ie, aloe vera gel, honey, and maltodextrin.
These results suggest that Device A, because of its specific formulation, has a faster mechanism of action than the other liquid products: this synergy efficiently interacts with the oesophageal epithelium and is able within 15 min to significantly modify the epithelial barrier effect, thereby reducing epithelial permeability and, more importantly, rapidly increasing epithelial protection, as evidenced by the LY paracellular flux.
The results of the present investigation show that an early readout has allowed us to better understand the oesophageal epithelium–product interactions at short timepoints after exposure, and to precisely underline differences in liquid formulation performance.
Conclusions
The results suggest that 3D reconstructed models can be useful to 1) sensitively address and predict the mechanism of action of medical devices on the part of the body they are intended to act on and 2) provide objective evidence of the mechanism of action of different formulations when interacting with the epithelium barrier. Although the data of the present study are obtained in an in vitro model, the results add new and relevant knowledge that supports the clinical use of mucosal protective agents in the great majority of GOR patients who do not have any oesophageal erosions at endoscopy and include a high rate of PPI nonresponders.32 The protective effects of these medical devices are also of great clinical relevance, as they are a valuable alternative in several conditions in which PPIs are not totally efficacious or not indicated (Table 2). Finally, the present study demonstrates the different behaviours in the timing and efficiency of the tested mucosal protective agents, with Device A being more effective than Device B and Device C. Having quantified the ability of these compounds to establish a persistent physical barrier on the oesophageal epithelium is indicative of the need for clinical studies to evaluate which medical device(s), in combination or not with other treatments, will be able to improve epithelial permeation and to more efficiently protect against epithelial damage caused by noxious components of the refluxate in GORD, being it present in ERD, NERD or hypersensitive oesophageal patients.
Abbreviations
CS, Chondroitin sulfate; EB, Evans blue; EMA, European Medicines Agency; EO, Erosive oesophagitis; ERD, Erosive gastro-oesophageal reflux disease; GOR, Gastro-oesophageal reflux; GORD, Gastro-oesophageal reflux disease; HA, Hyaluronic acid; H2RA, H2-receptor antagonists; HO2E, Human-reconstructed oesophageal epithelium; LY, Lucifer yellow; NERD, Non-erosive gastro-oesophageal reflux disease; OTC, Over the counter; PPIs, Proton pump inhibitors.
Disclosure
ESC acts as a scientific advisor to SOFAR S.p.A. The other authors have no conflicts of interest to declare in this work.
References
1. Nirwan JS, Hasan SS, Babar ZU, Conway BR, Ghori MU. Global prevalence and risk factors of Gastro-oesophageal Reflux Disease (GORD): systematic review with meta-analysis. Sci Rep. 2020;10(1):5814. doi:10.1038/s41598-020-62795-1
2. Savarino E, Marabotto E, Bodini G, et al. Epidemiology and natural history of gastroesophageal reflux disease. Minerva Gastroenterol Dietol. 2017;63(3):175–183. doi:10.23736/S1121-421X.17.02383-2
3. Patrick L. Gastroesophageal Reflux Disease (GERD): a review of conventional and alternative treatments. Altern Med Rev. 2011;16(2):116–133.
4. Mermelstein J, Chait Mermelstein A, Chait MM. Proton pump inhibitor-refractory gastroesophageal reflux disease: challenges and solutions. Clin Exp Gastroenterol. 2018;21(11):119–134. doi:10.2147/CEG.S121056
5. Deam BB, Gano AD, Knight K, et al. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol. 2004;2(8):656–664. doi:10.1016/S1542-3565(04)00288-5
6. Galmiche JP, Stephenson K. Treatment of gastroesophageal reflux disease in adults: an individualized approach. Dig Dis. 2004;22(2):148–160. doi:10.1159/000080314
7. Weiberg DS, Kadish SL. The diagnosis and management of gastroesophageal reflux disease. Med Clin N Amer. 1996;80:411–429. doi:10.1016/S0025-7125(05)70446-6
8. Wang C, Hunt RH. Medical management of gastroesophageal reflux disease. Gastroenterol Clin North Am. 2008;37:879–899. doi:10.1016/j.gtc.2008.09.001
9. Fass R, Shapiro M, Dekel R, et al. Systematic review: proton-pump inhibitor failure in gastro-oesophageal reflux disease-where next? Aliment Pharmacol Ther. 2005;25(2):79–94. doi:10.1111/j.1365-2036.2005.02531.x
10. Fass R, Sifrim D. Management of heartburn not responding to proton pump inhibitors. Gut. 2009;58(2):295–309. doi:10.1136/gut.2007.145581
11. Di Simone MP, Baldi F, Vasina V, et al. Barrier effect of Esoxx® on esophageal mucosal damage: experimental study on ex-vivo swine model. Clin Exp Gastroenterol. 2012;5:103–107. doi:10.2147/CEG.S31404
12. Mönkemüller K, Wex T, Kuester D, et al. Role of tight junction proteins in gastroesophageal reflux disease. BMC Gastroenterol. 2012;20(12):128. doi:10.1186/1471-230X-12-128
13. Björkman EV, Edebo A, Oltean M, Casselbrant A. Esophageal barrier function and tight junction expression in healthy subjects and patients with gastroesophageal reflux disease: functionality of esophageal mucosa exposed to bile salt and trypsin in vitro. Scand J Gastroenterol. 2013;48(10):1118–1126. doi:10.3109/00365521.2013.828772
14. Fang Y, Chen H, Hu Y, et al. Gastroesophageal reflux activates the NF-κB pathway and impairs esophageal barrier function in mice. Am J Physiol Gastrointest Liver Physiol. 2013;305(1):G58–G65. doi:10.1152/ajpgi.00438.2012
15. Asaoka D, Miwa H, Hirai S, et al. Altered localization and expression of tight-junction proteins in a rat model with chronic acid reflux esophagitis. J Gastroenterol. 2005;40(8):781–790. doi:10.1007/s00535-005-1628-6
16. EMA/CHMP/CVMP/JEG-3Rs/450091/2012 guideline on the principles of regulatory acceptance of 3Rs (replacement, reduction, refinement) testing approache; 2016.
17. Pellegatta G, Spadaccini M, Lamonaca L, et al. Evaluation of human esophageal epithelium permeability in presence of different formulations containing hyaluronic acid and chondroitin sulphate. Med Devices. 2020;13:57–66. doi:10.2147/MDER.S234810
18. Meloni M, Buratti P, Carriero F, Ceriotti L. In vitro modelling of barrier impairment associated with Gastro-Oesophageal Reflux Disease (GERD). Clin Exp Gastroenterol. 2021;14:361–373. doi:10.2147/CEG.S325346
19. Agostinis C, Bossi F, Mangogna A, et al. Protective and regenerative effects of a novel medical device against esophageal mucosal damage using in vitro and ex vivo models. Biomed Pharmacother. 2020;131:11075. doi:10.1016/j.biopha.2020.110752
20. Ackermann K, Lombardi Borgia S, Korting HC, Mewes KR, Schäfer-Korting M. The phenion full-thickness skin model for percutaneous absorption testing. Skin Pharmacol Physiol. 2010;23:105–112. doi:10.1159/000265681
21. Davies DJ, Heylings JR, Gayes H, McCarthy TJ, Mack MC. Further development of an in vitro model for studying the penetration of chemicals through compromised skin. Toxicol in vitro. 2017;38:101–107. doi:10.1016/j.tiv.2016.10.004
22. Casiraghi A, Ranzini F, Musazzi UM, Franzè S, Meloni M, Minghetti P. In vitro method to evaluate the barrier properties of medical devices for cutaneous use. Regul Toxicol Pharmacol. 2017;90:42–50. doi:10.1016/j.yrtph.2017.08.007
23. Meloni M, Granata A, Pelleviosin C, Ceriotti L. Human reconstructed mucosal models to assess drug permeation: caffeine case study on oral mucosa.
24. Palmieri B, Merighi A, Corbascio D, Rottigni V, Fistetto G, Esposito A. Fixed combination of hyaluronic acid and chondroitin-sulphate oral formulation in a randomized double blind, placebo controlled study for the treatment of symptoms in patients with non-erosive gastroesophageal reflux. Eur Rev Med Pharmacol Sci. 2013;17(24):3272–3278.
25. Boarino V, Raguzzi I, Marocchi M, Merighi A. Symptomatic response to GERDOFF® in patients with gastro-esophageal reflux disease and poor response to alginates: an exploratory, post-market, open-label study. Turk J Gastroenterol. 2020;31(6):466–473. doi:10.5152/tjg.2020.19327
26. Mansbridge JN, Knapp AM. Penetration of Lucifer yellow into human skin: a lateral diffusion channel in the stratum corneum. J Histochem Cytochem. 1993;41(6):909–914. doi:10.1177/41.6.8315281
27. Roger M, Fullard N, Costello L, et al. Bioengineering the microanatomy of human skin. J Anat. 2019;234(4):438–455. doi:10.1111/joa.12942
28. Mendel M, Karlik W, Chłopecka M. The impact of chlorophyllin on deoxynivalenol transport across jejunum mucosa explants obtained from adult pigs. Mycotoxin Res. 2019;35(2):187–196. doi:10.1007/s12550-019-00342-2
29. Zhao W, Han L, Bae Y, Manickam DS. Lucifer yellow - a robust paracellular permeability marker in a cell model of the human blood-brain barrier. J Vis Exp. 2019;150. doi:10.3791/58900
30. Alqahtani S, Roberts CJ, Stolnik S, Bosquillon C. Development of an in vitro system to study the interactions of aerosolized drugs with pulmonary mucus. Pharmaceutics. 2020;12(2):145. doi:10.3390/pharmaceutics12020145
31. Souza RF, Bayeh L, Spechler SJ, Tambar UK, Bruick RK. A new paradigm for GERD pathogenesis. Not acid injury, but cytokine-mediated inflammation driven by HIF-2alpha: a potential role for targeting HIF-2alpha to prevent and treat reflux esophagitis. Curr Opin Pharmacol. 2017;37:93–99. doi:10.1016/j.coph.2017.10.004
32. Boeckxstaens G, El-Serag HB, Smout AJ, Kahrilas PJ. Symptomatic reflux disease: the present, the past and the future. Gut. 2014;63(7):1185–1193. doi:10.1136/gutjnl-2013-306393
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.