Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Prospective Evaluation of Exacerbations Associated with Suboptimal Peak Inspiratory Flow Among Stable Outpatients with COPD
Authors Mahler DA , Niu X, Deering KL, Dembek C
Received 10 December 2021
Accepted for publication 28 February 2022
Published 15 March 2022 Volume 2022:17 Pages 559—568
DOI https://doi.org/10.2147/COPD.S353441
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Donald A Mahler,1 Xiaoli Niu,2 Kathleen L Deering,3 Carole Dembek2
1Geisel School of Medicine, Dartmouth, Hanover, NH, USA and Valley Regional Hospital, Claremont, NH, USA; 2Sunovion Pharmaceuticals Inc., Marlborough, MA, USA; 3EPI-Q, Inc, Oak Brook, IL, USA
Correspondence: Donald A Mahler, Emeritus Professor of Medicine, Geisel School of Medicine, Dartmouth, Director of Respiratory Services, Valley Regional Hospital, 1 Rope Ferry Road, Hanover, NH, 03755, USA, Tel +1 603 542-6777, Fax +1 603 543-5613, Email [email protected]
Purpose: A suboptimal peak inspiratory flow (PIF) against a dry powder inhaler (DPI) may result in ineffective inhalation of medications, which may affect outcomes. The primary objective of this study was to examine the association between PIF status and COPD exacerbations among outpatients with moderate to very severe COPD.
Patients and Methods: This was a prospective, observational study of patients from 7 US outpatient centers. PIF was measured using an inspiratory flow meter (In-Check™ DIAL G16) set to medium low resistance. Patients were classified by suboptimal (< 60 L/min) or optimal PIF (≥ 60 L/min). The primary outcome was the proportion of patients with moderate/severe COPD exacerbations collected by medical record review over 12 months. Secondary outcomes were time to first exacerbation and mortality.
Results: Of 474 patients screened, 38.8% had suboptimal PIF, and 71 patients with optimal PIF were excluded from the study. The enrolled sample included 184 and 219 patients with suboptimal and optimal PIF, respectively. Suboptimal PIF was associated with shorter stature (66.6± 4.1 vs 67.8± 3.8 inches, P = 0.002), female sex (45.1 vs 34.7%, P = 0.033), Black race (27.2 vs 11.0%, P < 0.001), and greater symptom burden (CAT: 22.3± 7.7 vs 19.0± 7.0, P < 0.001; mMRC: 2.0± 1.1 vs 1.7± 1.1, P = 0.003). The proportion of patients with COPD exacerbations at 12 months was not significantly different (29.3 vs 27.9%, P = 0.751). Suboptimal PIF was associated with shorter time to first COPD exacerbation (3.8± 2.7 vs 4.9± 3.0 months, P = 0.048). The mortality rate at 12 months was higher in the suboptimal cohort but not significantly different (6.5 vs 2.8%, P = 0.073).
Conclusion: Over one-third of outpatients with stable moderate to very severe COPD had a suboptimal PIF against a medium low resistance DPI. The phenotype of suboptimal PIF was short stature, female, and Black. Suboptimal PIF status was associated with shorter time to moderate/severe COPD exacerbations compared with optimal PIF.
Keywords: dry powder inhaler, exacerbations, prospective, mortality
Introduction
Individual medications and combinations have been approved for the treatment of COPD and are used to reduce COPD exacerbations.1 These molecules are available in pressurized metered-dose inhalers (pMDIs), soft mist inhalers (SMIs), dry powder inhalers (DPIs), and nebulizers.1 For the use of pMDIs and SMIs, the patient should inhale “slow and steady” to deliver the aerosol deep into the lower respiratory tract.2,3 In contrast, DPIs have internal resistances that require the patient to inhale “forcefully” or “hard and fast” for optimal benefit.3 The measurement of inspiratory flow (IF) has been used to assess whether the patient has the ability to create turbulent energy within the DPI to de-aggregate the powder medication into fine particles that can be inhaled deep into the lungs.4,5
In vitro testing has provided both minimal and optimal IFs for different DPIs.6 A minimal IF of 30 liters/minute is generally recommended to actuate most DPIs,6 whereas studies show that higher IFs create smaller drug particle sizes for greater deposition of the medication into the lower respiratory tract.7,8 For low to medium high resistance DPIs, a peak IF (PIF) of at least 60 liters/minute is recommended.9 Two studies have demonstrated greater improvements in lung function with nebulized therapy compared with DPI in patients with a suboptimal PIF.10,11
To our knowledge, three studies have investigated whether patients with suboptimal PIF experience more frequent COPD exacerbations. In these retrospective studies, PIF was measured using the In-Check DIAL in patients with COPD admitted to the Hospital for an exacerbation. Loh and colleagues12 found that 90-day COPD and all-cause readmission rates were significantly greater in 64 patients with a PIF <60 L/min measured at functional residual capacity at no resistance compared with 59 patients who had PIF >60 L/min. Sharma and colleagues13 measured PIF against a medium low resistance in a total of 170 patients prior to discharge. There were no differences in rehospitalization rates based on optimal (n=85) versus suboptimal (n=85) PIF status.13 Finally, Samarghandi and colleagues14 reported a non-significant trend for greater 30- and 90-day readmission rates in 42 patients with a suboptimal PIF compared with 33 patients who had an optimal PIF measured against a medium low resistance.
To address these conflicting data, we performed a prospective, observational trial in outpatients with COPD at seven sites within the United States (US). At baseline, PIF was measured against a medium low resistance while patients were taking their prescribed therapies for COPD. The hypothesis of this real-world study was that patients with a suboptimal PIF (<60 L/min) would experience more moderate and severe exacerbations over the one-year period. Secondary outcomes included time to first exacerbation and mortality.
Methods
Data
This multi-center, prospective observational study used data collected from seven US sites which were not using PIF as a tool to determine or monitor inhaler treatment appropriateness. Patients with COPD were invited to participate in the study during an outpatient visit. Prior to any study activities, the patient provided written informed consent to participate in the study. Patients were eligible for inclusion in the study if they were ≥40 years of age, had stable moderate to very severe (FEV1 <80% predicted) COPD, and were fluent in and able to read English. Patients were excluded from the study if they were unable or unwilling to provide informed consent, had experienced a COPD exacerbation within the last 8 weeks, were currently hospitalized, or were currently residing in a long-term care facility. Data were collected from May 1, 2019, to March 31, 2021. Data collection and analysis were approved by the following Institutional Review Boards (IRBs): VA Western New York Healthcare System IRB, The Washington University in St. Louis IRB, Cleveland Clinic IRB, Birmingham VA Medical Center IRB, and WCG IRB (3 sites). Study participants received honoraria to participate in the study consistent with IRB ethical guidelines. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice.
PIF Measurement
PIF was measured with an inspiratory flow meter (In-Check™ DIAL G16) set to medium low resistance. For performance of PIF, eligible patients were instructed “to exhale slowly and deeply,” seal their lips around the mouthpiece, and then “to inhale as fast and as hard as possible.” The inspiratory flow was recorded, and the device was reset. The procedure was repeated two more times allowing sufficient time for the patient to recover from each effort. If the final attempt was the highest PIF, the process was repeated until there was no subsequent increase in the PIF. PIF values were not shared with the investigator. Patients were classified into suboptimal PIF (<60 L/min) or optimal PIF (≥60 L/min) cohorts. Due to incorrect measurement of PIF at one study site which was later corrected, several patients were removed from the study, leading to slower enrollment.
Cohort enrollment was actively managed to achieve similar numbers of patients in the suboptimal and optimal PIF cohorts. Once the optimal PIF cohort was closed, additional patients with optimal PIF had their PIF measurements recorded but were not selected for participation in the remaining study activities. Prevalence of suboptimal PIF was calculated from all patients screened for enrollment. Consented, eligible patients were also asked to complete spirometry for eligibility screening purposes using the site’s spirometer or a supplied spirometer (Micro I Diagnostic Spirometer) with necessary training prior to recruiting or enrolling patients. Spirometry was not conducted post-bronchodilator. Recorded measurements included forced expiratory volume in one second (FEV1), FEV1% predicted, forced vital capacity (FVC), FEV1/FVC, and peak expiratory flow (PEF).
COPD Exacerbations and Other Variables
The primary outcome was the proportion of patients with moderate or severe COPD exacerbations collected by medical record review over 12 months. Exacerbation severity was based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) definitions.1 Moderate exacerbations were treatment as an outpatient with systemic corticosteroids and/or antibiotics, while severe were defined as COPD-related hospitalizations or evaluations in the emergency department. COPD exacerbations were recorded by study personnel based on the review of the patients’ medical records. Information was collected on hospitalizations, emergency department visits, urgent care visits, and outpatient visits (including pulmonologist visits). Due to the COVID-19 pandemic,15 the study protocol was updated to allow 12-month visits and patient interviews to be conducted in-person or by phone. When medical record reviews and/or interviews occurred after 12 months, only 12 months of data after enrollment were used to calculate COPD exacerbations.
Secondary outcomes included time to first exacerbation and mortality. Time to first COPD exacerbation was counted as the number of days from enrollment to the first day of the moderate or severe exacerbation. Both breathlessness with daily activities using the modified Medical Research Council (mMRC) dyspnea scale and COPD-related health status using the COPD Assessment Test (CAT) were measured at enrollment. Medication classes and inhaler delivery devices were also reported at enrollment. Demographic (age, race, gender, BMI) and clinical characteristics (smoking status and duration, comorbidities, duration of COPD, treatments for COPD, maintenance medications) were collected via review of patient’s medical record or patient interview at enrollment.
Statistical Analysis
The study was powered for a 10% difference in the primary outcome based on an estimated 12-month exacerbation rate of 25% (evenly distributed among the months), alpha=0.05, and beta=0.80. The rate of suboptimal PIF in screened patients was assumed to be 20%. Based on this information, we estimated that 1650 patients would be screened to enroll 330 patients in both the suboptimal and optimal PIF cohorts for a total of 660 patients. Due to slow enrollment and the effect of the COVID-19 pandemic, the decision was made to end enrollment on March 31, 2020, before the target sample size was reached.
Descriptive statistics at enrollment, 6-months, and 12-months were reported using mean and standard deviation (SD) for continuous variables and frequency and percentages for categorical variables. Differences between cohorts at each time point were tested using independent sample t-tests for continuous variables and chi-squared tests for categorical variables. Data were collected in Microsoft Excel (Microsoft Corporation, Seattle, Washington, USA). Analyses and statistical tests were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA). Statistical significance was defined as p<0.05.
Results
Analysis Sample
A total of 474 patients with moderate to very severe COPD were screened (Figure 1). Suboptimal PIF was observed in 38.8% (184/474) of the screened patients. To achieve a similar number of patients in each cohort, 71 patients with optimal PIF were included in the prevalence calculation but were not enrolled in the study. The final study cohort included 403 enrolled patients of which 184 (45.7%) patients had suboptimal PIF and 219 (54.3%) patients had optimal PIF. At 12 months, medical record review and patient visits were conducted for 399 and 331 patients, respectively.
 |
Figure 1 Patient Flow Diagram. Abbreviations: COPD, chronic obstructive pulmonary disease; PIF, peak inspiratory flow. |
Patient Characteristics at Baseline
The mean PIF for the 403 enrolled patients was significantly lower for patients with suboptimal PIF compared to optimal PIF (46.4 ± 9.3 vs 79.4 ± 13.2 L/min; P<0.001) (Table 1). Patients in the suboptimal PIF cohort performed an average of 3.7 attempts, while those in the optimal PIF cohort performed 4.3 attempts (P<0.001). A similar percentage of patients with suboptimal and optimal PIF used a DPI at baseline (44.4 vs 44.1%).
 |
Table 1 Patient Baseline Demographics and Clinical Characteristics at Enrollment by PIF |
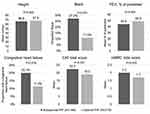
Patients with suboptimal PIF were more likely to be female (45.1 vs 34.7%; P=0.033), have shorter stature (66.6 ± 4.1 vs 67.8 ± 3.8 inches; P=0.002), and be Black (27.2 vs 11.0%; P<0.001) compared to patients with optimal PIF (Figure 2). Pulmonary function was lower for patients with suboptimal PIF compared to optimal PIF (FEV1% predicted=44.3 ± 16.0 vs 48.9 ± 16.2% predicted; P=0.004). Comorbidities were similar between patient cohorts except that congestive heart failure was more common in patients with suboptimal PIF (20.1 vs 11.4%; P=0.016). At baseline, patients with suboptimal PIF reported more shortness of breath (mMRC total score=2.0 ± 1.1 vs 1.7 ± 1.1; P=0.003) and had a greater burden of COPD (CAT total score=22.3 ± 7.7 vs 19.0 ± 7.0; P<0.001) compared to the optimal PIF cohort.
COPD Exacerbations at 12-Months Follow-Up
The proportion of patients with moderate/severe COPD exacerbations at 12-months follow-up was not statistically significantly different between the suboptimal and optimal PIF cohorts (29.3 vs 27.9%, P=0.751) (Table 2). Among patients with moderate/severe exacerbations, the average number of exacerbations was also not statistically different between the two cohorts (2.0 ± 1.6 vs 1.7 ± 1.1, P=0.361). However, the suboptimal PIF cohort had a statistically significantly shorter time to first moderate/severe COPD exacerbation compared to the optimal PIF cohort (3.8 ± 2.7 vs 4.9 ± 3.0 months, P=0.048). The mortality rate was not statistically significantly different between the suboptimal and optimal PIF cohorts (6.5 vs 2.8%, P=0.073).
 |
Table 2 COPD Exacerbations and Mortality at 6 and 12 Months |
Discussion
To our knowledge, this study is the first prospective assessment of the association between PIF status and exacerbations and mortality in outpatients with COPD. The novel findings of the study are that at baseline the suboptimal PIF cohort had a greater percentage of Blacks along with higher mMRC scores. Although there was no significant difference in the proportion of patients with moderate/severe exacerbations at 12 months between the cohorts, the suboptimal PIF cohort had a shorter time to the first COPD exacerbation compared with the optimal PIF cohort.
The results of our study confirm previous reports that the suboptimal PIF phenotype includes short stature, female sex, and reduced lung function.6,9,12–14,16–18 Additionally, our results reveal for the first time that Blacks (18% of the 403 screened subjects) are greater than twice more likely to have a suboptimal PIF compared to having an optimal value (27.2 vs 11.0%; P<0.001). Although previous studies evaluating PIF status included Blacks (13–54% of the study population), neither Sharma et al13 nor Duarte et al17 reported a relationship between race and PIF values. Samarghandi and colleagues14 studied 21 Whites and 54 Others (race not specified) and found no significant difference between race and PIF status. The greater frequency of a suboptimal PIF in Blacks observed in our study may be due, in part, to an absolute threshold for PIF (< and >60L/min) and lower overall values for lung function compared with Whites. For example, comparative studies show that Blacks have 10–15% lower spirometric values than Whites,19 and the American Thoracic Society has recommended a reduction of 12% in predicted values for disability evaluation in Blacks.20
Another novel finding was that patients who had a suboptimal PIF reported significantly more shortness of breath on the mMRC scale compared with the optimal PIF cohort. Previously, investigators consistently found numerically higher mMRC scores (ie, more shortness of breath) in the suboptimal cohorts, but the differences were not significant.12–14 These results may reflect small to modest numbers of patients (75 to 268).12–14 In contrast, our study included 403 patients at baseline, and the significant association between PIF status and mMRC scores is likely due to a larger sample size to demonstrate a small, but significant difference in breathlessness with daily activities.
PIF is determined by an individual’s inspiratory muscle strength and effort. Thus, any reason for reduced inspiratory muscle strength could explain a low or suboptimal PIF. One possibility is lung hyperinflation which shortens the vertical muscle fibers of the diaphragm and thereby adversely affects diaphragm strength. Previous studies have shown that inspiratory capacity, a marker of lung hyperinflation, is an independent predictor of PIF12,18 and is also a mechanism causing dyspnea in patients with COPD.21 Therefore, hyperinflation could contribute to both a suboptimal PIF as well as dyspnea. In this study we did not assess lung hyperinflation, and there may be other explanations for the observed difference in shortness of breath according to PIF status. One possibility is congestive heart failure which was more frequent in the suboptimal PIF cohort (20.1 vs 11.4%; P=0.016). Congestive heart failure is associated with reduced inspiratory muscle strength and can also contribute to shortness of breath.22
The results of our study showed no significant difference in the proportion of patients with moderate/severe exacerbations, the primary outcome, based on PIF status. However, the suboptimal PIF cohort had a shorter time to first exacerbation (3.8 ± 2.7 vs 4.9 ± 3.0 months, P=0.048). Both the proportion of patients with exacerbations and time to first exacerbation are commonly reported outcomes. Due to slow patient recruitment and the impact of the COVID-19 pandemic, the final number of patients recruited for the suboptimal and optimal PIF cohorts were below the sample size targets estimated by the power calculations. Overall, 28.6% of the enrolled patients experienced a moderate/severe exacerbation which approximates the estimated 25% over 12 months used for the power effect calculation.
Mortality data were collected as an exploratory outcome, and information on the specific causes of death was not recorded. It is possible that one or more differences in baseline characteristics in the suboptimal PIF cohort (shorter stature, more females, more Blacks, lower lung function, greater breathlessness, greater COPD burden, and a higher frequency of congestive heart failure) could explain the numerically but not significantly higher mortality rate compared to the optimal PIF cohort. Certainly, a history of congestive heart failure could have been an important factor as there is a high incidence of sudden cardiac death that is attributed to ventricular arrhythmias.23 In addition to lung hyperinflation and congestive heart failure, reduced inspiratory muscle strength could be a manifestation of generalized muscle weakness, a major component of frailty. Thus, suboptimal PIF may be a marker of frailty which is an independent predictor of death.24 Prospective studies are needed in a large number of patients to further evaluate the relationship between suboptimal PIF and mortality.
There are several limitations of this study. First, as previously stated, our study was underpowered as we were unable to recruit the planned number of patients in the two PIF cohorts. Therefore, we cannot exclude a type II statistical error. The COVID-19 pandemic affected patient enrollment, which was stopped after March 2020, and disrupted planned measurement of lung function and PIF at 12 months in some patients. Second, PIF was measured against a medium low resistance, so the results may not be generalizable to patients using DPIs with lower or higher resistance. Third, although research staff at each site were trained on how to use the inspiratory flow meter, differences in PIF measurement technique are possible. Fourth, COPD exacerbations that occurred outside the study clinics and affiliated hospitals where the patients typically received their medical care may have been missed. Due to the slower enrollment of the suboptimal PIF cohort, a larger proportion of the suboptimal PIF cohort follow-up occurred during the COVID-19 pandemic (after March 2020). Therefore, the suboptimal PIF cohort could have been differentially impacted by a greater number of unobserved exacerbations if patient behaviors changed (ie, use of different healthcare facilities or practitioners) during the pandemic.
Conclusion
Nearly 40% of all screened patients with stable moderate to very severe COPD had a PIF<60 L/min against a medium low resistance. A suboptimal PIF was frequent in Blacks with COPD (50 of 74 patients; 67.6%) and may be due to the known reduced lung function compared with Whites. Although the proportion of patients with COPD exacerbations was similar between cohorts, a suboptimal PIF was associated with a shorter time to first exacerbation compared with an optimal PIF. The numerically higher mortality observed in patients with a suboptimal PIF may be due to distinct baseline characteristics and/or a more frequent history of congestive heart failure. As a manifestation of inspiratory muscle weakness, suboptimal PIF could be a marker of frailty which is an independent predictor of death.
Acknowledgments
We thank the patients for their participation in the study and the principal investigators at each site (alphabetical order): J. Allen Cooper, Jr., MD of Birmingham VA Medical Center (Birmingham, AL, USA); James Gutmann, MD of Deaconess Clinic (Evansville, IN, USA); Umur Hatipoğlu, MD, MBA of Respiratory Institute at Cleveland Clinic (Cleveland, OH, USA); Laura Helman, MD of MOC Research (Mishawaka, IN, USA); Stacey L. House, MD, PhD of Washington University School of Medicine (St. Louis, MO, USA); Sanober Kable, MD of Premier Pulmonary Critical Care and Sleep Medicine (Denison, TX, USA); and Jeffery Mador, MD of VA Western NY Healthcare System (Buffalo, NY, USA). We also thank Barbara Blaylock from Blaylock Health Economics LLC for providing medical writing support.
Author Contributions
EPI-Q, an epidemiology and scientific consulting agency, managed the administration of the study, including site recruitment, site management, protocol design, data collection, and statistical analysis. KLD had full access to the data. All authors (DAM, XN, KLD, and CD) made significant contributions to the work including conception, study design, analysis, and interpretation; drafted, wrote, or substantially revised the article; agreed to the journal to which the article was submitted; reviewed and agreed to all versions of the article; and agreed to take responsibility and be accountable for the contents of the article.
Funding
This study was funded by Sunovion Pharmaceuticals Inc.
Disclosure
Donald A. Mahler has served on advisory boards for AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Mylan, Teva, Theravance, and Verona; has received royalties from Salem Media Group; has been on the speaker’s bureau for AstraZeneca, Boehringer Ingelheim, and Teva; and operates https://www.donaldmahler.com, an educational website for those with COPD and their families. Kathleen Deering is employed at EPI-Q, Inc., which received funding from Sunovion Pharmaceuticals Inc. for this analysis. Xiaoli (Charlene) Niu and Carole Dembek are full-time employees of Sunovion Pharmaceuticals Inc. The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the COPD diagnosis, management, and prevention of chronic obstructive pulmonary disease – 2020 report; 2020. https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf.
2. Hochrainer D, Hölz H, Kreher C, Scaffidi L, Spallek M, Wachtel H. Comparison of the aerosol velocity and spray duration of Respimat Soft Mist inhaler and pressurized metered dose inhalers. J Aerosol Med. 2005;18(3):273–282.
3. Laube BL, Janssens HM, de Jongh FH, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37(6):1308–1331.
4. Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. Lancet. 2011;377(9770):1032–1045.
5. Broeders ME, Sanchis J, Levy ML, Crompton GK, Dekhuijzen PN. The ADMIT series–issues in inhalation therapy. 2. Improving technique and clinical effectiveness. Prim Care Respir J. 2009;18(2):76–82.
6. Mahler DA. The role of inspiratory flow in selection and use of inhaled therapy for patients with chronic obstructive pulmonary disease. Respir Med. 2020;161:105857.
7. Al-Showair RA, Tarsin WY, Assi KH, Pearson SB, Chrystyn H. Can all patients with COPD use the correct inhalation flow with all inhalers and does training help? Respir Med. 2007;101(11):2395–2401.
8. Yokoyama H, Yamamura Y, Abe T, et al. Relationship between amount of drug delivered to lungs and amount released from Diskhaler by inhalation with tapping. Biol Pharm Bull. 2007;30(6):1167–1170.
9. Ghosh S, Ohar JA, Drummond MB. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30(6):381–387.
10. Mahler DA, Waterman LA, Ward J, Gifford AH. Comparison of dry powder versus nebulized beta-agonist in patients with COPD who have suboptimal peak inspiratory flow rate. J Aerosol Med Pulm Drug Deliv. 2014;27(2):103–109.
11. Mahler DA, Ohar JA, Barnes CN, Moran EJ, Pendyala S, Crater GD. Nebulized versus dry powder long-acting muscarinic antagonist bronchodilators in patients with COPD and suboptimal peak inspiratory flow rate. Chronic Obstr Pulm Dis. 2019;6(4):321–331.
12. Loh CH, Peters SP, Lovings TM, Ohar JA. Suboptimal inspiratory flow rates are associated with chronic obstructive pulmonary disease and all-cause readmissions. Ann Am Thorac Soc. 2017;14(8):1305–1311.
13. Sharma G, Mahler DA, Mayorga VM, Deering KL, Harshaw O, Ganapathy V. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4(3):217–224.
14. Samarghandi A, Ioachimescu OC, Qayyum R. Association between peak inspiratory flow rate and hand grip muscle strength in hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease. PLoS One. 2020;15(1):e0227737.
15. Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160.
16. Ghosh S, Pleasants RA, Ohar JA, Donohue JF, Drummond MB. Prevalence and factors associated with suboptimal peak inspiratory flow rates in COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:585–595.
17. Duarte AG, Tung L, Zhang W, Hsu ES, Kuo YF, Sharma G. Spirometry measurement of peak inspiratory flow identifies suboptimal use of dry powder inhalers in ambulatory patients with COPD. Chronic Obstr Pulm Dis. 2019;6(3):246–255.
18. Mahler DA, Waterman LA, Gifford AH. Prevalence and COPD phenotype for a suboptimal peak inspiratory flow rate against the simulated resistance of the Diskus® dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2013;26(3):174–179.
19. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187.
20. American Thoracic Society. Evaluation of impairment/disability secondary to respiratory disorders. Am Rev Respir Dis. 1986;133(6):1205–1209.
21. O’Donnell DE, Laveneziana P. Physiology and consequences of lung hyperinflation in COPD. Eur Respir Rev. 2006;15(100):61–67.
22. McParland C, Krishnan B, Wang Y, Gallagher CG. Inspiratory muscle weakness and dyspnea in chronic heart failure. Am Rev Respir Dis. 1992;146(2):467–472.
23. Packer M. What causes sudden death in patients with chronic heart failure and a reduced ejection fraction? Eur Heart J. 2020;41(18):1757–1763.
24. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365–1375.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.