Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Prospective memory in non-psychotic first-degree relatives of patients with schizophrenia: a meta-analysis
Authors Lin SZ, Wu YK, Su YA , Si TM
Received 31 January 2019
Accepted for publication 4 May 2019
Published 7 June 2019 Volume 2019:15 Pages 1563—1571
DOI https://doi.org/10.2147/NDT.S203729
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Shi-Ze Lin,1,2 Yan-Kun Wu,2–4 Yun-Ai Su,2–4 Tian-Mei Si2–4
1Quanzhou Mental Health Center, The Third Hospital of Quanzhou, Quanzhou, Fujian Province, People’s Republic of China; 2Institute of Mental Health, Peking University Sixth Hospital, Beijing, People’s Republic of China; 3National Clinical Research Center for Mental Disorders, Peking University Sixth Hospital, Beijing, People’s Republic of China; 4NHC Key Laboratory of Mental Health, Peking University, Beijing, People’s Republic of China
Abstract: Prospective memory (PM) could be impaired in the non-psychotic first-degree relatives of patients with schizophrenia. This meta-analysis systematically examined the PM of non-psychotic first-degree relatives of patients with schizophrenia. Both Chinese and English databases were systematically searched for articles from the inception of the databases through November 13, 2018. Case-control studies of PM in non-psychotic first-degree relatives of patients with schizophrenia were included in the analyses. Confidence intervals (CIs) and standardized mean differences (SMDs) were calculated utilizing the random effects model. Four studies (n=268) that compared PM performance between non-psychotic first-degree relatives of patients with schizophrenia (n=136) and healthy controls (n=132) were included. Three studies were rated as “high quality”, while the quality of evidence of the three outcomes included in this meta-analysis was moderate. Compared with the healthy controls, the non-psychotic first-degree relatives of patients with schizophrenia showed impairments in overall PM (two studies, n=127; SMD: −0.46; 95% CI=−0.82, −0.11, P=0.01; I=0%), event-based PM (EBPM) (four studies, n=268; SMD: −0.56; 95% CI=−0.80, −0.31, P<0.00001; I=0%), and time-based PM (TBPM) (four studies, n=268; SMD: −0.66; 95% CI=−0.90, −0.41, P<0.00001; I=0%). This meta-analysis demonstrated that the overall PM, EBPM, and TBPM might be impaired in the non-psychotic first-degree relatives of patients with schizophrenia.
Keywords: schizophrenia, relatives, prospective memory, endophenotypes
Introduction
Schizophrenia is a recurrent, prolonged, and chronic mental illness with strong heritability,1 and the genetic risk of schizophrenia is estimated to be as high as 80%.2 Therefore, the risk of developing schizophrenia in non-psychotic first-degree relatives of patients with schizophrenia is higher than that of the general population.3 Schizophrenia is often associated with various cognitive dysfunctions,4–8 which may be related to the heritability of schizophrenia. Relevant studies have found that the non-psychotic first-degree relatives of patients with schizophrenia also exhibit cognitive defects that are similar but less severe than those of the patients.9–14In terms of memory impairment, one of the most extensively researched areas of cognitive impairment, verbal memory, has been found to be as deficient as working memory in non-onset biological relatives or healthy siblings of patients with schizophrenia.15–19 Apparently, each of the studies have been restricted to retrospective memory, and there are few reports on prospective memory.
Prospective memory (PM) is defined as the capability of remembering to perform a planned action in the future.20 To complete a typical PM task, an individual first needs to form an intent (when and what needs to be done) and then begins to engage in an ongoing task. When performing ongoing tasks, individuals need to maintain focus and monitor PM cues. When a prospective memory cue appears and is detected, the individual should retrieve the intent and respond, which is of vital importance for everyday life, as research has shown that up to “50–80%” of daily activities are largely affected by PM.21 For example, remembering to attend a meeting tomorrow, remembering to sit for an exam next week, and remembering when to turn off the gas, etc., all require good PM function. We cannot imagine how severe and grave the consequences would be in our lives without PM. According to the nature of intention cues, PM falls into three categories: time-based prospective memory (TBPM), event-based prospective memory (EBPM), and activity-based prospective memory (ABPM).22,23 TBPM requires a person to remember to execute one intention after one specific time period (such as attending an academic conference next Saturday); EBPM requires a person to perform an intent when the prompt appears (such as purchasing a toothbrush when passing a supermarket, which is an external cue); and ABPM requires a person to do a planned action at the end of an activity (such as sending an email after a wedding).
We have developed an understanding of cognitive profiles of non-psychotic first-degree relatives of patients suffering from schizophrenia both in working and verbal memory. In recent years, the PM function of the non-psychotic first-degree relatives of patients with schizophrenia has attracted increasing attention. To date, only a small number of case-control studies have been published, and these studies had small sample sizes and conflicting results.24–27 By thoroughly searching the literature, no systematic review or meta-analysis can be located that specifically investigates the prospective memory of non-psychotic first-degree relatives of patients with schizophrenia, which provided an impetus for us to perform a comprehensive meta-analysis on the prospective memory of the non-psychotic first-degree relatives of patients with schizophrenia.
Methods
Search strategy and selection criteria
The inclusion criteria were identified by utilizing the PICOS acronym: Participants (P): non-psychotic first-degree relatives of patients with schizophrenia. Intervention (I): not applicable (NA). Comparison (C): healthy controls. Outcome (O): time-based PM, event-based PM, and overall PM. Study design (S): case-control studies exploring PM of non-psychotic first-degree relatives of patients with schizophrenia with the data required for the meta-analysis.
In accordance with the statement of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA),28 two investigators (SZL, YKW) searched Chinese (China National Knowledge Infrastructure, WanFang and Chinese Biomedical databases) and English databases (Embase, PubMed, Cochrane Library, and PsycINFO databases) independently, from database inception through November 13, 2018. The following search keywords were adopted: (schizophrenia OR psychotic disorder) AND (relatives OR siblings OR parents OR children OR offspring OR family members) AND (prospective memory OR prospective memories OR prospective remembering OR delayed intention). In addition, hand searching of reference lists of the identified studies and related reviews was performed.
Data extraction
SZL and YKW checked, extracted, and analyzed data independently. The data is presented in forest plots. Any inconsistency during the collection and analysis of the data was attempted to be addressed by a consensus; if the inconsistency was not solved, a third reviewer (TMS) would get involved. The related variables were extracted from the articles using a pre-designed standard form. If the same data was reported in more than one study, only the study with complete data would be incorporated into the analysis. Moreover, the corresponding or first author was contacted to obtain more information if required.
Statistical approaches
In accordance with the recommendations from the Cochrane Handbook for Systematic Reviews,29 the meta-analysis was performed utilizing Review Manager Version 5.3 software. Due to heterogeneity within the characteristics of this study (like sampling and sample size), a random effects model was adopted for each meta-analysis outcome.30 In terms of continuous outcome data, the standard mean difference (SMD) and the 95% CI between the non-psychotic first-degree relatives of patients with schizophrenia and the controls were calculated using the inverse variance method. Notable heterogeneity of the meta-analysis results was defined when I2 values were >50% or P<0.1 within Q statistics.31 In terms of primary outcome, a subgroup or sensitivity analysis was performed to investigate the cause of the heterogeneity.
Egger’s intercept and funnel plots were used to assess the publication bias.32 Each analysis was two-tailed, with 0.05 set as the significance level.
Study quality assessment
Two investigators (SZL, YKW) evaluated the quality of all studies utilizing the Newcastle-Ottawa Scale (NOS).33Following previous studies, an NOS score ≥7 points was rated as high-quality.34,35 In addition, the grading of recommendation evaluation, development, and assessment (GRADE) system was utilized to assess the quality of evidence,36,37and the meta-analysis data results were rated as “high quality”, “moderate quality”, “low quality”, and “very low quality”.
Results
Search results
Figure 1 shows the PRISMA flow diagram demonstrating that 369 articles were discovered by searching the abovementioned databases. Three studies were excluded because of overlapping data with other studies.38–40Four published English studies were finally included in the meta-analysis.24–27
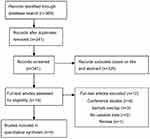 | Figure 1 PRISMA flow. |
Studies, characteristics of the relatives, and PM measures
The four case-control studies (n=268) that compared the non-psychotic first-degree relatives of patients with schizophrenia (n=136) with healthy controls (n=132) were performed in Hong Kong, China, and India. The average age of the participants was between 11.33 and 50.02 years, and the average length of education of the participants was 11.71–14.94 years. In one study,26 the Cambridge Prospective Memory Test-Chinese version (C-CAMPROMPT) was used for assessing PM.41 In addition, one laboratory computerized dual-task paradigm was used for assessing PM in three studies (Table 1).42–45
Table 1 Characteristics of the studies included in the meta-analysis
 | Table 1 Characteristics of the studies included in the meta-analysis |
Assessment of quality
NOS scores of the four studies were between 6 and 8 (Table 2), and three studies were rated as “high quality” (NOS ≥7).24–26The quality of evidence for the three meta-analysis outcomes was “moderate” according to the GRADE approach (Table 3).
 | Table 2 Methodological quality assessment by the Newcastle-Ottawa scale |
 | Table 3 GRADE analyses: prospective memory in non-psychotic first-degree relatives of patients with schizophrenia |
Table 2 Methodological quality assessment by the Newcastle-Ottawa scale
Table 3 GRADE analyses: prospective memory in non-psychotic first-degree relatives of patients with schizophrenia
Overall PM
In the two studies that had data available about overall performance of PM (n=127), the performance of non-psychotic first-degree relatives of patients with schizophrenia was poorer than that of healthy controls with a SMD of −0.46 (95% CI=−0.82, −0.11, P=0.01; I2= 0%, Figure 2).
Time-based PM
In the four studies that had data available about time-based PM (n=268), the performance of non-psychotic first-degree relatives of patients with schizophrenia was poorer than that of healthy controls with a SMD of −0.66 (95% CI=−0.90, −0.41, P<0.00001; I2=0%, Figure 2).
Event-based PM
In the four studies that had data available about event-based PM (n=268), the performance of non-psychotic first-degree relatives of patients with schizophrenia was poorer compared to that of healthy controls with a SMD of −0.56 (95% CI=−0.80, −0.31, P<0.00001; I2=0%, Figure 2).
Activity-based PM
In the two studies that had data available about activity-based PM (n=127), the performance of non-psychotic first-degree relatives of patients with schizophrenia did not differ from healthy controls with a SMD of −0.11 (95% CI=−0.46, 0.24, P=0.55; I2=0%, Figure 2).
Publication bias
Because only four studies were incorporated in the meta-analysis, the publication bias of the meta-analysis outcomes could not be carried out using Egger’s test or a funnel plot.46
Discussion
To the best of our knowledge, this is the first meta-analysis on the PM performance of first-degree relatives of patients with schizophrenia. The analysis results demonstrated that the overall prospective memory and the two categories of PM were markedly impaired with a medium effect size (overall PM: SMD=−0.46; EBPM: SMD=−0.56; TBPM: SMD=−0.66). These results are in line with the meta-analysis on working memory, verbal memory, and executive function in non-psychotic first-degree relatives of patients with schizophrenia.13,17 The performance of the overall PM, TBPM, and EBPM in first-degree relatives was markedly lower than that of normal controls, which was similar to that in schizophrenic patients.43,47,48 Although this impairment is less severe, this indicates that PM might be related to the heritability of schizophrenia. Additionally, the ABPM task was considered to be the simplest among the three memory tasks. Only two articles confirmed that the ABPM of first-degree relatives of patients with schizophrenia was not impaired in this task,24,25 suggesting that the first-degree relatives of patients suffering from schizophrenia had certain neurocognitive impairments, but their impairments were not as serious as those of patients with schizophrenia.
We discovered that the effect size of TBPM (SMD=−0.66) was larger than that of EBPM (SMD=−0.56), which showed a similar trend as that observed in any other neuropsychiatric disorder. For instance, one meta-analysis on the prospective memory of patients with schizophrenia revealed that TBPM impairment (Cohen’s d=−1.33) was more severe than EBPM impairment (Cohen’s d=−0.827).49 A larger effect size of TBPM than of EBPM was also reported in depression (SMD=−0.89 vs −0.44),50 bipolar disorder (SMD=−0.82 vs −0.51),51 Parkinson’s disease (Hedges’ g=−0.71 vs −0.55) and autism spectrum disorder (Hedges’ g=−0.87 vs −0.41) .52,53 This tendency can be explained by various TBPM and EBPM cognitive processes and their load on the prefrontal cortex.54,55TBPM has a more complicated cognitive process than EBPM, because there is not any specific external cue to trigger it. When performing tasks, much more effort is required to supervise time as well as self-initiate a prospective intention. In contrast, EBPM includes fewer demands for self-initiation, as it relies on one external cue as the trigger,22 leading to less demanding EBPM in the prefrontal cortex.55Therefore, TBPM is more demanding and is more likely to be compromised in patients suffering from pathological or other neuropsychiatric disorders, which includes first-degree relatives of patients with schizophrenia.22,55 This hypothesis was confirmed in the meta-analysis,49which suggested that the differences between TBPM and EBPM were heterogeneous.
Schizophrenia is an inherited disease,1but the results of conventional linkage and association studies on susceptibility loci are not consistent.56 Therefore, in recent years, researchers have adopted endophenotype approaches to investigate the genetic basis of schizophrenia.57,58 An endophenotype is closer to the function of gene action than the diagnosis of schizophrenia itself; thus, it is easier to locate the locus of the endophenotype than the locus of schizophrenia.57,59 There are a number of endophenotypes, and cognitive endophenotypes have been extensively studied. So far, some research has shown that the impairment of PM exists within schizophrenia patients, regardless of chronic,47,60–63 first-episode, or even first-episode drug-naive status,64–66 and meta-analyses and systematic reviews have been conducted.49,67 In this study, it was found that the non-psychotic first-degree relatives of patients with schizophrenia had defects in their prospective memory. Based on the above studies, we can determine that the characteristics of PM impairment of schizophrenia seem to conform to the disease association, state independence (regardless of first or chronic stages), family association, and the high-risk group of the endophenotype criterion proposed by Gottsman and Gould.57 In summary, it is reasonable to speculate that PM might be an endophenotype of schizophrenia.
Due to some methodological limitations, the meta-analysis results should be interpreted cautiously. First, the sample sizes involved in this study were comparatively small, and all meta-analysis results were rated only as “moderate quality” according to the GRADE score. Future studies may require larger samples and may be required to distinguish between parents, siblings, and offspring of the patients suffering from schizophrenia, since genetic studies have discovered that parents, siblings, and offspring of people with schizophrenia have different prevalence rates.3Second, only Chinese and English databases were searched, and only four studies were incorporated; therefore, the publication bias could not be tested. Third, different studies employed different PM measurement approaches, and the results were not the same. Therefore, we used a random effects model to provide conservative estimates.
Conclusion
The meta-analysis revealed that PM, especially TBPM, might be impaired in non-psychotic first-degree relatives of patients with schizophrenia. Further high-quality large sample studies are worthwhile and necessary. Furthermore, it is recommended that psychiatrists place more emphasis on early detection of PM deficits in first-degree relatives of patients with schizophrenia, and take into account the effective implementation of interventions to mitigate negative outcomes related to PM deficits.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81630031), Beijing Municipal Science and Technology Project (Z171100000117016), and The Capital Foundation of Medicine Research and Development (2016-1-4111).
Disclosure
Dr Shi-Ze Lin reports grants from the National Natural Science Foundation of China, Beijing Municipal Science and Technology Project, and The Capital Foundation of Medicine Research and Development during the conduct of the study; grants from the National Natural Science Foundation of China, Beijing Municipal Science and Technology Project, and The Capital Foundation of Medicine Research and Development outside the submitted work. Dr Yan-Kun Wu reports grants from the National Natural Science Foundation of China, Beijing Municipal Science and Technology Project, and The Capital Foundation of Medicine Research and Development during the conduct of the study; grants from The Capital Foundation of Medicine Research and Development, Beijing Municipal Science and Technology Project, and the National Natural Science Foundation of China, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Kendler KS, Diehl SR. The genetics of schizophrenia: a current, genetic-epidemiologic perspective. Schizophr Bull. 1993;19(2):261–285.
2. Cannon TD, Kaprio J, Lonnqvist J, Huttunen M, Koskenvuo M. The genetic epidemiology of schizophrenia in a Finnish twin cohort. A population-based modeling study. Arch Gen Psychiatry. 1998;55(1):67–74.
3. Vogel F. Schizophrenia genesis: The origins of madness. Am J Hum Genet. 1991;48(6):1218.
4. Fioravanti M, Carlone O, Vitale B, Cinti ME, Clare L. A meta-analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychol Rev. 2005;15(2):73–95. doi:10.1007/s11065-005-6254-9
5. Heinrichs RW. The primacy of cognition in schizophrenia. Am Psychol. 2005;60(3):229–242. doi:10.1037/0003-066X.60.3.229
6. Green MF, Harvey PD. Cognition in schizophrenia: past, present, and future. Schizophr Res Cogn. 2014;1(1):e1–e9. doi:10.1016/j.scog.2014.02.001
7. Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23(3):315–336. doi:10.1037/a0014708
8. Schulz SC, Murray A. Assessing cognitive impairment in patients with schizophrenia. J Clin Psychiatry. 2016;77(Suppl 2):3–7. doi:10.4088/JCP.14074su1c.01
9. Davalos DB, Compagnon N, Heinlein S, Ross RG. Neuropsychological deficits in children associated with increased familial risk for schizophrenia. Schizophr Res. 2004;67(2–3):123–130. doi:10.1016/S0920-9964(03)00187-7
10. Egan MF, Goldberg TE, Gscheidle T, et al. Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biol Psychiatry. 2001;50(2):98–107.
11. Fis NP, Cetin FC, Erturk M, Erdogan E, Dedeoglu C, Yazgan Y. Executive dysfunction in Turkish children at high risk for schizophrenia. Eur Child Adolesc Psychiatry. 2008;17(7):424–431. doi:10.1007/s00787-008-0684-x
12. Groom MJ, Jackson GM, Calton TG, et al. Cognitive deficits in early-onset schizophrenia spectrum patients and their non-psychotic siblings: a comparison with ADHD. Schizophr Res. 2008;99(1–3):85–95. doi:10.1016/j.schres.2007.11.008
13. Sitskoorn MM, Aleman A, Ebisch SJ, Appels MC, Kahn RS. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophr Res. 2004;71(2–3):285–295. doi:10.1016/j.schres.2004.03.007
14. Wolf LE, Cornblatt BA, Roberts SA, Shapiro BM, Erlenmeyer-Kimling L. Wisconsin Card Sorting deficits in the offspring of schizophrenics in the New York high-risk project. Schizophr Res. 2002;57(2–3):173. doi:10.1016/S0920-9964(01)00301-2
15. Conklin HM, Curtis CE, Katsanis J, Iacono WG. Verbal working memory impairment in schizophrenia patients and their first-degree relatives: evidence from the digit span task. Am J Psychiatry. 2000;157(2):275–277. doi:10.1176/appi.ajp.157.2.275
16. Conklin HM, Curtis CE, Calkins ME, Iacono WG. Working memory functioning in schizophrenia patients and their first-degree relatives: cognitive functioning shedding light on etiology. Neuropsychologia. 2005;43(6):930–942. doi:10.1016/j.neuropsychologia.2004.09.013
17. Trandafir A, Meary A, Schurhoff F, Leboyer M, Szoke A. Memory tests in first-degree adult relatives of schizophrenic patients: a meta-analysis. Schizophr Res. 2006;81(2–3):217–226. doi:10.1016/j.schres.2005.09.005
18. Whyte MC, McIntosh AM, Johnstone EC, Lawrie SM. Declarative memory in unaffected adult relatives of patients with schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2005;78(1):13–26. doi:10.1016/j.schres.2005.05.018
19. Wittorf A, Klingberg S, Wiedemann G. Secondary verbal memory: a potential endophenotype of schizophrenia. J Psychiatr Res. 2004;38(6):601–612. doi:10.1016/j.jpsychires.2004.03.005
20. Brandimonte M, Einstein G, McDaniel M, eds. Prospective Memory.
21. Kliegel M, Martin M. Prospective memory research: why is it relevant?. Int J Psychol. 2010;38(4):193–194. doi:10.1080/00207590344000114
22. Brandimonte M, Einstein GO, McDaniel MA. Varieties of intention: some distinctions and classifications. In: Prospective Memory: Theory and Applications. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 1996:23–51.
23. Einstein GO, McDaniel MA, Richardson SL, Guynn MJ, Cunfer AR. Aging and prospective memory: examining the influences of self-initiated retrieval processes. J Exp Psychol Learn Mem Cogn. 1995;21(4):996–1007.
24. Lui SS, Wang Y, Liu AC, et al. Prospective memory in patients with first-onset schizophrenia and their non-psychotic siblings. Neuropsychologia. 2011;49(8):2217–2224. doi:10.1016/j.neuropsychologia.2011.04.002
25. Wang Y, Chan RC, Cui J, et al. Prospective memory in non-psychotic first-degree relatives of patients with schizophrenia. Psychiatry Res. 2010;179(3):285–290. doi:10.1016/j.psychres.2009.07.011
26. Zhou FC, Hou WM, Wang CY, et al. Prospective memory performance in non-psychotic first-degree relatives of patients with schizophrenia: a controlled study. PLoS One. 2014;9(11):e111562. doi:10.1371/journal.pone.0111562
27. Saleem S, Kumar D, Venkatasubramanian G. Prospective memory in first-degree relatives of patients with schizophrenia. Clin Neuropsychol. 2018;32(5):993–1001. doi:10.1080/13854046.2017.1406145
28. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi:10.1016/j.jclinepi.2009.06.005
29. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. UK:Wiley ;2008.
30. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):
31. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558.
32. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):
33. Connell O. D. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Appl Eng Agric. 2002;18(6):727–734.
34. Hernandez AV, Pasupuleti V, Benites-Zapata VA, Thota P, Deshpande A, Perez-Lopez FR. Insulin resistance and endometrial cancer risk: A systematic review and meta-analysis. Eur J Cancer. 2015;51(18):2747–2758. doi:10.1016/j.ejca.2015.08.031
35. Zhu J, Su X, Li G, Chen J, Tang B, Yang Y. The incidence of acute myocardial infarction in relation to overweight and obesity: a meta-analysis. Arch Med Sci. 2014;10(5):855–862. doi:10.5114/aoms.2014.46206
36. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. Bmj. 2004;328(7454):1490. doi:10.1136/bmj.328.7445.934
37. Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi:10.1016/j.jclinepi.2010.07.015
38. Lui SSY, Wang Y, Liu ACY, et al. Prospective memory in patients with early-stage schizophrenia and their unaffected first-degree siblings. Schizophr Bull. 2011;37:217.
39. Wang Y, Chan RCK, Cui J, et al. Prospective memory in schizophrenia and their non-psychotic first-degree relatives. Aust N Z J Psychiatry. 2010;44:A47–A48.
40.
41. Lou Z, Dou Z, Zheng J, Man D The Study of The Chinese Version of Cambridge Prospective Memory Test (CAMPROMPT) for traumatic brain injury (unpublished Master thesis). Guangzhou, PR China, Sun Yat Sen University; 2009.
42. Chan RC, Wang Y, Ma Z, et al. Objective measures of prospective memory do not correlate with subjective complaints in schizophrenia. Schizophr Res. 2008;103(1–3):229–239. doi:10.1016/j.schres.2008.02.019
43. Wang Y, Chan RC, Hong X, et al. Prospective memory in schizophrenia: further clarification of nature of impairment. Schizophr Res. 2008;105(1–3):114–124. doi:10.1016/j.schres.2008.07.002
44. Wang Y, Chan RC, Xin Y, Shi C, Cui J, Deng Y. Prospective memory deficits in subjects with schizophrenia spectrum disorders: a comparison study with schizophrenic subjects, psychometrically defined schizotypal subjects, and healthy controls. Schizophr Res. 2008;106(1):70–80. doi:10.1016/j.schres.2007.07.020
45. Kumar D, Nizamie HS, Jahan M. Event-based prospective memory in schizophrenia. J Clin Exp Neuropsychol. 2005;27(7):867–872. doi:10.1080/13803390490919100
46. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Bmj. 2011;343:d4002. doi:10.1136/bmj.d4002
47. Shum D, Ungvari GS, Tang WK, Leung JP. Performance of schizophrenia patients on time-, event-, and activity-based prospective memory tasks. Schizophr Bull. 2004;30(4):693–701.
48. Ungvari GS, Xiang YT, Tang WK, Shum D. Prospective memory and its correlates and predictors in schizophrenia: an extension of previous findings. Arch Clin Neuropsychol. 2008;23(5):613–622. doi:10.1016/j.acn.2008.06.005
49. Wang Y, Cui J, Chan RC, et al. Meta-analysis of prospective memory in schizophrenia: nature, extent, and correlates. Schizophr Res. 2009;114(1–3):64–70. doi:10.1016/j.schres.2009.07.009
50. Zhou FC, Wang YY, Zheng W, et al. Prospective memory deficits in patients with depression: A meta-analysis. J Affect Disord. 2017;220:79–85. doi:10.1016/j.jad.2017.05.042
51. Zhou FC, Wang YY, Zheng W, et al. Prospective memory in bipolar disorder: A meta-analysis. Psychiatry Res. 2018;259:184–190. doi:10.1016/j.psychres.2017.09.073
52. Ramanan S, Kumar D. Prospective memory in Parkinson’s disease: a meta-analysis. J Int Neuropsychol Soc. 2013;19(10):1109–1118. doi:10.1017/S1355617713001045
53. Landsiedel J, Williams DM, Abbot-Smith K. A meta-analysis and critical review of prospective memory in autism spectrum disorder. J Autism Dev Disord. 2017;47(3):646–666. doi:10.1007/s10803-016-2987-y
54. Zhou FC, Xiang YT, Wang CY, et al. Characteristics and clinical correlates of prospective memory performance in first-episode schizophrenia. Schizophr Res. 2012;135(1–3):34–39. doi:10.1016/j.schres.2011.12.001
55. Einstein GO, McDaniel MA, Richardson SL, Guynn MJ, Cunfer AR. Aging and prospective memory: examining the influences of self-initiated retrieval processes. J Exp Psychol Learn Mem Cogn. 1995;21(4):996–1007.
56. Luo X, Huang L, Han L, et al. Systematic prioritization and integrative analysis of copy number variations in schizophrenia reveal key schizophrenia susceptibility genes. Schizophr Bull. 2014;40(6):1285–1299.
57. Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi:10.1176/appi.ajp.160.4.636
58. Gould TD, Gottesman II. Psychiatric endophenotypes and the development of valid animal models. Genes Brain Behav. 2006;5(2):113–119. doi:10.1111/j.1601-183X.2005.00186.x
59. Gur RE, Calkins ME, Gur RC, et al. The consortium on the genetics of schizophrenia: neurocognitive endophenotypes. Schizophr Bull. 2007;33(1):49–68. doi:10.1093/schbul/sbl055
60. Au RW, Man D, Shum D, et al. Assessment of prospective memory in schizophrenia using the Chinese version of the Cambridge prospective memory test: a controlled study. Asia Pac Psychiatry. 2014;6(1):54–61. doi:10.1111/j.1758-5872.2012.00217.x
61. Au RW, Man D, Xiang YT, et al. Prospective memory predicts the level of community living skills in schizophrenia. Psychiatry Res. 2014;219(1):86–91. doi:10.1016/j.psychres.2014.04.055
62. Au RWC, Xiang YT, Ungvari GS, et al. Prospective memory performance in persons with schizophrenia and bipolar disorder and healthy persons. Perspect Psychiatr Care. 2017;53(4):266–274. doi:10.1111/ppc.12172
63. Demeter G, Szendi I, Domjan N, et al. Preserved intention maintenance and impaired execution of prospective memory responses in schizophrenia: evidence from an event-based prospective memory study. Front Psychol. 2016;7:593. doi:10.3389/fpsyg.2016.00593
64. Cheung EFC, Lui SSY, Wang Y, Yang TX, Shum DHK, Chan RCK. Time-based but not event-based prospective memory remains impaired one year after the onset of schizophrenia: a prospective study. Schizophr Res. 2015;169(1–3):147–152. doi:10.1016/j.schres.2015.09.015
65. Liu D, Ji C, Zhuo K, et al. Impaired cue identification and intention retrieval underlie prospective memory deficits in patients with first-episode schizophrenia. Aust N Z J Psychiatry. 2017;51(3):270–277. doi:10.1177/0004867416640097
66. Lui SS, Wang Y, Yang TX, et al. Problems in remembering to carry out future actions in first-episode schizophrenia: primary or secondary impairment? J Psychiatr Res. 2015;61:141–149. doi:10.1016/j.jpsychires.2014.11.007
67. Ordemann GJ, Opper J, Davalos D. Prospective memory in schizophrenia: a review. Schizophr Res. 2014;155(1–3):77–89. doi:10.1016/j.schres.2014.03.008
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

