Back to Journals » OncoTargets and Therapy » Volume 12
Protective effect of Chuquiraga spinosa Lessing associated with simvastatin on N-Nitroso-N-methylurea (NMU)-induced prostate cancer in rats
Authors Arroyo-Acevedo JL , Rojas-Armas JP , Herrera-Calderón O , Chávez-Asmat R , Justil-Guerrero HJ, Aguilar-Carranza C, Enciso-Roca E , Tinco-Jayo JA , Yuli-Posadas RÁ, Franco-Quino C , Chumpitaz-Cerrate V
Received 9 April 2019
Accepted for publication 19 July 2019
Published 15 August 2019 Volume 2019:12 Pages 6555—6562
DOI https://doi.org/10.2147/OTT.S211642
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Jorge Luis Arroyo-Acevedo,1 Juan Pedro Rojas-Armas,1 Oscar Herrera-Calderón,2 Roberto Chávez-Asmat,3 Hugo Jesús Justil-Guerrero,1 Cristian Aguilar-Carranza,4 Edwin Enciso-Roca,5 Johnny Aldo Tinco-Jayo,5 Ricardo Ángel Yuli-Posadas,6 Cesar Franco-Quino,7 Victor Chumpitaz-Cerrate8
1Laboratory of Pharmacology, Faculty of Medicine, Universidad Nacional Mayor De San Marcos, Lima, Peru; 2Laboratory of Pharmacognosy and Traditional Medicine, Faculty of Pharmacy and Biochemistry, Universidad Nacional Mayor De San Marcos, Lima, Peru; 3Association for the Development of Student Research in Health Sciences (ADIECS), Lima, Peru; 4Department of Pathology, National Cardiovascular Institute, Lima, Peru; 5Faculty of Health Sciences, Universidad Nacional De San Cristóbal De Huamanga, Ayacucho, Peru; 6Universidad Continental, Huancayo, Peru; 7Faculty of Public Health and Administration, Universidad Peruana Cayetano Heredia, Lima, Peru; 8Laboratory of Pharmacology, Universidad Científica Del Sur, Lima, Peru
Background and objective: Chuquiraga spinosa Lessing (ChS) has shown protective effect on N-Nitroso-N-methylurea (NMU)-induced prostate cancer in rats. Currently, statins are being studied for their pro-apoptotic and antimetastatic effects. The main objective of this research was to determine the protective effect associated with the oral administration of simvastatin and ethanolic extract of the aerial parts of ChS in the prevention of prostate cancer.
Methods: Fifty-six albino male rats were randomized into seven groups: I) negative control: physiological serum: 2 mL/kg; II) TCN: testosterone 100 mg/kg + cyproterone 50 mg/kg + NMU 50 mg/kg; III) TCN + S40 (simvastatin 40 mg/kg); IV) TCN + ChS250 (ChS 250 mg/kg); V) TCN + ChS50 (ChS 50 mg/kg) + S40; VI) TCN + ChS250 (ChS 250 mg/kg) + S40; and VII) TCN + ChS500 (ChS 500 mg/kg) + S40. The antioxidant activity was tested by using (2,2-diphenyl-1-picrylhydrazyl) (DPPH) assay. Hematology, toxicological biochemical parameters, prostate-specific antigen (PSA), histology and prostate size were evaluated as main indicators of protective effect.
Results: Triglyceride values were decreased in the groups receiving ChS, being significant (P=0.02) in IV and VII group compared to cancer-inducing group (TCN). In groups that received ChS, PSA levels (P=0.71) were significant compared with TCN group. The VII group had the lowest prostate volume by sonography. The TCN group showed multiple foci of high-grade prostatic intraepithelial neoplasia (HG-PIN) with the presence of cells in mitosis; whilst, groups V and VI had few areas of HG-PIN.
Conclusion: In experimental conditions, the ethanolic extract of C. spinosa in association with simvastatin showed a protective effect on prostate cancer through hypolipidemic and antioxidant activity.
Keywords: prostate cancer, anti-tumor, Chuquiraga spinosa, simvastatin
Introduction
Prostate cancer is the most frequently diagnosed malignancy and is the fifth leading cause of death in men worldwide and one of the leading causes of cancer death in the United States;1,2 also considering prostate cancer presents slow growth, several strategies include monitoring initially and reducing complications associated with surgery and radiation therapy can generate eventually resistance to androgen deprivation;3,4 In addition, these treatments can cause the risk of creating side effects.
Cholesterol is the main sterols and structural component of cell membranes; its biosynthetic pathway is indirectly related to cell-growth processes.5 Statins, which are a class of lipid-lowering drug reduce not only serum cholesterol but also mevalonate synthesis by inhibiting 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMG-CoA). Mevalonate is a precursor of several major products regulating the cell cycle, including dolichol, geranyl pyrophosphate (GPP), and farnesyl-pyrophosphate (FPP).6 Dolichol, GPP, and FPP have a stimulatory effect on DNA synthesis and is linked to several tumor cell proteins, for instance, isoprenylating the intra-cellular G-proteins Ras and Rho, which are involved in cell proliferation, differentiation, and apoptosis.7
Syväläa et al, studied the effectiveness of the combination of simvastatin and enzalutamide in prostate cancer cells, observing a reduction on growth and signaling, with large induction of autophagy.8 Pennanen et al, evaluated the association of simvastatin and metformin, demonstrating a synergism by reducing the cytosolic ATP, induction of necrosis and autophagy on tumor cell lines of prostate cancer.9 Murtola et al, determined that a lipophilic statin (Simvastatin) exhibits a greater inhibition of cell growth compared to one hydrophilic (Rosuvastatin).10 Miyasawa et al, determined the expression of annexin A10, which is associated to inhibit the proliferation, migration, and invasion into cells of human prostate cancer.11
The World Health Organization (WHO) states that about 65% of the population worldwide prefer to use medicinal plants, approximately 60% of the agents used against cancer derived from medicinal plants and other natural resources.12 The National Cancer Institute of the United States (NCI) identified 3000 plants with anticancer properties, the majority of tropical origin (70%). Traditionally Chuquiraga spinosa is used as a treatment of urinary diseases in northern Peru.13
On the other hand, C. spinosa (Family: Asteraceae) known as “huamanpinta” in Peru has shown protective effect on N-methyl-N-nitrosourea (NMU)-induced prostate and gastric cancer in rats as well as cytotoxicity on prostatic carcinoma (DU-145).14
Cave et al, indicate that the presence of polyphenols can generate beneficial effects in reducing the risk of cancer due to its anti-inflammatory and antioxidant capacity.15 Prevention of cancer diseases can be favorable from the economic approach because it allows spending less on specialized human resources, centers and expensive treatments that are usually prolonged, so more investment is required.
The main objective of this research was to determine the protective effect in the prevention of prostate cancer associated with the oral administration of simvastatin and the ethanolic extract of the aerial parts of C. spinosa Lessing (ChS).
Materials and methods
Chemicals
NMU; Ferric chloride (FeCl3); Folin-Ciocalteu; 2,2′-diphenyl-1-picrylhydrazyl (DPPH); thiobarbituric acid; and trichloroacetic acid were purchased from Sigma Co. (St. Louis, MO, USA). Sulfuric acid; sodium carbonate (Na2CO3); sodium hydroxide (NaOH); were obtained from Merck Co. (Darmstadt, Germany). Other solvents used were of analytical grade and purchased from Merck Co. (Darmstadt, Germany). Simvastatin was purchased from Medifarma Co (Lima-Peru).
Plant collection
The plant was collected in April 2018 from Huanta, Ayacucho, Peru. The plant material was botanically identified by Dr. Mario Benavente, from the Universidad Nacional Mayor de San Marcos (Lima-Peru). A voucher specimen (25-USM-2018) was deposited at the National Herbarium of Natural History of the UNMSM.
Extract preparation
The fresh, whole plant (2 kg) was collected and shade dried to obtain 500 g dry sample which was later powdered in a blade mill and used for solvent extraction. For sample preparation, 500 g of dried sample was extracted once (3000 mL for each) with 96% ethanol at 22°C for 72 hrs and concentrated using a rotary evaporator under reduced pressure at 40°C to yield the ChS extract (19.5%). The extract was stored in amber vial until further use.
Qualitative phytochemical screening of the ethanolic extract
Qualitative phytochemical screening was done using standard procedures described by Evans15 to determine the phytochemicals present. The extract (5 mg) was dissolved in 10 mL of the respective solvents used for their extraction. The solution was made ready for qualitative phytochemical analysis for carbohydrates, phenols, anthracene derivatives, saponins, tannins, flavonoids, alkaloids, and terpenes.
Evaluation of antioxidant activity
The antioxidant activity of the ethanolic extract was evaluated by the method proposed by Herrera et al.16
1,1-diphenyl-2-picrylhydrazyl (DPPH) was used at a concentration of 20 µg/mL in methanol and the extract at concentrations of 1.0, 10.0, and 50.0 µg/mL in methanol was used in the reactions.
Animals
Fifty-six male Holtzman rats with an average weight of 200 g ±20 g, from the National Institute of Health, acclimated for 5 days in large-ventilated cages and food ad libitum were used. The study was conducted in the Bioterio of the Faculty of Medicine, Universidad Nacional Mayor de San Marcos (Lima-Peru). Rats were maintained in periods of 12 hrs’ light/dark cycle and a temperature of 21±2°C.
Induction of prostate cancer in rats
Tumor induction was carried out following the method of Bosland and Prinsen17 with slight modifications. Rats received cyproterone acetate daily (50 mg/kg body weight in sesame oil) by intraperitoneal injection for 18 consecutive days, 1 day after the final dose of cyproterone acetate, rats received daily subcutaneous injections of testosterone propionate (100 mg/kg in sesame oil) for 3 days; next day, each rat received a single intraperitoneal injection of NMU (50 mg/kg body weight in sterile saline, pH 5.0). The groups were named according to the treatment and dosed in mg/kg.
Experimental groups
Rats were randomized into seven groups with eight rats per group; according to the following experimental design: 1) Negative control: PS 2 mL/kg; 2) TCN: Testosterone 100 mg/kg + cyproterone 50 mg/kg + NMU 50 mg/kg; 3) TCN + ChS250 (ChS 250 mg/kg); 4) TCN + S40 (Simvastatin 40 mg/kg); 5) TCN+ ChS50 (ChS 50 mg/kg) + S40; 6) TCN + ChS250 (ChS 250 mg/kg) + S40; 7) TCN+ ChS500 (ChS 500 mg/kg) + S40. The treatment was received by 20 weeks. At the end of the experimental period, the rats were weighed. Blood samples were obtained to assess biochemical and hematological indicators. The animals were sacrificed by pentobarbital anesthetic (100 mg/kg).
Hematological and biochemical
Drawing blood in rats was by intracardiac puncture, the animals were previously subjected to a state of anesthesia using pentobarbital 30 mg/kg.
Hematological parameters were determined spectrophotometric method; total leucocyte was counted in a Neubauer chamber; total cholesterol by the modified method of Roeschlau et al,18 cholesterol high-density lipoproteins was determined based on the method of Trinder;19 triglycerides were estimated by the enzymatic method GPO-PAP, as described by Høstmark et al.20 Alanine aminotransferase by using the method of Mohun et al;21 alkaline phosphatase activity was evaluated by the method of Jacoby et al;22 the determination of urea according to the cleavage of urea with urease (Berthelot reaction) according to Fawcett and Scott.23
The amount of specific antigen (prostate-specific antigen, PSA) was quantified using an ELISA kit available commercially (Diagnostics Biochem, Dorchester, ON, Canada) against a standard curve (0.2–50 ng/mL PSA).
Prostate ultrasonography
After the tumor induction, a high-level system (CHISON D600 VET, JIANG SU, CHINA) was used with a linear 10 MHz transducer. Before the examinations, rats were shaved in the lower abdomen and then placed in the supine position on a heating pad. A B-mode test was performed to determine the site and size of the tumor within the prostate.
Statistical analysis
Data are presented as mean ± standard deviation (SD in triplicate from three independent in vitro experiments and for each in vivo experimental group. Statistical analysis of data with SPSS software version 21.00 were realized using the one-way analysis of variance followed by Tukey’s post hoc test for multiple comparisons. Statistical significance of differences was considered at a value <0.05.
Ethical considerations
This research was approved by the Ethics Committee of the Faculty of Medicine of San Marcos, Acta 0310 (4 November 2017). During the study, the specifications proposed by the Institutional Committee for the Care and Use of Animals (CICUA, ILAR) were followed, and the current regulations of the Animal Protection Act (Law 27,265) were respected.
Results
The qualitative phytochemical analyses revealed that ChS ethanolic extract contains, tannins, alkaloids, terpenes, quinones, flavonoids, and phenols as main phytochemical components. Moreover, it can be observed that the efficacy concentrations of ChS extract which result in 50% of the scavenging (EC50) are 25.4 μg/mL (DPPH). In Figure 1, percentage uptake of DPPH was dependent doses 10.0 µg/mL (41.5%) 50.0 µg/mL (68.1%), and 100.0 µg/mL (85.0%).
 |
Figure 1 Antioxidant effect of Chuquiraga spinosa Lessing on DPPH radical. |
 |
Table 1 Effects of Chuquiraga spinosa Lessing (ChS) extract associated with simvastatin on hematological parameters after 20 weeks of treatment |
 |
Table 2 Effects of Chuquiraga spinosa Lessing (ChS) extract associated with simvastatin on some toxicological biochemical parameters after 20 weeks of treatment |
Discussion
Many researches have demonstrated that statins to be beneficial as anticancer agents.24 The anti-cancer effects of this kind of drugs may be due to various biological processes, such as inhibition of cell proliferation, induction of apoptosis, inhibition of angiogenesis as well as stop of metastasis, improvement of the immunity system or targeting some receptor to combat malignant cells.25
In the development of strategies for the chemoprevention of prostate cancer, a series of animal models have been developed to evaluate such strategies, thus emerging sequential regimes as described by McCormick DL et al (1998)26 in their research with the use of NMU induces an incidence of 3% of dorsolateral adenocarcinoma that increases to 18% in the NMU + Testosterone association. In the research of Bosland, M.C. and Prinsen, MK (1990),27 the experimental groups that received injections of MNU, after the pre-treatment with Cyproterone and Testosterone, presented a combined incidence of adenocarcinomas and carcinomas in situ of the dorsal side of 30% with a low incidence of focal atypical hyperplasia. In our study, we found in the TCN group, an incidence of 25% of the high-grade intraepithelial neoplasia, confirming the adequate development of the animal model.
The hematological parameters are identified within the normality between the groups, there are no toxic effects with the association between ChS and Simvastatin (Table 1). Concerning the hepatic and lipid profile, a decrease in the total cholesterol and triglycerides levels is evidenced in the ChS+ simvastatin groups compared to the TCN group, being only a significant difference for triglycerides (Table 2). Statins inhibit cell cycle proliferation, induce apoptosis, inhibit angiogenesis and metastasis, delay the progression of intraductal pancreatic neoplasia in KC transgenic mouse model, avoid hepatocellular carcinogenesis, inhibit azoxymethane-induced colonic preneoplastic lesions in obese C57BL/KsJ-db/db mice.27 In our study, the synergistic effect in the reduction of triglyceride levels when using ChS + simvastatin, which is associated with its antioxidant capacity would explain the results of the promising ones.
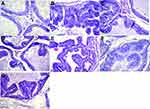
PSA is one of the most used biomarkers that has revolutionized the management of prostate cancer, only a destruction of the basement membrane of the epithelial cells of the prostate can cause excessive leakage of PSA into the bloodstream;28 In our study, there were no differences between the levels of PSA, which contradicts the results of previous investigations where the PSA in the TCN group reached 1.2±0.2 ng/mL (Figure 2), so it was analyzed in a complementary way. The dimensions of the prostate, in various studies an inverse relationship between prostate volume and the incidence of prostate cancer has been demonstrated, we can see a smaller volume in the TCN + H500+ group S40 and TCN + H250+ S40; with several foci of high-grade intraepithelial neoplasia compared to the TCN + H50+ S40 group, which has a higher volume and few focal points of PIN AG (Figure 3), these findings associated with the absence of PIN in the group receiving a tamponade and few centers of BG-PIN in the groups receiving simvastatin, evidence the chemoprotective effect of C. spinosa at low doses (Table 3).
 |
Table 3 Histological parameters of Chuquiraga spinosa Lessing associated with simvastatin |
Previous phytochemical studies have revealed that C. spinosa contains nine types of flavonoids (quercetin-3-O-glucuronide, quercetin-3-O-rutinoside, quercetin-3-O-glucoside, kaempferol-3-O-glucuronide, kaempferol-3-O-rutinoside, kaempferol-3-O-glucoside, isorhamnetin-3-O-glucuronide, isorhamnetin-3-O-rutinoside, and isorhamnetin-3-O-glucoside) and a phenolic compound (p-hydroxyacetophenone), which the flavonoid isorhamnetin-3-O-glucuronide has anti-inflammatory activity in vitro and the flavonoids kaempferol and quercetin are inversely associated with lung cancer.29,30 Finally, experimental trials have shown antioxidant, anti-inflammatory, and antifungal activity of the methanolic extract. Limitations of this study included the lack of determination of metastasis and vascular flow prostate also increased induction time of prostate cancer and dysplasia may show elevated levels of PSA.
In conclusion experimental conditions, the extract C. spinosa associated with simvastatin has a chemopreventive effect on prostate cancer through the hypolipidemic and antioxidant activity.
Acknowledgment
The authors thank the Research Vice-chancellor office of the Universidad Nacional Mayor de San Marcos, for having supported this research by Rectoral Resolution No. 04274-R-17 of July 24, 2017, and project code A17011541.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
2. De Marzo AM, Platz EA, Sutcliffe S, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7(4):256–269. doi:10.1038/nrc2090
3. Parikh NR, Mandal A, Bhatia D, Siveen KS, Sethi G, Bishayee A. Oleanane triterpenoids in the prevention and therapy of breast cancer: current evidence and future perspectives. Phytochem Rev. 2014;13(4):793–810. doi:10.1007/s11101-014-9337-5
4. Nevedomskaya E, Baumgart SJ, Haendler B. Recent advances in prostate cancer treatment and drug discovery. Int J Mol Sci. 2018;19(5):1359. doi:10.3390/ijms19051359
5. Park HS, Schoenfeld JD, Mailhot RB, et al. Statins and prostate cancer recurrence following radical prostatectomy or radiotherapy: a systematic review and meta-analysis. Ann Oncol. 2013;24(6):1427–1434. doi:10.1093/annonc/mdt077
6. Kantor ED, Lipworth L, Fowke JH, Giovannucci EL, Mucci LA, Signorello LB. Statin use and risk of prostate cancer: results from the Southern community cohort study. Prostate. 2015;75(13):1384–1393. doi:10.1002/pros.23019
7. Tan P, Zhang C, Wei SY, et al. Effect of statins type on incident prostate cancer risk: a meta-analysis and systematic review. Asian J Androl. 2017;19(6):666–671. doi:10.4103/1008-682X.190327
8. Syväläa H, Pennanen P, Blauer M, Tammela TL, Murtola TJ. Additive inhibitory effects of simvastatin and enzalutamide on androgen-sensitive LNCaP and VCaP prostate cancer cells. Biochem Biophys Res Commun. 2016;481(1–2):46–50. doi:10.1016/j.bbrc.2016.11.021
9. Pennanen P, Syvälä H, Bläuer M, et al. The effects of metformin and simvastatin on the growth of LNCaP and RWPE-1 epithelial cell lines prostate. Eur J Pharmacol. 2016;788:160–167. doi:10.1016/j.ejphar.2016.06.036
10. Murtola TJ, Syvälä H, Pennanen P, et al. Comparative effects of high and low-dose simvastatin on prostate epithelial cells: the role of LDL. Eur J Pharmacol. 2011;673(1–3):96–100. doi:10.1016/j.ejphar.2011.10.022
11. Miyasawa A, Sekine Y, Kato H, Furuya A, Koike H, Suzuki K. Simvastatin up-regulates annexin A10 that can inhibit the proliferation, migration, and invasion in androgen-independent human prostate cancer cells. Prostate. 2017;77(4):337–349. doi:10.1002/pros.23273
12. Kamble SS, Gacche RN. Evaluation of anti-breast cancer, anti-angiogenic and antioxidant properties of selected medicinal plants. Eur J Integr Med. 2019;25:13–19. doi:10.1016/j.eujim.2018.11.006
13. Arroyo J, Herrera O, Chavez R, Anampa A, Chumpitaz V, Enciso E. Protective effect of Chuquiraga spinosa extract on N-methyl-nitrosourea (NMU) prostate cancer induced in rats. Prostate Int. 2017;5(2):47–52. doi:10.1016/j.prnil.2017.01.005
14. Rodríguez-García C, Sánchez-Quesada C, Gaforio J. Dietary flavonoids as cancer chemopreventive agents: an updated review of human studies. Antioxidants. 2019;8(5):
15. Almora-Pinedo Y, Arroyo-Acevedo J, Herrera-Calderon O, et al. Preventive effect of Oenothera rosea on N-methyl-N-nitrosourea-(NMU) induced gastric cancer in rats. Clin Exp Gastroenterol. 2017;10:327–332. doi:10.2147/CEG.S142515
16. Herrera-Calderon O, Alvarado-Puray C, Arroyo-Acevedo JL, et al. Phytochemical screening, total phenolic content, antioxidant, and cytotoxic activity of five peruvian plants on human tumor cell lines. Pharmacognosy Res. 2018;10(2):161–165. doi:10.4103/pr.pr_109_17
17. Bosland MC, Prinsen MK. Induction of dorsolateral prostate adenocarcinomas and other accessory sex gland lesions in male Wistar rats by a single administration of N-methyl-N-nitrosourea, 7,12-dimethylbenz(a)anthracene, and 3,20-dimethyl-4-aminobiphenyl after sequential treatment with cyproterone acetate and testosterone propionate. Cancer Res. 1990;50:691e–699e.
18. Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total cholesterol in serum. Clin Chem. 1974;20(4):470–475.
19. Trinder P. Determination of total cholesterol. Ann Clin Biochem. 1969;6:24e–27e. doi:10.1177/000456326900600108
20. Høstmark AT, Lunde MS, Haug A. Increased serum triglycerides and reduced HDL cholesterol in male rats after intake of ammonium chloride for 3 weeks. Lipids Health Dis. 2013;12:92. doi:10.1186/1476-511X-12-92
21. Mohun AF, Cook IJ. Simple methods for measuring serum levels of the glutamic-oxalacetic and glutamic-pyruvic transaminases in routine laboratories. J Clin Pathol. 1957;10(4):394–399. doi:10.1136/jcp.10.4.394
22. Jacoby F, Martin BF. The relationship of bile alkaline phosphatase to histochemically detectable alkaline phosphatase in the biliary tract; including reference to the histology of the gall-bladder epithelium. J Anat. 1951;85(4):391–399.
23. Fawcett JK, Scott JE. A rapid and precise method for the determination of urea. J Clin Path. 1960;13:156e–159e. doi:10.1136/jcp.13.2.156
24. Gong J, Sachdev E, Robbins LA, Lin E, Hendifar AE, Mita MM. Statins and pancreatic cancer. Oncol Lett. 2017;13(3):1035–1040. doi:10.3892/ol.2017.5572
25. Stine JE, Guo H, Sheng X, et al. The HMG-CoA reductase inhibitor, simvastatin, exhibits anti-metastatic and anti-tumorigenic effects in ovarian cancer. Oncotarget. 2016;7(1):946–960. doi:10.18632/oncotarget.5834
26 Wang F, Liu W, Ning J, et al. Simvastatin suppresses proliferation and migration in non-small cell lung cancer via pyroptosis. Int J Biol Sci. 2018;14(4):406–417. doi:10.7150/ijbs.23542
27. Trogden KP, Battaglia RA, Kabiraj P, Madden VJ, Herrmann H, Snider NT. An image-based small-molecule screen identifies vimentin as a pharmacologically relevant target of simvastatin in cancer cells. Faseb J. 2018;32(5):2841–2854. doi:10.1096/fj.201700663R
28. Pron G. Prostate-Specific Antigen (PSA)-based population screening for prostate cancer: an evidence-based analysis. Ont Health Technol Assess Ser. 2015;15(10):1–64.
29. Al-Khalil S, Ibilibor C, Cammack JT, de Riese W. Association of prostate volume with incidence and aggressiveness of prostate cancer. Res Rep Urol. 2016;8:201–205. doi:10.2147/RRU.S117963
30. Costea T, Nagy P, Ganea C, Szöllősi J, Mocanu MM. Molecular mechanisms and bioavailability of polyphenols in prostate cancer. Int J Mol Sci. 2019;20(5:1062.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.