Back to Journals » Cancer Management and Research » Volume 14
Prostate Cancer Disparities and Management in Southern Africa: Insights into Practices, Norms and Values
Authors Marima R, Mbeje M, Hull R , Demetriou D , Mtshali N, Dlamini Z
Received 19 July 2022
Accepted for publication 1 November 2022
Published 28 December 2022 Volume 2022:14 Pages 3567—3579
DOI https://doi.org/10.2147/CMAR.S382903
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Rahaba Marima,1 Mandisa Mbeje,1,2 Rodney Hull,1 Demetra Demetriou,1 Nompumelelo Mtshali,3 Zodwa Dlamini1
1SAMRC Precision Oncology Research Unit (PORU), DSI/NRF SARChI Chair in Precision Oncology and Cancer Prevention (POCP), Pan African Cancer Research Institute (PACRI), University of Pretoria, Pretoria, South Africa; 2Department of Medical Oncology, Faculty of Health Sciences, Steve Biko Academic Hospital, University of Pretoria, Pretoria, South Africa; 3Department of Anatomical Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
Correspondence: Zodwa Dlamini, Tel +27 12 319 2614, Email [email protected]
Abstract: Prostate cancer (PCa) is a leading cause of mortality in men of African origin. While men of African descent in high-income countries (HICs) demonstrate poor prognosis compared to their European counterparts, African men on the African continent, particularly Southern Africa have shown even higher PCa mortality rates. Extrinsic factors such as the socioeconomic status, education level, income level, geographic location and race contribute to PCa patient outcome. These are further deepened by the African norms which are highly esteemed and may have detrimental effects on PCa patients’ health. Insights into African cultures and social constructs have been identified as key elements towards improving men’s health care seeking behaviour which will in turn improve PCa patients’ outcome. Compared to Southern Africa, the Eastern, Western and Central African regions have lower PCa incidence rates but higher mortality rates. The availability of cancer medical equipment has also been reported to be disproportionate in Africa, with most cancer resources in Northern and Southern Africa. Even within Southern Africa, cancer management resources are unevenly available where one country must access PCa specialised care in the neighbouring countries. While PCa seems to be better managed in HICs, steps towards effective PCa management are urgently needed in Africa, as this continent represents a significant portion of low–middle-income countries (LMICs). Replacing African men in Africa with African American men may not optimally resolve PCa challenges in Africa. Adopting western PCa management practices can be optimised by integrating improved core-African norms. The aim of this review is to discuss PCa disparities in Africa, deliberate on the significance of integrating African norms around masculinity and discuss challenges and opportunities towards effective PCa care in Africa, particularly in Southern Africa.
Keywords: prostate cancer, PCa, Africa, disparities, low–middle-income countries, high-income countries, masculinity, African norms
Introduction
The number of new prostate cancer (PCa) cases in Southern Africa has been reported to have increased by 60% between 2002 and 2018. Although Northern and Southern Africa seem to have high PCa prevalence, the Eastern, Western and Central African regions have been reported to have higher PCa mortality rates.1 The availability of health care resources and medical devices play a significant role in these reported discrepancies. According to the World Bank, Africans’ life expectancy has been growing more than the global rate. This is evidenced by the increased life expectancy of the Kwa-Zulu Natal Province in South Africa, from 49 to 60 years. This province was previously hard-struck by the Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome (HIV/AIDS) pandemic.2 While the AIDS defining cancers (ADCs) have been recorded to be decreasing in Africa, the non-AIDS defining cancers (NADCs) such as PCa have been reported to be on the rise, revealing HIV/AIDS and cancer as colliding pandemics and as public health burdens in Southern Africa. Rebbeck et al demonstrated that African American men were reluctant and less likely to seek PCa treatment, compared to European American men.3 This is one of the significant external contributing factors that warrants prudent actions to redress it. This reluctance is coupled with other factors such as lack of health insurance, financial barriers, low education levels, disadvantaged socioeconomic backgrounds and men’s overall poor health seeking behavior.1,3
It has become evident that high-income countries’ (HICs) PCa management practices may not necessarily be applicable or beneficial to LMICs such as African countries. Cancer research in Africa has not been adequately prioritized as compared to communicable diseases, despite the high incidence and mortality rates. This lack of prioritization may be attributed to a lack of awareness, a lack of adequate preventative strategies, delayed diagnosis, transforming to westernized lifestyle and increased life expectancies. It was observed in a study by Hamdi et al that the Southern and Northern African regions had similar patterns of cancer incidences and mortality rates.1 While breast, liver and bladder cancer were recorded to be the most prevalent in Northern Africa, prostate, lung and colorectal cancers were recorded to be predominant in Southern Africa. Additionally, both prostate and cervical cancers’ mortality rates have been reported to have increased in Southern Africa. It has also been revealed that the African continent has a shortage of cancer research resources, medical equipment and epidemiological expertise.1 Although it cannot be ignored that risk factors may be interrelated, it was also highlighted that focusing on specific risk factors for specific cancers may be a better preventative and therapeutic approach for the continent. Furthermore, integrating African norms towards improved healthcare practices is largely unelucidated. This review will focus on PCa disparities in Africa, deliberate on the significance of integrating African norms around masculinity and discuss challenges and opportunities towards effective PCa care in Africa.
Global PCa Epidemiology: HICs Vs LMICs
Worldwide, 1,414,259 PCa cases were recorded in 2020 with a worldwide ASR of 36.0 per 100,000 people. There were also an estimated 375 304 deaths related to PCa recorded in 2020. The regions with the highest incidence of PCa include Southern Africa (65.9), Australia and New Zealand (75.8), the Caribbean (75.8), North America (73) and South America (62.5) and Western Europe (77.6) and Northern Europe (83.4). The Northern and Western European regions have the highest incidence rates. However, these regions also have very low mortality rates as does the North American region. Australia and New Zealand have slightly higher mortality rates but it is still low compared to the incidence rates of PCa in that region. The remaining high incidence areas, South America, the Caribbean and Southern Africa, all have higher mortality rates with the Caribbean region having the highest mortality rates of all these regions. This region also has the highest mortality rate of any geographical region. With reference to the African continent, Southern Africa has the highest incidence of PCa but not the highest mortality rate of all the African regions with Middle Africa having the highest mortality rate of any African region. Both Western and Eastern Africa have lower incidence rates than Southern Africa but have comparatively high mortality rates. Northern Africa on the other hand has low incidence and mortality rates. The Pacific Island regions of Melanesia, Polynesia and Micronesia all have relatively high incidence rates (Figure 1).4
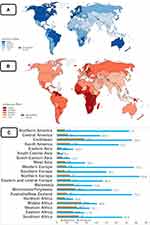 |
Figure 1 Global PCa prevalence for the year 2020. (A) Age-standardized rate (ASR) (per 100,000 men) for PCa incidence. (B) ASR for mortality. (C) Incidence and mortality rates based on geographical location. HICs have high incidence rates (IR) but lower mortality rates (MR), compared to LMICs with relatively lower IR but high MR. Reproduced from World Health Organization. Global cancer observatory: cancer Today; 2014. Available from: https://gco.iarc.fr.4 |
Factors Contributing to PCa in Africa
Increased Risk of PCa in the African Population
Several factors have been identified to contribute to PCa. It has been reported that specific factors such as diversity, ethnicity and population origin may influence cancer prevalence, drug response, molecular pathways, survival and treatment outcome.1,5 This is exacerbated by the underrepresentation of African populations in most genomic and epidemiologic studies and the underrepresentation of African men with PCa in such studies is no exception.6,7 It is undeniable that a significant portion of resources have been invested in the fight against communicable diseases, and this further broadens the cancer research gap between Africa and HICs.1 Notably, cancers affecting women’s health seems to have better advocacy accompanied by the channeled resources, compared to men’s cancer health in Africa. PCa can thus be used as a good model to address cancer health disparities within the African continent itself, and to study the specific risk factors, epidemiology and genomic components specific to African populations compared to European populations. PCa genomic disparities and lack of targeted therapies in LMICs have been reviewed by Marima et al, while precision oncology opportunities towards PCa management in LMICs have been reviewed by Marima et al.6,8
Africa also reports a higher percentage of PCa cases in young patients and more aggressive form of the disease compared to the HICs.9 In Africa, three major risk factors have been identified to play a significant role in cancer incidence rates: population genomics, environmental factors and infectious agents. Environmental exposures such as the continued use of dichloro-diphenyl-trichloroethane (DDT) have also been reported to increase PCa risk in the South African VhaVenda population. DDT has potential carcinogenic effects and has been banned in most countries.6,10 It has been reported that PCa, together with breast and colon, are the most predominant cancers in Southern Africa and Northern Africa, and this trend is like the Western countries. Contrarily, cancers with a viral etiology are most dominant in other parts of Africa such as Eastern, Western and Central Africa. Despite various reports on PCa linked genetic loci, little representation of African genomes in PCa genomic studies needs urgent counteraction by primarily building trust with the PCa research community, appropriate awareness and education as well as the acknowledgement of the masculine territory.
Socio-Economic Status and Health Care Resources
Human Development Index (HDI) is one of the factors affecting PCa patterns. HDI can be defined as the summary measure of achievement in fundamental areas of human development. These include education levels, socioeconomic status and a long and healthy life. Generally, HICs have higher HDIs compared to the LMICs. Hamdi et al measured the association between HDI and PCa incidence and mortality rates. This group reported an inverse relationship between HDI and PCa mortality, even though PCa incidence rates were still high in HDI countries.1 This pattern was reported to be similar in Northern and the Southern Africa. In Africa, Southern Africa (South Africa in particular) and Northern Africa have the highest HDIs, compared to Eastern, Western and Central Africa. Southern Africa has the highest PCa incidence and mortality rates. Unlike the LMICs with high HDIs, HICs have high PCa incidence rates but low mortality rates. South Africa is an upper middle-income country with high PCa incidence and mortality rates. PCa mortality rates significantly decline with increasing HDI, correlating with early detection and a broad spectrum of available therapeutic options. As a low HDI continent, PCa incidence rates in Africa often go undiagnosed and unreported. Thus, with improved PCa diagnostic equipment in Africa, it is likely to have increased PCa incidence rates.
While for many decades esophageal cancer was the most prevalent cancer in South African men, PCa is currently the most common cancer in South African men, across all nine provinces. One of the most identified key areas in the underreporting of PCa cases may be due to the primary reliance on the pathology-based cancer registries, as radiologically and clinically diagnosed PCa cases may go unreported.11,12 In Eswatini, a Southern African country, costs towards PCa management are devastating. Radiation therapy is not available in Eswatini and as a result the Eswatini government is compelled to refer PCa patients to South African private hospitals. Surgical procedures such as the transurethral resection of prostate (TURP) or bladder (TURB) can be performed in Eswatini. Additionally, chemotherapy is also available locally in Eswatini through either public or private healthcare. It was however revealed that most PCa patients from Eswatini were also receiving chemotherapy from South Africa. It has been reported that the total annual cost to Eswatini due to PCa was about $6 million. The direct costs for patients’ radiotherapy, chemotherapy and non-medical costs such as transport and accommodation were excessive. Indirect costs such as sick leave and premature mortality all resulted in a productive loss that also contributed to these overall costs. These devastating effects warrant urgent action by the government, policy makers and all stakeholders to reduce the PCa burden by amending therapeutic procedures. This also highlights the need for innovative strategies to optimize the limited resources for improved health care services and directly alleviate the economic constraints. While Southern Africa has the highest PCa prevalence, the availability of multimodal PCa treatment in various African countries is key to the effective management of this disease.13
Availability of Specific Medical Devices for Cancer Care in Africa
Data regarding the distribution of medical devices was extracted from the Global Health Observatory 2014. The following data describes the number of cancer medical devices in African health care facilities per 1000,000. These include mammographs, radiotherapy units, computed tomography (CT), gamma camera units, magnetic resonance imaging (MRI) and positron emission tomography (PET). Figure 2 refers to the availability of different cancer medical devices per region in Africa. It has also been reported that oncologists in Africa have a significantly greater amount of clinical work and lower job satisfaction than their counterparts in various parts of the world. This may in part be attributed to limited medical devices and a higher doctor: patient ratio. For example, it was reported that African oncologists have about a median number of 325 annual consults (which include PCa cases) compared to the 175 in other developed countries. Furthermore, while HICs oncologists sub-specialises in cancer care management, African oncologists often must treat and manage a broad spectrum of cancers. There are less than adequate available cancer care resources in Africa, including resources for managing PCa.14,15
 |
Figure 2 Medical devices available across the globe for cancer care treatment-Global Health Observatory 2014. These include (A) Mammography units per million females aged between 50 and 69 years old. 2014. (B) Radiotherapy units per million population. (C) Computed tomography (CT) units per million population. (D) Gamma camera units per million population. (E) Magnetic resonance imaging (MRI) units per million population. (F) Positron emission tomography (PET) units per million population. Data from Global Health Observatory.4 |
Differences in Screening and Diagnosis Practices Between LMICs and HICs
Healthcare costs are a contributing factor in the incidence and mortality rates of cancers. In a study by Ebell, Thai and Royalty,16 cancer screening recommendations in HICs were compared. They highlighted that due to better resources, HICs have implemented vast cancer screening programs. However, with PCa, in particular, there is a greater variation in screening recommendations within these HICs. If this is the case only within HICs, how much greater variation exists between HICs and LMICs. In HICs such as Canada, the United States and Australia, the introduction of PSA testing resulted in the rapid increase of PCa incidence between the late 1980s and early 1990s.17 The late 2000s saw a decrease in incidence rates which was most likely due to the decrease of PSA testing17 as changes to the screening recommendations occurred.18 An opposing trend was seen in LMICs such as Kenya, Zimbabwe and Mozambique. The increasing PCa incidence rates were seen during and after the mid-1990s till 2018 and this is thought to be due to improving healthcare systems and thus more PSA tests.19 This trend of incidence rates across HICs and LMICs is linked to availability of screening and adequate treatment options which is directly associated with resource availability.20 In some LMICs, the economic burden of PCa cannot be accurately determined due to insufficient evidence associated with resources inaccessibility and shortage.13 In these countries, knowledge on PCa, the availability of screening and diagnostic tools, and the funds for these are also limited. This may explain the high mortality rates in LMICs as the cancer is not detected and treated early.
Men of African ancestry have been shown to be more predisposed to developing PCa.21 Unfortunately, many of these men reside in countries that are considered to be LMICs. Considering that they have an increased risk as well as poor availability of resources for the screening and diagnosis of PCa, they are at an even greater disadvantage than those in HICs with less risk of developing PCa and greater access to medical care.
Global debates around PSA testing are ongoing regarding PSA threshold for biopsy, potential harm to patients, the concepts of over-diagnosis and overtreatment. For example, the broad adoption of the 4ng/mL PSA threshold has led to significant tumours being missed while also alarmingly instigating attention on the benign conditions over urgent and serious ones.22,23 It has been reported that normal PSA levels are below 4ng/mL with data showing 0.9 ng/mL for men in their 40s and 50s, while more than 1 ng/mL PSA levels would still identify aggressive cancers that may otherwise go undetected.24,25 Thus, the standard 4ng/mL PSA threshold does not cater for high-risk patients that may benefit from the test. For instance, a 3.6 ng/mL PSA level in a 78-year-old and a 41-year-old may not yield optimal outcomes for each of these individual patients without proper assessment. Such debates have led to the development of age-specific PSA thresholds for PCa testing, to aid in the identification of men at high risk of developing PCa. Health and social care bodies such as England’s National Institute of Clinical Excellence (NICE) has revised guidelines on specific age group PSA thresholds. The age-specific PSA thresholds are as follows: 3ng/mL for 50–59 years; 4ng/mL for 60–70 years and 5ng/mL for 70 years and older.26,27
PCa Treatment
Non-communicable diseases (NCDs) such as cancer, including PCa have been identified as a major challenge to sustainable development. Cancer prevention and management to prevent premature mortality and loss of productive years of life is an urgent matter in Africa as this will alleviate the devastating socioeconomic burden and move towards sustainable development. In addition to early detection, correct diagnosis and multimodal cancer treatment, the World Health Organization (WHO) identifies palliative care as an additional key principle for effective cancer control in LMICs. Palliative care improves quality of life of cancer patients including PCa patients and their families. It has been recognized that palliative care considers and approaches cancer patients as holistic beings, with health, medical physiological, social and cultural needs. It has been reported that about 40 million people globally need palliative care, and 32 million reside in LMICs. Palliative care holds the promise of serving as a bridge between Western practices and African norms towards effective PCa management.28
PCa patients go through different pathways of treatment. These include hormone therapy (androgen deprivation therapy (ADT)), surgery (prostatectomy), radiation therapy (internal radiation or external beam radiation therapy), active surveillance and watchful waiting.29 The difference between active surveillance (AS) and watchful waiting (WW) is that AS extends PCa life expectancy as it involves serial PCa testing for disease progression, thus offering selective delayed treatment with a curative intent.30 The androgen hormone plays a significant role in prostate gland growth and prostate tumourigenesis.31 Androgen signaling has therefore remained the cornerstone of PCa treatment.32 These treatment interventions against androgen signaling include the direct targeting of the androgens (ligand), or the androgen receptor (AR), or the androgen/AR signaling regulatory mechanisms.33–35 Substantial efforts directly targeting the constitutively expressed AR signaling components are being made, as this is the key driver of castration resistance cells that lead to advanced PCa. Numerous studies have reported on compensating pathways to AR signaling in castration-resistant cells. Furthermore, the upregulated human epidermal growth factor receptor 2 (HER2/neu) pathway and insulin growth factor 1 (IGF-1) pathway have been reported to activate AR signaling, while the MAPK pathway plays a role in the overexpression of PSA under androgen depravation.36,37 IL-6 expression in castration-resistant cells has also been reported.37,38 Various other findings reported decreased protein phosphatase 2 (PP2A) signaling, overexpression of MYB oncogenic transcription factor, estrogen and glucocorticoid signaling in AR-independent conditions.39–46
Barriers and Opportunities to PCa Care in Africa
Distinguishing between clinically insignificant and significant PCa remains challenging, with the existing diagnostic tests limited by false negatives or false positives. The African populations represent some of the most genetically and culturally diverse globally.47 Significant efforts still need to be done in Africa to appreciate the seriousness of PCa in men, to reach the same level of knowledge as that of women’s cancers, breast and cervical, that have been attained through high levels of funding.
In South Africa, efforts have been made to have men of African origin undergo PSA testing from 40 years, as it has been recommended by the PCa foundation of South Africa.10 Despite efforts to promote early screening, it has been reported that most PCa cases in Africa and South Africa remain undiagnosed and therefore unreported. It has also been reported that access to standard PCa treatment for Southern Africa men is a problem.48 Poor PCa prognosis also remains a challenge in African men.49,50
While most PCa patients access health care through the public health system in South Africa, the order in which PCa patients eventually are referred to, through the public health system is multilayered with multiple referral levels before the patient can make the first visit to the oncologist. This involves point of contact or the primary healthcare center which usually is the local clinic, then the district hospital followed by the regional hospital and eventually the tertiary hospital with oncology facilities, Figure 3.51,52 This may partly be attributed to the already burdened public health system, while most African countries do not even have adequate oncology facilities to ultimately meet the needs of PCa patients. Nigeria also uses a similar referral system to South Africa. In Nigeria, the National Development Plans (NDP) fragmented public health care services into three levels of care, primary, secondary and tertiary. These levels reflect the three-step hierarchy of government, which are, Local, State and Federal governments.53 The shortage of specialist urologists and oncologists is acute in Africa.47 This is further exacerbated by the centralisation of oncology facilities to the big urban cities, compelling PCa patients from rural areas to incur unnecessary costs for transport and accommodation.51,52 This is worsened if such patients get to be admitted in the urban areas’ hospital, as their families are mostly unable to visit them during their hospital stay. This creates unfavourable psychological conditions that black patients battle with in conjunction with their physical health status.
 |
Figure 3 Referral hierarchy of PCa patients in South African public health care system. Patients commence at primary level, followed by subsequent multiple levels before their first oncology visit at the tertiary public hospital. Data from these studies.51,52 |
In South Africa, black patients particularly from disadvantaged backgrounds have been reported to desire more information regarding their prostate cancer status in health care facilities. It has been thus reported that language and communication barriers leave such patients with unanswered questions such as the implications of having prostate cancer (particularly as African men), severity of the disease, treatment options available, follow-up procedures, tests and appointments. The lack of adequate community education to address the misconceptions about cancer is also another barrier. Part of this fragmented communication is the South African healthcare workers using questionnaires from developed countries in local South African settings with most of these patients coming from disadvantaged socio-economic backgrounds.54–59 Furthermore, upholding traditional and cultural norms by African men, in particular, impedes effective PCa care management. For instance, such patients have expressed concerns of the association between PCa and their manhood. Some patients reject treatment because of erectile dysfunction fears, even when their health is deteriorating. Manhood superiority overrides the choice for good health in such settings. The disparity between black men seeking PCa health care compared to other races in South Africa is alarming. This warrants significant efforts to reach out to these men using various approaches such as religious, physical and cultural. Generally, men respond more poorly to healthcare needs than women and this is even more evident in African men. Challenges and solutions to this imbalance are discussed in the next section.
Roles of African Norms and Masculinity in PCa Management
The social construction theory plays an integral part in shaping African populations. This theory states that people develop knowledge and depend on shared ways of thinking, in a social context driving African populations to develop collaboratively rather than individually.60,61 The social construction theory is strongly supported by an African proverb “I am because we are”. Therefore, individuals in a society adopt perceptions, beliefs and meanings based on a construct of their existing society.62 In line with the social construct theory practices in the African population, peers play a significant role in men’s decision-making to seek health care.
Socioeconomic, geographical and education levels play a significant role in men’s response to good health practices. However, it has been documented that men in general have poorer health seeking behaviours than women and African men are no exception.63,64 In an African context, men are perceived as leaders and heads of families and this is tightly linked to their masculinity. These factors are corroborated by healthcare facilities physical accessibility, employment status, cultural beliefs, income level and politics. A study conducted in rural parts of the KwaZulu Natal (KZN) province in South Africa demonstrated that key health-related behaviors are influenced by dominant masculinity which identifies with social constructs such as excessive drinking, drug abuse and practicing unprotected sex. This study identified the lack of education and men’s awareness of their health. There is therefore an urgent need to introduce innovative methods that will reach out to both educated and uneducated men, regarding their overall health and in particular prostate health. Bridging the gap between men’s behavior and men’s poor health seeking practices is key to improving men’s health.64
Another key factor highlighted by this study is that South African local clinics are women-dominated and have potentially become unfavourable environment for men. In these clinics, most nurses are females, and females also make up most counselors. This is corroborated by the fact that most clinics’ users are females. Furthermore, in most African cultures, it seems demeaning for African men to disclose problems, which in this case include sexual/reproductive health problems to women. This is seen as a weakness in their own sight, and strongly conflicts with masculinity. As local clinics are the first point of contact for most of these men who often present with advanced disease, policy makers could intervene to better advocate for men’s health. Targeting communities in which men reside with rigorous PCa education and awareness campaigns may be a good start. This should be supported by structural changes in local clinics that will accommodate both males and females in clinics. This will even broaden the chances of qualitative studies engaging men and their own health in an African context and this may alleviate the PCa health care cost associated with poor prognosis and treatment of advanced disease. Notably, in Africa, there is a lack of qualitative studies to underpin poor men’s health seeking behavior. Such studies must include community leaders (men), health care workers, cultural, traditional and spiritual leaders and social scientists.
In most communities, African men would rather primarily resort to self-remedy, where men would find their own cure following consultation with elderly men as they are considered approachable and trustworthy. Traditional medicine in some African countries is still considered the best option, better than Western medicine. African populations believe that traditional medicine is readily accessible, affordable, easy to use, trustworthy and provides healing to the holistic being, soul, body and spirit. The scientific discourse around PCa in the African continent should involve the African traditional understanding of health, disease and whole human aspect.65 This is often followed by consulting with traditional and spiritual leaders, where patients believe that sickness, PCa in this case, is associated with supernatural forces such as witchcraft, and therefore do not need the Western approach. Formal health care is often the last resort and usually is taken when the disease (PCa) could not be cured by the preceding options and is often at an advanced stage.
Challenges and Limitations
It is evident that in HICs, men of African ancestry have higher PCa incidence and mortality rates. Challenges in Africa include demonstrating the precise PCa prevalence in black men compared to other populations. Arguably, if more white men respond to PCa screening and testing than black men, then higher incidence rates favor the most tested population. This pattern will also affect reported PCa mortality rates in Africa. Figure 4 illustrates the disproportion of PCa incidence in South Africa. While incidence rates in white men appear to be higher than black men in South Africa, the reluctance of black men to seeking medical advice exacerbates PCa disparities in Africa. Overcoming masculinity barriers in African populations is key to addressing these challenges and limitations towards effective PCa care in the African populations. Accurate reporting of population-based PCa mortality rates by the African cancer registries would also be beneficial towards unlocking PCa disparities in Africa.
 |
Figure 4 The South African cancer statistics for 2019 as per the National Cancer Registry (NCR-SA). NCR indicates that South Africa has a very high incidence rate for PCa with relatively lower mortality rates in the region. These NCR cancer statistics show that the White and Colored population groups have the highest incidence rates compared to the black population.86 |
Furthermore, it has been documented that most of the translational cancer research is funded by industry in LMICs including African LMICs. This disadvantages the greater public as industry-related questions will primarily be addressed and only the privileged will benefit from such initiatives. Additionally, there is a lack of representation of African populations in the global clinical trials platform.66 Excessive costs associated with new drug development is another contributing factor to Africa’s underrepresentation in clinical trials. The reluctance of African descended populations to participate in medical and clinical research in HICs may be due to fear and mistrust founded on unethical treatment and experimentation on Africans by the medical research communities.67–80 Excessive alcohol use is also an emerging public health problem, globally and in South Africa. It has been reported that globally more than 5% of the cancer cases and deaths are attributed to alcohol.81,82 South Africa has been reported as one of the LMICs with excessive alcohol use. Although the scientific evidence lacks between alcohol use and PCa, excessive use of alcohol in South African men has been identified as an additional factor to poor health habits.
Despite these problems, advances in PCa management in Africa are embraced by the Human Heredity and Health in Africa (H3Africa) initiative. H3Africa has initiated projects such as Genome Wide Association Studies (GWAS) for prostate and breast cancers in the African American populations.83,84 Although African American and African populations may share a common genetic ancestry, external factors such as environmental and socioeconomic status may contribute to the significant differences in the overall PCa theranostics. For example, high-risk PCa genetic loci identified in European men have also been identified in African American populations but not in Southern African men.47 The SSA region has established the Men of the African Descent Carcinoma of the prostate (MADCaP) Network to share data and address the disparities in PCa management. In addition, the African Cancer Registry Network (AFRCN) has been established in SSA. AFRCAN’s mandate is to collate the existing cancer registries from SSA and promote the establishment of new ones.85 Furthermore, early PCa diagnosis especially in high-risk populations such as African populations is a challenge. The relatively low advocacy for men’s health in Africa compared to women’s health also warrants an urgent redress.
Conclusions
PCa in Africa is not just a medical problem, solutions that are inclusive of all stakeholders are key to combating the devastating PCa effects, particularly in Southern Africa. A shift in policy making, men’s advocacy campaigns to focus on men’s health is an urgent matter. Awareness concerning PCa is needed to de-stigmatize PCa in African communities. Programmes that will focus on behavioural change and build from masculine discourse will positively impact on men’s health behaviour and outcomes. Integrative research on elucidating how western PCa healthcare practices can be integrated into African cultural and traditional practices towards PCa management is warranted. In Africa, factors leading to poor PCA management are interconnected. This calls for collaborative efforts to effectively manage this disease, Figure 5.
There is an urgent need in Africa to unite governments, policy makers and global organisations. Furthermore, effective and electronic data collection and storing can aid in the identification of PCa patterns, providing opportunities for early interventions. Like the case of breast and cervical cancers, PCa mortality rates in Africa are also influenced by traditional, cultural and social factors that influence cancer screening, diagnosis, willingness/reluctance to seek PCa treatment and response to treatment. Indeed, in Africa, effective PCa management goes beyond the health care practitioners and should therefore involve other stakeholders such as religious, traditional and cultural leaders, social workers, social scientists and unique communities. Southern Africa bears the brunt of this disease, and lessons can be shared across the entire African continent and even beyond on effective PCa management, customized for African populations. Therefore, future work should be directed towards an all-round care that will include the norms, beliefs and world views of PCa patients.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception and/or study design, took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This project was funded by the South African Medical Research Council (SAMRC) Grant Number 23108, and the National Research Foundation (NRF) Grant Number 138139.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Hamdi Y, Abdeljaoued-Tej I, Zatchi AA, et al. Cancer in Africa: the untold story. Front Oncol. 2021;11:650117. doi:10.3389/fonc.2021.650117
2. Bor J, Herbst AJ, Newell ML, Bärnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science. 2013;339(6122):961–965. doi:10.1126/science.1230413
3. Rebbeck TR, Devesa SS, Chang BL, et al. Global patterns of prostate cancer incidence, aggressiveness, and mortality in men of African descent. Prostate Cancer. 2013;2013:560857. doi:10.1155/2013/560857
4. World Health Organization. Global cancer observatory: cancer Today; 2014. Available from: https://gco.iarc.fr.
5. Parker SL, Davis KJ, Wingo PA, Ries LA, Heath CW
6. Marima R, Hull R, Mathabe K, et al. Prostate cancer racial, socioeconomic, geographic disparities: targeting the genomic landscape and splicing events in search for diagnostic, prognostic and therapeutic targets. Am J Cancer Res. 2021;11(4):1012–1030.
7. Bentley AR, Callier S, Rotimi C. The emergence of genomic research in Africa and new frameworks for equity in biomedical research. Ethn Dis. 2019;29(Suppl 1):179–186. doi:10.18865/ed.29.S1.179
8. Marima R, Hull R, Mbeje M, et al. Role of precision oncology in type II endometrial and prostate cancers in the African population: global cancer genomics disparities. Int J Mol Sci. 2022;23(2). doi:10.3390/ijms23020628
9. Corbex M, Bouzbid S, Boffetta P. Features of breast cancer in developing countries, examples from North-Africa. Eur J Cancer. 2014;50(10):1808–1818. doi:10.1016/j.ejca.2014.03.016
10. Tindall EA, Monare LR, Petersen DC, et al. Clinical presentation of prostate cancer in black South Africans. Prostate. 2014;74(8):880–891. doi:10.1002/pros.22806
11. Ramaliba TM, Sithole N, Ncinitwa A, Somdyala NIM. Prostate cancer patterns and trends in the Eastern Cape Province of South Africa; 1998–2017. Original research. Front Public Health. 2022;10. doi:10.3389/fpubh.2022.882586
12. Babb C, Urban M, Kielkowski D, Kellett P. Prostate cancer in South Africa: pathology based national cancer registry data (1986–2006) and mortality rates (1997–2009). Prostate Cancer. 2014;2014:419801. doi:10.1155/2014/419801
13. Ngcamphalala C, Östensson E, Ginindza TG. The economic burden of prostate cancer in Eswatini. BMC Health Serv Res. 2022;22(1):483. doi:10.1186/s12913-022-07817-6
14. Stefan DC. Cancer care in Africa: an overview of resources. J Glob Oncol. 2015;1(1):30–36. doi:10.1200/JGO.2015.000406
15. Vanderpuye V, Hammad N, Martei Y, et al. Cancer care workforce in Africa: perspectives from a global survey. Infect Agent Cancer. 2019;14(1):1–8. doi:10.1186/s13027-019-0227-8
16. Ebell MH, Thai TN, Royalty KJ. Cancer screening recommendations: an international comparison of high income countries. Public Health Rev. 2018;39:7. doi:10.1186/s40985-018-0080-0
17. Zhou CK, Check DP, Lortet-Tieulent J, et al. Prostate cancer incidence in 43 populations worldwide: an analysis of time trends overall and by age group. Int J Cancer. 2016;138(6):1388–1400. doi:10.1002/ijc.29894
18. Screening for prostate cancer: U.S. Preventive services task force recommendation statement. Ann Intern Med. 2008;149(3):185–191. doi:10.7326/0003-4819-149-3-200808050-00008
19. Seraphin T, Joko Fru YW, Kamaté B, et al. Rising prostate cancer incidence in sub-Saharan Africa: a trend analysis of data from the African cancer registry network. Cancer Epidemiol Biomarkers Prev. 2020;30:158–165. doi:10.1158/1055-9965.EPI-20-1005
20. Cassell A, Yunusa B, Jalloh M, et al. Management of advanced and metastatic prostate cancer: a need for a sub-saharan guideline. J Oncol. 2019;2019:1785428. doi:10.1155/2019/1785428
21. Rebbeck TR, Devesa SS, Chang B-L, et al. Global patterns of prostate cancer incidence, aggressiveness, and mortality in men of African descent. Prostate Cancer. 2013;2013:560857. doi:10.1155/2013/560857
22. Lajous M, Cooperberg MR, Rider J, et al. Prostate cancer screening in low- and middle-income countries: the Mexican case. Salud Publica Mex. 2019;61(4):542–544. doi:10.21149/10373
23. Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level ≤4.0 ng per milliliter. N Eng J Med. 2004;350(22):2239–2246. doi:10.1056/NEJMoa031918
24. Preston MA, Batista JL, Wilson KM, et al. Baseline prostate-specific antigen levels in midlife predict lethal prostate cancer. J Clin Oncol. 2016;34(23):2705–2711. doi:10.1200/jco.2016.66.7527
25. Vickers AJ, Ulmert D, Sjoberg DD, et al. Strategy for detection of prostate cancer based on relation between prostate specific antigen at age 40–55 and long term risk of metastasis: case-control study. BMJ. 2013;346:f2023. doi:10.1136/bmj.f2023
26. National Institute for H, Clinical E. Prostate Cancer: Diagnosis and Treatment (CG 175). National Institute for Health and Care Excellence London; 2014.
27. Gilbert R, Tilling K, Martin RM, et al. Developing new age-specific prostate-specific antigen thresholds for testing for prostate cancer. Cancer Causes Control. 2018;29(3):383–388. doi:10.1007/s10552-018-1014-3
28. Shah SC, Kayamba V, Peek RM
29. Washington CM, Leaver DT. Principles and Practice of Radiation Therapy-e-Book. Elsevier Health Sciences; 2015.
30. Loeb S, Zhou Q, Siebert U, et al. Active surveillance versus watchful waiting for localized prostate cancer: a model to inform decisions. Eur Urol. 2017;72(6):899–907. doi:10.1016/j.eururo.2017.07.018
31. La Vignera S, Condorelli RA, Russo GI, Morgia G, Calogero AE. Endocrine control of benign prostatic hyperplasia. Andrology. 2016;4(3):404–411. doi:10.1111/andr.12186
32. Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22(4):232–240. doi:10.3322/canjclin.22.4.232
33. Heemers HV. Targeting androgen receptor action for prostate cancer treatment: does the post-receptor level provide novel opportunities? Int J Biol Sci. 2014;10(6):576–587. doi:10.7150/ijbs.8479
34. Liang T, Heiss CE. Inhibition of 5 alpha-reductase, receptor binding, and nuclear uptake of androgens in the prostate by a 4-methyl-4-aza-steroid. J Biol Chem. 1981;256(15):7998–8005. doi:10.1016/S0021-9258(18)43378-9
35. Phillips R. Novel targeting of androgen signalling in CRPC. Nat Rev Urol. 2014;11(6):303. doi:10.1038/nrurol.2014.107
36. Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5(3):280–285. doi:10.1038/6495
37. Lin DL, Whitney MC, Yao Z, Keller ET. Interleukin-6 induces androgen responsiveness in prostate cancer cells through up-regulation of androgen receptor expression. Clin Cancer Res. 2001;7(6):1773–1781.
38. Ueda T, Bruchovsky N, Sadar MD. Activation of the androgen receptor N-terminal domain by interleukin-6 via MAPK and STAT3 signal transduction pathways. J Biol Chem. 2002;277(9):7076–7085. doi:10.1074/jbc.M108255200
39. Bhardwaj A, Singh S, Srivastava SK, et al. Restoration of PPP2CA expression reverses epithelial-to-mesenchymal transition and suppresses prostate tumour growth and metastasis in an orthotopic mouse model. Br J Cancer. 2014;110(8):2000–2010. doi:10.1038/bjc.2014.141
40. Bhardwaj A, Singh S, Srivastava SK, Honkanen RE, Reed E, Singh AP. Modulation of protein phosphatase 2A activity alters androgen-independent growth of prostate cancer cells: therapeutic implications. Mol Cancer Ther. 2011;10(5):720–731. doi:10.1158/1535-7163.Mct-10-1096
41. Huang J, Jia J, Tong Q, et al. Knockdown of cancerous inhibitor of protein phosphatase 2A may sensitize metastatic castration-resistant prostate cancer cells to cabazitaxel chemotherapy. Tumour Biol. 2015;36(3):1589–1594. doi:10.1007/s13277-014-2748-5
42. Srivastava SK, Bhardwaj A, Singh S, et al. Myb overexpression overrides androgen depletion-induced cell cycle arrest and apoptosis in prostate cancer cells, and confers aggressive malignant traits: potential role in castration resistance. Carcinogenesis. 2012;33(6):1149–1157. doi:10.1093/carcin/bgs134
43. Fradet A, Bouchet M, Delliaux C, et al. Estrogen related receptor alpha in castration-resistant prostate cancer cells promotes tumor progression in bone. Oncotarget. 2016;7(47):77071–77086. doi:10.18632/oncotarget.12787
44. Bonkhoff H. Estrogen receptor signaling in prostate cancer: implications for carcinogenesis and tumor progression. Prostate. 2018;78(1):2–10. doi:10.1002/pros.23446
45. Hu J, Chen Q. The role of glucocorticoid receptor in prostate cancer progression: from bench to bedside. Int Urol Nephrol. 2017;49(3):369–380. doi:10.1007/s11255-016-1476-8
46. Puhr M, Hoefer J, Eigentler A, et al. The glucocorticoid receptor is a key player for prostate cancer cell survival and a target for improved antiandrogen therapy. Clin Cancer Res. 2018;24(4):927–938. doi:10.1158/1078-0432.Ccr-17-0989
47. Hayes VM, Bornman MSR. Prostate cancer in Southern Africa: does Africa hold untapped potential to add value to the current understanding of a common disease? J Glob Oncol. 2018;4:1–7. doi:10.1200/JGO.2016.008862
48. Cassell A, Yunusa B, Jalloh M, et al. A review of localized prostate cancer: an African perspective. World J Oncol. 2019;10(4–5):162–168. doi:10.14740/wjon1221
49. Olender J, Lee NH. Role of alternative splicing in prostate cancer aggressiveness and drug resistance in African Americans. Adv Exp Med Biol. 2019;1164:119–139. doi:10.1007/978-3-030-22254-3_10
50. Cooperberg MR. Re-examining racial disparities in prostate cancer outcomes. J Clin Oncol. 2013;31(24):2979–2980. doi:10.1200/jco.2013.50.7723
51. Government SA Regulations relating to categories of hospitals. 469. Available from: https://www.gov.za/documents/national-health-act.
52. Government SA. Regional, tertiary and central hospital services. Republic of South Africa;2015 Available from: https://www.health.gov.za/index.php/nhi/category/267-nhi-016?download=1142:annexure-b-tertiary-services-definitions-package-of-care-december-2015.
53. Koce F, Randhawa G, Ochieng B. Understanding healthcare self-referral in Nigeria from the service users’ perspective: a qualitative study of Niger state. BMC Health Serv Res. 2019;19(1):1–14. doi:10.1186/s12913-019-4046-9
54. King AJ, Evans M, Moore TH, et al. Prostate cancer and supportive care: a systematic review and qualitative synthesis of men’s experiences and unmet needs. Eur J Cancer Care. 2015;24(5):618–634. doi:10.1111/ecc.12286
55. Rüesch P, Schaffert R, Fischer S, et al. Information needs of early-stage prostate cancer patients: within- and between-group agreement of patients and health professionals. Support Care Cancer. 2014;22(4):999–1007. doi:10.1007/s00520-013-2052-8
56. Chauhan M, Holch P, Holborn C. Assessing the information and support needs of radical prostate cancer patients and acceptability of a group-based treatment review: a questionnaire and qualitative interview study. J Radiother Pract. 2018;17(2):151–161. doi:10.1017/S1460396917000644
57. Kassianos AP, Raats MM, Gage H. An exploratory study on the information needs of prostate cancer patients and their partners. Health Psychol Res. 2016;4(1):4786. doi:10.4081/hpr.2016.4786
58. Feldman-Stewart D, Capirci C, Brennenstuhl S, et al. Information needs of early-stage prostate cancer patients: a comparison of nine countries. Radiother Oncol. 2010;94(3):328–333. doi:10.1016/j.radonc.2009.12.038
59. Dale J, Jatsch W, Hughes N, Pearce A, Meystre C. Information needs and prostate cancer: the development of a systematic means of identification. BJU Int. 2004;94(1):63–69. doi:10.1111/j.1464-410X.2004.04902.x
60. Leeds-Hurwitz W. Social construction of reality. Encyc Comm Theor. 2009;2:891–894.
61. Miller G. Reconsidering Social Constructionism: Social Problems and Social Issues. Routledge; 2017.
62. Ezeugwu CR, Ojedokun O. Masculine norms and mental health of African men: what can psychology do? Heliyon. 2020;6(12):e05650. doi:10.1016/j.heliyon.2020.e05650
63. Mofolo N, Betshu O, Kenna O, et al. Knowledge of prostate cancer among males attending a urology clinic, a South African study. SpringerPlus. 2015;4:67. doi:10.1186/s40064-015-0824-y
64. Nzama N. Masculinity and men’s health seeking behaviours amongst black/African men: the case of Durban, KwaZulu-Natal, South Africa; 2013.
65. Tetteh DA, Faulkner SL. Sociocultural factors and breast cancer in sub-Saharan Africa: implications for diagnosis and management. Womens Health. 2016;12(1):147–156. doi:10.2217/whe.15.76
66. Loree JM, Anand S, Dasari A, et al. Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol. 2019;5(10):e191870–e191870. doi:10.1001/jamaoncol.2019.1870
67. Harris Y, Gorelick PB, Samuels P, Bempong I. Why African Americans may not be participating in clinical trials. J Natl Med Assoc. 1996;88(10):630–634.
68. Wissing MD, Kluetz PG, Ning YM, et al. Under-representation of racial minorities in prostate cancer studies submitted to the US Food and Drug Administration to support potential marketing approval, 1993–2013. Cancer. 2014;120(19):3025–3032. doi:10.1002/cncr.28809
69. Branson RD, Davis K
70. Gorelick PB, Harris Y, Burnett B, Bonecutter FJ. The recruitment triangle: reasons why African Americans enroll, refuse to enroll, or voluntarily withdraw from a clinical trial. An interim report from the African-American Antiplatelet Stroke Prevention Study (AAASPS). J Natl Med Assoc. 1998;90(3):141–145.
71. Allen M. The dilemma for women of color in clinical trials. J Am Med Womens Assoc. 1994;49(4):105–109.
72. O’Donnell PH, Dolan ME. Cancer pharmacoethnicity: ethnic differences in susceptibility to the effects of chemotherapy. Clin Cancer Res. 2009;15(15):4806–4814. doi:10.1158/1078-0432.Ccr-09-0344
73. Sekine I, Yamamoto N, Nishio K, Saijo N. Emerging ethnic differences in lung cancer therapy. Br J Cancer. 2008;99(11):1757–1762. doi:10.1038/sj.bjc.6604721
74. Mulder NJ, Adebiyi E, Adebiyi M, et al. Development of bioinformatics infrastructure for genomics research. Glob Heart. 2017;12(2):91–98. doi:10.1016/j.gheart.2017.01.005
75. Nordling L. Putting Genomes to Work in Africa. Nature. 2017;544:20–22.
76. Mukhwana AM, Kariuki T, Kay S, Silva AJ, Kirkland J. The African academy of sciences research management programme in Africa. J Res Manag Gov. 2019;2(1):31–33.
77. Hoppe TA, Litovitz A, Willis KA, et al. Topic choice contributes to the lower rate of NIH awards to African-American/black scientists. Sci Adv. 2019;5(10):eaaw7238.
78. Carnethon MR, Kershaw KN, Kandula NR. Disparities research, disparities researchers, and health equity. JAMA. 2020;323(3):211–212.
79. Ginther DK, Schaffer WT, Schnell J, et al. Race, ethnicity, and NIH research awards. Science. 2011;333(6045):1015–1019.
80. Savitt TL. The use of blacks for medical experimentation and demonstration in the Old South. J South Hist. 1982;48(3):331–348.
81. Sivaram S, Perkins S, He M, et al. Building capacity for global cancer research: existing opportunities and future directions. J Cancer Educ. 2021;36(Suppl 1):5–24. doi:10.1007/s13187-021-02043-w
82. LoConte NK, Brewster AM, Kaur JS, Merrill JK, Alberg AJ. Alcohol and cancer: a statement of the American society of clinical oncology. J Clin Oncol. 2018;36(1):83–93.
83. Tan S-H, Petrovics G, Srivastava S. Prostate cancer genomics: recent advances and the prevailing underrepresentation from racial and ethnic minorities. Int J Mol Sci. 2018;19(4):1255.
84. Zheng Y, Walsh T, Gulsuner S, et al. Inherited breast cancer in Nigerian women. J Clin Oncol. 2018;36(28):2820.
85. Ambele MA, Van Zyl A, Pepper MS, Van Heerden MB, Van Heerden WFP. Amplification of 3q26. 2, 5q14. 3, 8q24. 3, 8q22. 3, and 14q32. 33 are possible common genetic alterations in oral cancer patients. Front Oncol. 2020;10:683.
86. Diseases N. National Cancer Registry; 2022. Available from: https://www.nicd.ac.za/centres/national-cancer-registry/.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.