Back to Journals » Drug Design, Development and Therapy » Volume 13
Protective effects of ethyl gallate and pentagalloylglucose, the active components of Qingwen Baidu Decoction, against lipopolysaccharide-induced acute lung injury in rats
Authors Zhang Q , Nie J, Chen S, Li Q
Received 31 August 2018
Accepted for publication 12 November 2018
Published 19 December 2018 Volume 2019:13 Pages 71—77
DOI https://doi.org/10.2147/DDDT.S186029
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Anastasios Lymperopoulos
Qi Zhang,1 Jing Nie,2 Su-Juan Chen,2 Qiang Li2
1Department of Natural Medicine, School of Pharmacy, Fujian Medical University, Fuzhou, Fujian 350122, China; 2School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing 100102, China
Background: The aim of this study was to investigate the bioactive constituents of Qingwen Baidu Decoction (QBD) in a rat model of acute lung injury (ALI) induced by lipopolysaccharide (LPS). Our previous studies showed that ethyl gallate (EG) and pentagalloylglucose (PGG) were the active components of QBD for the treatment of ALI.
Materials and methods: We isolated two compounds and identified the structures of them by nuclear magnetic resonance and mass spectrometer. Lung injury was induced by intratracheal instillation with LPS (5 mg/kg). Rats were randomly divided into six groups: Control group; LPS group; 5 mL/kg DEX + LPS group; 6.6 g/kg QBD extract + LPS group; 17.16 mg/kg PGG + LPS group; 7.26 mg/kg EG + LPS group. The effects of compounds on LPS-induced the number of total cells, neutrophils influx, protein leakage, W/D weight ratio and pulmonary histological changes were examined.
Results: The results demonstrated that pretreatment with EG and PGG could notably inhibit lung edema and attenuate the pulmonary histological changes (P<0.05). The pretreatment also decreased the number of total cells and polymorphonuclear leukocytes in bronchoalveolar lavage fluid (BALF) (P<0.05).
Conclusion: Ethyl gallate and pentagalloylglucose of QBD played a protective role in preventing LPS-induced ALI. The results supported further study of EG and PGG as potential candidates for preventing ALI.
Keywords: ethyl gallate, pentagalloylglucose, acute lung injury, Qingwen Baidu Decoction
Introduction
Acute lung injury (ALI) and its severe form, acute respiratory distress syndrome (ARDS), cause high mortality in critically ill patients.1,2 ALI is characterized by severe hypoxemia, pulmonary edema, and neutrophil accumulation in the lungs, which in sequence result in respiratory dysfunction.3 The release of lipopolysaccharide (LPS) from Gram-negative bacteria has been recognized as a primary pathogen resulting in ALI/ARDS.4,5 Acute exposure to LPS can induce the apoptosis of lung epithelial cells and rapid influx of polymorphonuclear leukocytes (PMNs).6,7 Then, the activation initiated a cascade of inflammatory cell influx and finally resulted in pulmonary edema, which well stimulated the pathophysiological activity of ALI patients.8,9
So far, there are only few effective therapies for ALI. Therefore, the development of novel effective therapeutic agents is urgently required. In response, we have screened for potential drugs from traditional Chinese medicines, which have multicomponent and multitarget characteristics for treating a disease. We found that Qingwen Baidu Decoction (QBD) is effective in the treatment of H1N1 influenza and ARDS, with good outcome and patient prognosis.10 Our previous studies showed that both high dose and medium dose of QBD extract had significantly ameliorated LPS-induced ALI in rats by anti-inflammatory effects and reducing pulmonary edema (P<0.05). Moreover, the result of principal component analysis (PCA) showed that ethyl gallate (EG), pentagalloylglucose (PGG), galloylpaeoniflorin, mudanpioside C, and harpagoside of QBD were effective in the treatment of ALI.11 Hence, we isolated and identified the polyphenols from QBD to verify that the potential compounds were effective for the treatment of ALI. We also demonstrated their key role in protecting against ALI in rats.
Materials and methods
Chemicals and reagents
LPS (Escherichia coli, serotype 055:B5) was obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Dexamethasone (DEX) was purchased from Tianjin KingYork Group Co. Ltd. (Tianjin, China). The ELISA kit for tumor necrosis factor-α (TNF-α) was obtained from Abcam (Cambridge, UK). The Bio-Rad protein assay kit was obtained from Bio-Rad Laboratories Inc. (Hercules, CA, USA). PBS and acetonitrile (HPLC grade) were obtained from Thermo Fisher Scientific (Waltham, MA, USA). Distilled water was purchased from Watson’s Food & Beverage Co. (Guangzhou, China). Formic acid was obtained from Beijing Reagent Company (Beijing, China). The LTQ-Orbitrap mass spectrometer was acquired from Thermo Fisher Scientific. NMR spectra were recorded on a Varian VNMRS 600 MHz (Varian, Palo Alto, CA, USA) in DMSO-d6 solvent using TMS as a reference.
Extraction and isolation
As both compounds 1 and 2 were the constituents of Moutan cortex radicis of QBD by HPLC, they can be separated from Moutan cortex radicis. Moutan cortex radicis (5 kg) were crushed and extracted with 50% EtOH (3×50 L) at 85°C for 2 hours. The extract was partitioned with petroleum ether, EtOAc, and nBuOH. The n-BuOH-soluble fraction (220 g) was chromatographed with an HP-20 column using a stepwise gradient to give the following six fractions: A (H2O), B (10% EtOH-H2O), C (30% EtOH-H2O), D (50% EtOH-H2O), E (70% EtOH-H2O), and F (95% EtOH-H2O). Fraction D (30.1 g) was chromatographed on an RP-18 column using 0–100% MeOH (10% stepwise increase in MeOH) to afford subfractions D1-10 via HPLC-DAD analyses. Fraction D5 (6.1 g) was chromatographed on an LH-20 column using 80% MeOH–H2O to obtain 100 subfractions (Fr. D5.1-Fr. D5.100). Subfraction Fr. D5.20 was purified by preparative HPLC using 30% MeOH-H2O to yield compound 1 (500 mg). Similarly, the subfraction Fr. D5.95 was purified by preparative HPLC to yield compound 2 (600 mg).
Content analysis of bioactive components in QBD by HPLC
Standard solutions were prepared by adding accurately weighed amounts of EG and PGG to a volumetric flask and dissolving with methanol (10 mL) to give final concentrations 2.9100 and 1.2000 mg/mL, respectively. Content analysis of potential bioactive components in QBD was performed by HPLC. Similarly, the sample of QBD extract was accurately weighed to a volumetric flask and dissolved with methanol (10 mL) to give a final concentration of 0.06336 mg/mL. All samples were filtered through a 0.22-μm membrane before analysis.
The mobile phase system was composed of (A) acetonitrile and (B) 0.1% formic acid in water. The HPLC chromatogram elution profile was as follows: 2% A (0–10 minutes); 2%–7% A (10–20 minutes); 7%–9% A (20–40 minutes); 9 and 10% A (40–50 minutes); 10%–16% A (50–60 minutes); 16 and 17% A (60–65 minutes); 17%–20% A (65–80 minutes); and 20%–24% A (80–112 minutes). The flow rate of mobile phase was 1.0 mL/min, and the UV spectra were 254 nm.11
Animals and housing
Sprague Dawley (SD) male rats weighing 200±20 g were purchased from Vital River Laboratories Co., Ltd. (Beijing, China). The rats were maintained under standard laboratory conditions (temperature 21°C–23°C, relative humidity 45%–65%, and controlled lighting 12 hours light/dark cycle) with food and water freely available. All of the experiments involving animals were carried out in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH publication no 85-23, revised 1985), and the Institutional Animal Care and Use Committee of Beijing University of Chinese Medicine approved the protocol.
Preparation of QBD extracts and its compounds
QBD was obtained from Tong Ren Tang (Beijing, China). QBD is composed of Gypsum fibrosum 30 g, Radix rehmanniae 12 g, Cornu bubali 12 g, Rhizoma coptidis 4.5 g, Radix platycodonis 12 g, Radix scutellariae 15 g, Rhizoma anemarrhenae 15 g, Fructus gardeniae 12 g, Cortex moutan radicis 12 g, Radix paeoniae rubra 15 g, Radix scrophulariae 15 g, Fructus forsythia 15 g, Herba lophatheri 15 g, and Radix glycyrrhizae 6 g. The crude components of the formula were extracted three times by refluxing with boiling distilled water (1:12, g/mL) for 1 hour. The extract was filtered, concentrated, freeze-dried, and stored at 4°C until use. According to the contents of bioactive constituents in QBD, the concentrations of EG and PGG were 0.725/mL and 1.98 mg/mL, respectively.
Experimental design
The rats were maintained under standard laboratory conditions (temperature 21°C–23°C, relative humidity 45%–65%, controlled lighting 12 hours light/dark cycle) with food and water freely available. The rats were divided randomly into the following six groups: 1) normal saline (NS) group (control group); 2) LPS-challenged group (LPS group); 3) DEX (5 mg/kg) + LPS group; 4) F (QBD extract 6.6 g/kg) + LPS group; 5) PGG (17.16 mg/kg) + LPS group; and (6) EG (7.26 mg/kg) + LPS group. QBD extracts and compounds were given intragastrically 48, 24, and 1 hours before the intraperitoneal (ip) injection of sterile saline (0.1 mL/10 g body weight), and DEX (5 mg/kg) was ip given by injection 1 hour before LPS administration. LPS (5 mg/kg) was administered by intratracheal instillation to induce lung injury, while saline was used as the control. Then, all rats were euthanized at 12 hours after LPS administration, and the samples were collected for subsequent analysis.
Measurement of cell counts and protein concentration in bronchoalveolar lavage fluid (BALF)
At the end of the experiment, the right lungs were ligated at the right main bronchus. Then, the left lungs were lavaged with 1 mL of autoclaved PBS five times. The recovery ratio of BALF was 4 mL (about 80%), the fluid was immediately centrifuged at 300 g, and the supernatants were stored at −80°C for analysis.12 The cell pellets were re-suspended in PBS, and the total cell number was counted and classified using the automated hematology analyzer. Neutrophil and lymphocyte counts were tested using a modified Giemsa method. The total protein concentrations in BALF were measured using a bicinchoninic acid (BCA) assay according to the company protocol.
Wet-to-dry (W/D) lung weight ratio
The water content of the lungs was tested by calculating the W/D weight ratio of the lung tissues. After the rats were euthanized, the right upper lungs were excised, rinsed briefly in PBS, blotted, weighed to obtain the wet weight, and then placed in an oven at 80°C for 48 hours to get the dry weight. The ratio of the wet lung to the dry lung was calculated to determine the magnitude of pulmonary edema.
Histological analysis of lungs
The lung tissues were obtained and fixed with 10% formalin, imbedded in paraffin, and sliced (5-mm thick) at 12 hours after instillation of LPS. After H&E staining, pathological changes in mammary tissues were observed under a light microscope.
Statistical analysis
All data were expressed as mean ± SD and analyzed with one-way ANOVA using the SPSS v. 17.0 software (SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
Results
Extraction and isolation
The structure of compound 1 was determined by NMR and mass spectrometer (MS) spectral data. The molecular formula C41H32O26 of compound 1 {m/z 939.1130 [M–H]−} was determined by high-resolution electrospray ionization mass spectrometer (HRESIMS) and 13C NMR data. The 1H and 13C NMR assignments of compound 1 were determined using 1H, 13C, heteronuclear multiple quantum correlation spectrum, and heteronuclear multiple bond correlation spectrum spectra. The 1H and 13C NMR and electrospray ionization mass spectrometer (ESI-MS) data of 1 were identical to those of PGG (C41H32O26) in the literature.13 1H and 13C NMR spectral data are as follows: 1H NMR (DMSO-d6, 600 MHz): d 4.46 (1H, dd, J=4.5, 10.0 Hz, glc. H-6a), 4.75 (1H, m, glc. H-5), 4.46 (1H, d, J=10.0 Hz, glc. H-6b), 5.59 (1H, t, J=9.0, glc. H-2), 5.61 (1H, t, J=9.0, glc. H-4), 6.13 (1H, t, J=9.0, glc. H-3), 6.54 (1H, d, J=9.0, glc. H-1), 6.93, 6.98, 7.00, 7.07, 7.13 (every 2 hours, s, galloyl H); 13C NMR (DMSO-d6, 150 MHz): δ (ppm): 91.73 (glc. C-1), 67.79 (glc. C-4), 70.6 (glc. C-2), 71.96 (glc. C-3), 72.16 (glc. C-5), 61.59 (glc. C-6), 108.83, 108.93, 108.78, 108.75, 109.06 (galloyl C-2, C-6), 118.93, 118.13, 118.1, 117.96, 117.38 (galloyl C-1), 138.81, 139.16, 138.95, 139.19, 139.7 (galloyl C-4), 145.6, 145.54, 145.42, 145.15, 145.70 (galloyl C-3, C-5), 165.5, 164.5, 164.9, 164.6, 163.97 (–COO–). The structure of compound 1 is shown in Figure 1.
  | Figure 1 Chemical structure of EG and PGG. |
The structure of compound 2 was determined by NMR and MS spectral data. The molecular formula, C9H10O5, of compound 2 {m/z 197.0457 [M–H]−} was determined by HRESIMS and 13C NMR data. The 13C NMR and ESI-MS data of compound 2 were identical to those of EG (C9H10O5) in the literature.14 13C NMR spectral data are as follows: 13C NMR (DMSO-d6, 150 MHz): 119.9 (C-1), 108.3 (C-2), 145.63 (C-3), 138.58 (C-4), 145.63 (C-5), 108.27 (C-6), 168.04 (C-7), 60.64 (C-8), 14.17 (C-9). The structure of compound 2 is shown in Figure 1.
Content analysis of bioactive components in QBD by HPLC
Content analysis of the main bioactive components in QBD was performed by HPLC. The contents of EG and PGG in QBD were determined to be 0.11% and 0.26%, respectively. The linear calibration curves were constructed with at least six different concentrations of chemical markers in triplicate. Good linear correlation and high sensitivity at these chromatographic conditions were confirmed by the correlation coefficients (r2 >0.999) (Table 1).
  | Table 1 Content analysis of bioactive components in QBD |
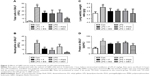
Effects of QBD and its compounds on PMN infiltration into the lungs in LPS-induced ALI rat model
Recruitment of neutrophils into the pulmonary compartment is an important feature of ALI. As shown in Figure 2A and B, LPS resulted in a dramatic increase in the number of either total cells or neutrophils in BALF compared with the normal group (P<0.05). Treatment with the extract of QBD, PGG, and EG significantly reduced LPS-induced cell infiltration (P<0.01), especially PGG. These data indicated that QBD, PGG, and EG substantially prevented PMN infiltration into the lung tissues in LPS-induced ALI rat model.
Effects of the extract of QBD and its compounds in reducing pulmonary edema in LPS-induced ALI rat model
As shown in Figure 2C and D, the W/D ratio and total protein concentrations in BALF significantly increased after inducing LPS compared with the normal control group. Pretreatment with the extract of QBD, PGG, and EG could significantly decrease protein level (pulmonary edema) and inhibit the increase in the lung W/D ratio and protein concentrations in BALF (P<0.05), when the extract of QBD and PGG is given orally, respectively (P<0.01). These results were in accordance with the histological changes.
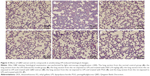
Effects of the extract of QBD and its compounds on reducing pathological damages in ALI rat model
Rats in the normal control group showed no significant morphologic damages (Figure 3). Compared with the normal control group (A), the lungs of ALI mice in the LPS group showed marked inflammatory responses characterized by the presence of alveolar hemorrhage, a marked swelling of the alveolar walls and remarkable recruitment of neutrophils into the alveolar spaces (B). Histological damage was improved by DEX (C) and the extract of QBD and its compounds’ (PGG and EG) administration (D–F).
Discussion
To our knowledge, this is the first report of the effects of EG and PGG in protecting against LPS-induced ALI in rats. EG and PGG, the gallate compounds that were derived from the Moutan Cortex, were the main effective components of QBD. Previously, PGG was found to be effective in protecting mice from a lethal challenge by LPS and significantly decreased the plasma endotoxin level both in endotoxemic mice and rats. The decrease in endotoxin level in rats was significantly associated with the TNF-α level.15 EG alleviates inflammatory condition in ALI by significantly reducing BALF neutrophils, ROS, proinflammatory cytokines, and albumin levels in both in vivo (BALB/c) and in vitro models (human monocytes).16 Pretreatment with EG and PGG inhibited lung edema, decreased lung W/D ratio and protein in BALF, and played a key role in preventing LPS-induced ALI in rat models.
To explore the underlying constituents of QBD, we analyzed the constituents and related pharmacology by PCA method. The results showed that EG, PGG, galloyl paeoniflorin, mudanpioside C, and harpagoside can effectively protect against ALI.11 On the basis of the previous studies, we further verify the effects of EG and PGG on ALI in rats. The results demonstrated that pretreatment with EG and PGG could notably inhibit lung edema and attenuate pulmonary histological changes by reducing the total number of cells and infiltration of activated PMNs in BALF. EG and PGG are the potential agents for preventing LPS-induced ALI. They had significantly potent anti-inflammatory effects and reduced pulmonary edema caused by ALI in rats (P<0.05).
ALI/ARDS is characterized by the influx of serous fluid into the air spaces, which leads to alveolar edema.17,18 To quantify the magnitude of pulmonary edema, the lung W/D ratio and protein concentration in BALF were detected. Consistent with the previous studies, we observed that instillation of LPS into lungs caused severe histological alterations in lungs including increased pulmonary permeability and lung edema evidenced by the elevation of lung W/D ratio and protein level in BALF.19,20 Our results showed that pretreatment with EG and PGG decreased the lung W/D ratio and protein concentration, which indicate that EG and PGG could attenuate the development of pulmonary edema.
Conclusion
These polyphenols, including EG and PGG, have anti-inflammatory effects. Our results showed that pretreatment with EG and PGG decreased the lung W/D ratio and protein concentration, which indicate that EG and PGG could attenuate the development of pulmonary edema to protect against LPS-induced ALI in rat models. EG and PGG were the potential agents of QBD decoction for preventing ALI.
Acknowledgment
This work was supported in part by grants from the National Science Foundation of China (grant no 81173562) and Fujian Province health and family planning research talent training project (grant no 2018-1-73).
Disclosure
The authors report no conflicts of interest in this work.
References
Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. | ||
Frutos-Vivar F, Ferguson ND, Esteban A. Epidemiology of acute lung injury and acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27(4):327–336. | ||
Piantadosi CA, Schwartz DA. The acute respiratory distress syndrome. Ann Intern Med. 2004;141(6):460–470. | ||
El-Agamy DS. Nilotinib ameliorates lipopolysaccharide-induced acute lung injury in rats. Toxicol Appl Pharmacol. 2011;253(2):153–160. | ||
Eiznhamer DA, Flavin MT, Jesmok GJ, et al. Effective attenuation of endotoxin-induced acute lung injury by 2,3-diacetyloxybenzoic acid in two independent animal models. Pulm Pharmacol Ther. 2004;17(2):105–110. | ||
Graziano FM, Cook EB, Stahl JL. Cytokines, chemokines, RANTES, and eotaxin. Allergy Asthma Proc. 1999;20(3):141–146. | ||
Berkow RL, Dodson MR. Biochemical mechanisms involved in the priming of neutrophils by tumor necrosis factor. J Leukoc Biol. 1988;44(5):345–352. | ||
Kabir K, Gelinas JP, Chen MH, et al. Characterization of a murine model of endotoxin-induced acute lung injury. Shock. 2002;17(4):300–303. | ||
Gustavo MB, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295(3):379–399. | ||
Chu GK. Clinical observation on treating 13 cases of influenza A (H1N1) patients with respiratory distresssyndrome in TCM. Clin J Chin Med. 2012;4(3):103–104. | ||
Zhang Q, Lei H-M, Wang P-L, et al. Bioactive components from qingwen baidu decoction against LPS-induced acute lung injury in rats. Molecules. 2017;22(5):692–711. | ||
Tsai CL, Lin YC, Wang HM, Chou TC. Baicalein, an active component of Scutellaria baicalensis, protects against lipopolysaccharide-induced acute lung injury in rats. J Ethnopharmacol. 2014;153(1):197–206. | ||
Cho J-Y, Sohn M-J, Lee J, Kim W-G. Isolation and identification of pentagalloylglucose with broad-spectrum antibacterial activity from Rhus trichocarpa Miquel. Food Chem. 2010;123(2):501–506. | ||
Ren ML. Study on Chemical Constituents and Bioactivities of Radix Paeoniae Alba. ShenYang: Shenyang Pharmaceutical University; 2009. | ||
Genfa L, Jiang Z, Hong Z, et al. The screening and isolation of an effective anti-endotoxin monomer from Radix Paeoniae Rubra using affinity biosensor technology. Int Immunopharmacol. 2005;5(6):1007–1017. | ||
Mehla K, Balwani S, Agrawal A, Ghosh B. Ethyl gallate attenuates acute lung injury through Nrf2 signaling. Biochimie. 2013;95(12):2404–2414. | ||
Geiser T, Atabai K, Jarreau PH, et al. Pulmonary edema fluid from patients with acute lung injury augments in vitro alveolar epithelial repair by an IL-1beta-dependent mechanism. Am J Respir Crit Care Med. 2001;163(6):1384–1388. | ||
Monnet X, Anguel N, Osman D, et al. Assessing pulmonary permeability by transpulmonary thermodilution allows differentiation of hydrostatic pulmonary edema from ALI/ARDS. Intensive Care Med. 2007;33(3):448–453. | ||
Leung PO, Lee HH, Kung YC. Therapeutic effect of Cphycocyanin extracted from blue green algae in a rat model of acute lung injury induced by lipopolysaccharide. Evid Based Complement Alternat Med. 2013; 2013:916590. | ||
Magalhães CB, Riva DR, Depaula LJ, et al. In vivo anti-inflammatory action of eugenol on lipopolysaccharide-induced lung injury. J Appl Physiol. 2010;108(4):845–851. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.