Back to Journals » Journal of Blood Medicine » Volume 11
Proportion of Acute Transfusion Reaction and Associated Factors Among Adult Transfused Patients at Felege Hiwot Compressive Referral Hospital, Bahir Dar, Northwest Ethiopia: A Cross-Sectional Study
Authors Gelaw Y , Woldu B, Melku M
Received 20 February 2020
Accepted for publication 17 June 2020
Published 30 June 2020 Volume 2020:11 Pages 227—236
DOI https://doi.org/10.2147/JBM.S250653
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Yemataw Gelaw,1,2 Berhanu Woldu,2 Mulugeta Melku2
1Amhara Regional Health Bureau, Bahir Dar, Ethiopia; 2Department of Hematology & Immunohematology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Yemataw Gelaw
Amhara Regional Health Bureau, Bahir Dar, Ethiopia
Email [email protected]
Introduction: Acute transfusion reactions are adverse events occurring within 24 hrs of transfusion and cause simple-to-severe complications. They may vary with the blood component transfused and recipient factors. In Ethiopia, there is a limited evidence about the incidence and associated factors of transfusion reactions.
Objective: To determine the proportion of acute transfusion reactions and associated factors among adult transfused patients.
Methods: A total of 384 study participants were included in the study. Structured questionnaires were used for socio-demographic and past medical history data collection. Vital signs were measured as a baseline for every study participants and monitored and followed for 24 hrs. Laboratory tests like complete blood count, direct anti-human globulin test and urine hemoglobin were done as a baseline for suspected patients. Cross-match, blood grouping, and blood culture for patients and donors sample were also done for suspected patients. Descriptive statistics, bivariable and multivariable binary logistic regression were analyzed using SPSS version 20. P-value of < 0.05 in the multivariable model was considered as statistically significant.
Results: Acute transfusion reactions were observed in 5.2% of patients. Of total cases of acute transfusion reaction, the majority developed allergic reactions (65%) and febrile non-hemolytic transfusion reaction (30%). It was significantly associated with transfusion history (AOR=3.4; 95% CI: 1.2– 9.7), abortion history (AOR=5.0; 95% CI: 1.5– 16.4), longer blood storage time (AOR=5.1; 95% CI: 1.7– 15.2) and receiving three or more unit of blood (AOR= 4.1; 95% CI: 1.5– 11.2).
Conclusion: Acute transfusion reactions were observed in 5.2% of patients (allergic reactions (65%), febrile non-hemolytic transfusion reaction (30%) and alloimmunization (5%)). Patients with a history of transfusion, abortion, transfused with blood stored ≥ 14 days and multi-transfused patients should be closely monitored.
Keywords: acute transfusion reaction, adverse event, blood transfusion, transfusion reaction, Ethiopia
Introduction
Blood component transfusion is an essential part of modern health care which can save lives and improve health.1,2 The main goal of blood transfusion is to treat chronic anemia and ineffective erythropoietin,3,4 coagulopathy, life-threatening bleeding diathesis. It is also used for the treatment of von Willebrand’s disease, Hemophilia A, Factor XIII deficiency and hypofibrinogenemia, especially when recombinant products are not available.5 Blood transfusions are also used for supportive care in cardiovascular and transplant surgery, massive trauma, and therapy for solid and hematological malignancies and treating pregnancy-related complications.6
The collection of blood only from low-risk voluntary non-remunerated donors7 and good laboratory practice1 minimizes the risk of transfusion reactions. However, each blood product carries a risk of transfusion reactions.8 A transfusion reaction is any undesirable effect occurring in a patient during or after blood transfusion.9,10 These reactions may happen as an immune response to the blood cell antigens or as a non-immune response caused by a circulation overload, transfusion siderosis, or transmission of infections.11
The type and severity of transfusion reactions vary with the transfused blood product, the clinical condition of the recipient, past medical history and age of the recipient.12,14 They are 1000 times more likely to occur than blood transfusion infection complications.15 A national blood collection and utilization survey in United State estimated that there are more than 60,000 transfusion reactions annually in 2009, of which 16,000 were serious reactions.16 These adverse effects can cause mortality and morbidity which has a social and economic impact on the patient and the public.9
Transfusion reactions are classified as acute and late transfusion reactions based on time of onset.8 Acute transfusion reactions (ATRs) have been found to occur during or within 24 hrs of transfusion and include acute hemolytic transfusion reaction (AHTR), allergic reactions, febrile non-hemolytic transfusion reaction (FNHTR), transfusion-associated circulatory overload (TACO), transfusion-related acute lung injury (TRALI) and anaphylactic.15 These may be simple like FNHTR or life-threatening complications such as transfusion-related acute lung injury (TRALI) and AHTR,17 blood sepsis (BS), which may be associated with death.10 The incidence may occur in 1–2% of transfused patients.18
Previously transfused Patients and multi-parous women are at risk of ATRs. Multi-transfused patients are at high risk for febrile reactions while elderly and patients with cardiovascular disease are at high risk for volume overload.19 In low- and middle-income countries, one of the most frequently transfused patient groups are females aged between 15 and 45 years.20 ATR is more common in females and patients who have been transfused whole blood. Around 68.8% of the ATR is occurred due to whole blood transfusion.21 In Ethiopia the most commonly transfused blood component is whole blood (85% of patients were transfused whole blood component). Furthermore, more than half of the patients transfused were females and multi-transfusion is common.2
Ethiopia established the National Blood Bank Service in 1969. In 2013, the blood bank established hemovigilance systems and signed a memorandum of understanding with all of the hospitals who received blood from the National Blood Bank and its networks. Hospital transfusion committees were also established for patient hemovigilance and reporting of transfusion reactions with its type and integrated into the national health management information system. However, the hemovigilance system is still poor in the regional blood banks because of non-functional hospital transfusion committee and gaps in reporting adverse reactions.22
Ethiopia also adopted the World Health Organization transfusion guideline and developed National guidelines for appropriate clinical use of blood. According to the guideline, the right blood component should be given to the right patient by checking its compatibility with complete cross-match including antibody screening of the recipient serum.23 However, practically all cross- matches are performed by immediate spine technique. This may miss some unexpected incomplete antibodies in the patient’s serum, which may cause ATR. Moreover, to the best of our knowledge, there is no research done about the proportion and associated factors of ATRs, not well investigated and reported in Ethiopia. Therefore, the main objective of the current study was to determine the proportion and associated factors of ATRs among adult transfused patients which are important to reduce transfusion reactions and its complications. Besides, the study can also be used as a baseline data for further studies.
Materials and Methods
Study Setting and Population
An institutional-based cross-sectional study was conducted at Felege Hiwot Comprehensive Referral Hospital from February to April 2019. The hospital is one of the biggest tertiary hospitals in Amhara region, Ethiopia, which provides health services for over 7 million people from the surrounding area and acts as the referral centre for other district hospitals. It provides obstetrics, pediatrics, internal medicine, ophthalmology, general, gynecology, oncology, otolaryngology (Ear, Nose, and Throat specialize department) and orthopedic surgical services. It has about 464 beds, and 25,962 patients are admitted annually and 12.3% of them need a blood transfusion. All adult patients receiving blood transfusion service and voluntary to participate were included in the study. Unconscious and critically ill transfused patient, those requiring blood transfusion intra-operatively, less than 24-hrs post-operatively transfused patients and patients undergoing hemodialysis were excluded from the study.
Sample Size Determination and Sampling Technique
All adult blood transfused patients during the study period who were voluntary to participate were included in the study. The study period was chosen by authors by assuming that ATR did not be affected by seasonally.
Data Collection Materials and Procedures
Socio-Demographic and Clinical Data Collection Procedures
Relevant socio-demographic data (gender, age, residence, and current marital status) and past medical history including transfusion history, previous pregnancy history and abortion history were collected with structured questionnaires. A general physical and clinical examination was also carried out on each of them before commencing the transfusion. Vital signs like body temperature, blood pressure, pulse rate, respiratory rate, and oxygen saturation percentage were measured as baseline data and recorded on data collection checklist. During the transfusion, these vital signs were monitored within 15 mins after the commencement of the transfusion, then subsequently half an hour to the end of the transfusion and 4-hrs interval after the end of transfusion for 24 hrs. At the same time, patients were examined for features of ATR, which included signs of fever, chills/rigours, itching, urticaria, nausea, tachycardia, restlessness, vomiting, dyspnea, anxiety and headache.
Laboratory Examinations
Data on the date of blood donation, blood group of the donor, blood group of the recipient, and number of units transfused and type of blood component was recorded on data collection checklist from transfusion service centre logbook and patient chart. Blood and urine samples were collected from each patient before and after transfusion for investigation of ATRs in suspected cases.
Pre-transfusion complete blood count (CBC), direct antiglobulin test (DAT) results were collected either from patient medical record or requested for those who did not have on their medical record. Most of the study participants had pre-transfusion CBC result from their chart and only a few had no CBC result and requested by the investigator. None of the patients were having DAT result on their chart. The baseline CBC and DAT were performed from a specimen collected for cross-match for those who did not have on their chart. The complete blood count was analyzed by Sysmex-500i five differential hematological analyzer (Sysmex Corporation Kobe, Japan) machines at FHCRH hematology laboratory before cross-match was done. All the pre-transfusion cross-matches were performed by immediate-spin techniques. However, for those patients suspected for transfusion reaction, the pre-transfusion samples were re-cross-matched with polyclonal antihuman-globulin (AGH) technique parallel with post-transfusion samples. DAT was tested by mixing post-transfusion patient’s washed RBCs with polyclonal AGH sera (both anti-IgG and anti-complement) (Spinreact, Spain) for the detection of in vivo sensitization of patient RBCs. Five mL urine specimen was also collected with clean urine cup container for Pre-transfusion urine hemoglobin (Hb) determination with urine strip test.
Patients with suspected ATRs either clinically or from the screening of urine Hb, first the blood pack labels or the patient’s identity was checked and the pre-transfusion sample of suspected patients was retested for their ABO and Rh blood type. Eight mL of post-transfusion blood specimen was collected from the suspected patients and 5mL of blood was added to a special blood culture tube for blood culture. The remaining 3 mL of blood was added to tri-potassium ethylenediaminetetraacetic acid (K3-EDTA) test tube and it was used for CBC, malaria parasite, regrouping, re-cross match and screening for in vivo sensitization of RBCs (DAT). The collected blood sample was checked for proper labelling, hemolytic, clotting pre analytically. Five mL urine specimen was also collected with clean urine cup container for post-transfusion urine Hb level determination. The donor blood sample was also sent for blood culture, regrouping, cross-match.
All blood culture bottle manipulations were carried out in the Class II biosafety cabinet at FHCRH Medical Microbiology Laboratory. The blood culture was incubated at 35–37°c on enrich media (Trypticase Soya Agar) for 24 hrs and examined for the presence of bacterial growth like turbidity and hemolysis. At the end of 24 hrs, the result was inoculated into blood agar, chocolate agar and MacKonkey agar and the result was reported as negative if there is no growth at the end of 5 days.
Data Analysis
The data were checked for completeness, cleaned, sorted, and categorized daily and entered into SPSS version 20 for analysis. Descriptive statistics were used to summarize the socio-demographic and clinical characteristics of study participants and presented by tables and texts. Bivariable and multivariable binary logistic regression analyses were performed to determine the association between the dependent and independent variables. The multivariable binary logistic regression model was analyzed with backward likelihood and stepwise method for variables with a P-value of < 0.25 in the bivariable binary logistic regression. The model fitness of the final multivariable logistic regression was checked using Hosmer and Lemeshow test. A p-value < 0.05 in the multivariable regression model was considered as statistically significant.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Research and Ethical Review Committee of School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar. Permission letter was also obtained from the hospital. To ensure confidentiality of the study participant’s information, anonymous typing was applied so that the name of the participant and any identifier of participants were not written on the questionnaire. Written informed consent was also obtained from each participant.
Result
Socio-Demographic Characteristics of Study Participants
A total of 384 participants were included in the study. More than half, 233 (60.7%), of them were female. Two hundred fifty-eight (67.2%) of the study participants were rural residents. Most of the study participants, 293 (76.3%), were married. The median age of the transfused patients was 31 (Interquartile range 15years) (Table 1).
 |
Table 1 Socio-Demographic Characteristics of Transfused Participants at FHCRH from February to April 2019 (N =384) |
Clinical Characteristics of Study Participants
Of the study participants, 163 (42.4%) had previous transfusion history of which 85 (22.1%) had transfusion history episode of one, 48 (12.5%) were had transfusion history episode of two and the remaining 30 (7.8%) had transfusion history episode of three and more. Among 233 female study participants, 197 (84.55%) were had pregnancy history and 52 (22.3%) of them were had previous abortion history. The most transfusion indicators were maternal complications (35.4%) followed by hematological cases (16.9%) (Anemia, without a diagnosis of the underline cause, hematological malignancy and idiopathic thrombocytopenia purpura) (Table 2).
 |
Table 2 Clinical Characteristics of Transfused Patients at FHCRH February to April 2019 |
Type and Characteristics of Transfused Blood and Frequency of Transfused Patients
Total of 638 units of blood components were transfused to 384 patients with a mean number of units transfused 1.66 (1.47 for male vs 1.79 for female). Most patients were transfused whole blood (non-leukocyte reduced) 364 (94.8%), followed by concentrated RBC (CRC) (non-leukocyte reduced) 8(2.1%), platelet (prepared from whole blood) 5(1.3%) and mixed 7(1.8%). The median storage time of the transfused whole blood and CRC was 11days whereas; the transfused platelet was 2 days. One hundred fifty-six (40.6%) patients were transfused with long-stored blood (storage time ≥14 days). Most patients were transfused a single unit blood/blood component (59.1%) (Table 3).
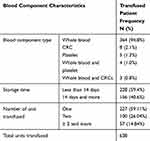 |
Table 3 Type and Characteristics of Transfused Blood Component with Frequency of Transfused Patients at FHCRH, February to April 2019 (N =384) |
Transfusion Reactions and Associated Factors
There were 20 ATRs cases which account for a total of 5.2% ATR proportion. Most of the reactions were allergic 13 (65%) followed by FNHTR 6 (30%) (Table 4). The proportion of ATR was higher in females than males (6% vs 4%). It was also higher in, patients who had transfusion history (8.6% vs 2.7%), patients who had pregnancy history (6.6% vs 3.7%), patients who had abortion history (13.5% vs 3.9%) and patients who transfused more than 2 units (14% vs 3.7%). Seventy-five percent (15/20) of the ATRs were observed in patients who transfused with long-stored blood. Ninety percent of the ATR was observed in patient transfused whole blood component. Only 10% of ATR has occurred in patients transfused with other blood component (1 in platelet-transfused patient and 1 in mixed transfused patient, data not showed) (Table 5). One possible transfusion-related mortality was observed in patients with allergic reactions (data not showed).
 |
Table 4 Reaction Incidence and Type Among Adult Transfused Patients at FHCRH, February to April 2019 (N =384) |
 |
Table 5 Bivariable and Multivariable Binary Logistic Regression Analysis of Associated Factors of ATRs Among Adult Transfused Patients at FHCRH, February to April 2019 |
In the bivariable binary logistic regression analysis, ATR was associated with transfusion history (COR = 3.4; 95% CI: 1.3–9.0), abortion history (COR =3.8; 95% CI: 1.4 −10.0), storage time (COR = 4.7; 95% CI: 1.7–13.3) and transfusion of 3 or more of units of blood/blood components (COR = 4.3; 95% CI: 1.7 −11) (Table 5). In multivariable model controlling the confounding factor, transfusion history (AOR = 3.4; 95% CI: 1.2 −9.7), abortion history (AOR = 5.0; 95% CI: 1.5–16.4), storage time of transfused blood for more than 13 day (AOR= 5.1; 95% CI: 1.7 −15.2) and number of transfused units (≥ 3 units of blood/blood component) (AOR = 4.1; 95% CI: 1.5–11.2) were significantly associated with development of ATR (Table 5).
Discussion
A blood transfusion reaction is an undesirable response in a patient, temporally associated with blood component transfusion. Nowadays, the greatest risk to the patient lies in non-infectious complications of transfusions that account for significant morbidity and mortality. Acute blood transfusion reactions are responsible for most serious adverse events and its incidence varies widely in the world.13
In the current study, the total proportion of ATR was found to be 5.2% of transfused patients (95% CI, 3–7.4%). The find was consistent with studies conducted in Japan (5.05%),12 India (3.4%),24 Belgaum (4.41%),25 and Nigeria (3.6%).26 However, this finding was lower than a study conducted in different areas, namely, USA (16.5%)27 and Nigeria (26.3%).28 The observed difference might be due to a difference in study participants. In the USA, the study was conducted on elderly patients with a mean age of 82 ± 9 which may be the cause of the higher incidence, but our study participant had median age 31 years old and reported with a few elder age groups (16, 4.2%) whereas, in Nigeria, all participants were pregnant women (86% of the subjects were multigravid). Moreover, most of the transfusions administered were women who had an incomplete abortion and placenta previa with fetal-maternal hemorrhage which might be associated with ATR.28 Multigravid women may produce alloantibodies to leukocyte, or platelet antigens as a result of an overt fetal-maternal hemorrhage. Hence, women who developed leukocyte antibodies following pregnancy or abortion are more likely to have allergic and FNHTR if subsequently transfused with leukocyte-containing blood components.29
On the other hand, the current study had a higher incidence than a study conducted in Japan (2.6%).30 The difference might be due to the difference in the transfused blood component. The transfused blood component in Japan was leukocyte-reduced blood component while in the current study it was non-leukocyte reduced blood component. Non-hemolytic febrile transfusion reactions are usually caused by cytokines released from leukocytes in transfused RBCs or platelet components, and causing fever, chills, or rigours,31 and secreted interleukin-8 which is a chemotactic cytokine for Neutrophil and Eosinophil.32 In non-leukocyte-reduced blood, cytokines are highly elevated in stored blood component and they induce leucocyte recruitment and cause an allergic reaction, when such a blood component is transfused.33
In the current study, allergic (65%) and FNHTR (30%) were the most frequently observed ATRs. This was similar with studies conducted in India (55.1% vs 35.7%),34 USA (45.5% vs 19.5%),14 Japan (70% vs.13.1%)30 and Malaysia (50.2% vs 38%)35 which revealed that these ATRs were the most frequently observed transfusion reactions. On the other hand, studies conducted in Saudi Arabia,36 Nigeria37 and Zimbabwe21 were vice versa with our study in which FNHTR was more frequently observed reaction in these studies.
In this study, a single case of possible transfusion-related mortality was observed due to anaphylactic shock. An anaphylactic reaction is a severe form of allergic reaction and Mukherjee S et al reported that IgA deficiency was the most cause of anaphylactic reactions.38 In addition, the recipient pre-formed antibodies to transfused haptoglobin,39 and cytokines in stored products can also cause an anaphylactic reaction.40 However, in the current study, the possible causes of death were not identified (of course there was no pre-transfused blood incompatibility, no BS in a donor blood sample and DAT was negative), but the patient showed signs of allergic reaction like itching, urticaria flushing and difficult to breath (dyspnea) and died within minutes of transfusion.
Alloantibody incompatibility other than ABO incompatibility was observed in one patient (0.3% of study participants or 5% of ATRs). In line with the importance of minor RBC antigen to the occurrence of other alloantibody, Reyhaneh K et al reported that alloimmunization (alloantibody detection) was 1% in Iran population and was more frequently observed in patients with pre-transfusion history, pregnancy history and abortion history41 which was similar to the patient history in this study. Dajak S et al reported 0.2% non-Rh RBC antibodies in pregnant women and it was also associated with transfusion history.42 Alloimmunization was also reported in India and it was 0.005% of the total incidence of 3.4%.24
Blood sepsis, TRALI, AHTR, and TACO were not observed in our study. This might be due to low sample size compared to studies conducted in different countries. Indeed, ABO mismatch and BS were not reported in India from 30,470 unit of transfusion.8 A single case of TRALI, two cases of TACO and 5 cases of AHTR were reported by Mafirakureva et al from 670,625 units of transfusion.21 Therefore, it was not surprising that the current study reported no cases of BS, TRALI, AHTR, and TACO from 638 units of transfusion.
Patients with transfusion history were 3.4 times more likely to be at risk for ATR. The possible reason might be due to the fact that patients with pre-transfusion history were exposed to different antigens like leukocyte antigen, RBCs alloantigen, platelet antigens, and plasma proteins and sensitized the antibody production against these antigens. When patient transfused blood or blood component for the second time, immune-mediated transfusion reactions are typically occurred due to incompatibility of the transfused blood or blood product and the recipient (antibodies made in response to foreign antigens or alloantibodies). These alloantibodies account for many reactions including mild allergic, FNHTR and anaphylactic reactions.43 Studies conducted in the USA,44 India8 and Nigeria26,28,37 were reported similar findings.
Abortion was also statistically associated with ATR. Patients with abortion history were 5.0 times more likely to develop ATR than those without abortion history. This might be due to women with abortion history that may form alloantibody to leukocyte, red cells or platelet antigens as a result of an overt or in apparent fetal-maternal hemorrhage. These antibodies produced following pregnancy or abortion are more likely to cause blood transfusion reaction like allergic and FNHTR when the patients are subsequently transfused with leukocyte-containing blood components.45 Ibrahim N et al reported that multi gravidity was a risk factor for transfusion reaction as against prim gravidity, presumably, due to sensitization and subsequent formation of antibiotics resulting from feta maternal transfusion that may occur in previous pregnancies that might have abortion history.28
There was an association between the storage time of blood component and ATR. Acute transfusion reaction was 5.1 times more likely to occur among patients transfused with long-stored blood (stored ≥14 days) than patients transfused with short-stored blood. Two studies conducted in Nigeria by Ibrahim et al28 and Gwaram et al26 have reported a similar finding. Ended the former was a comparison between stored blood and none stored blood (fresh). The possible reason might be due to leukocyte bio-chemicals increased with long-stored blood as reported by Chang C-C et al According to Chang C-C et al study, there was a statistically significant difference of leukocyte biochemicals between pre-storage leukocyte-reduced and post-storage leukocyte-reduced blood component. IL-1β and IL-8 levels were significantly elevated in the post-storage leukocyte-reduced blood component. These leukocyte bio-activators were associated with ATR, especially in FNHTR and allergic reaction.33 In our study, the transfused blood components were non-leukocyte-reduced blood component and the most frequented observed ATRs were allergic and FNHTR. So, these biochemicals might be elevated in the long-stored blood component and might cause higher ATR incidence in patients transfused with long-stored blood component. Indeed, there was a contrary report by Heddle NM et al, there no statistically significant effect on the patient outcome between short-stored and long-stored blood component. Of course, this study was the mortality of patient after transfusion, not the transfusion reaction.46 Therefore, long-stored blood might not be significantly associated with death but might be associated with transfusion reaction.
Numbers of units transfused per patient were statically significant to the occurrence of ATR and was reported by Menis et al44 and Arewa et al37 which was similar to current finding. One unit increment in transfusion for the patient transfused more than 2 units increased the likelihood of ATR by 4.1 times. Patients experiencing allergic transfusion reactions have been sensitized to the antigens in the donor unit. These antigens are soluble, and the associated reaction is dose-dependent. The fever is caused by leukocytes and cytokines in the donor blood. As a result, the more units transfused the more leukocytes and cytokine are induced to the recipient. Hence, FNHTR occurs more often in patients who have been transfused repeatedly. The antibodies to the antigens of the ABO blood group or alloantibodies to other RBC antigens are produced after sensitization through a previous transfusion or pregnancy. In patient transfused repeatedly, sensitization increases because of exposures to different antigens.15
Limitation of the Study
In a given study, the sample size was small compared to most studies conducted in other countries. The low sample size causes inadequate elder patients/participants proportionally, which might have an effect on some ATRs like TRALI and TACO. In addition to sample size, the small outcome of interest (ATR) might have an effect to predict the association between ATRs and explanatory variables. Besides, antibody screening and identification were not also performed for non-ABO alloantibody. We did not also analyze the association between the explanatory variables and types of ATR, because the number of participants who developed the outcome of interest, ATR, are small (n=20) for some variables.
Conclusion and Recommendation
To the best of our best knowledge, this study was the first study conducted in Ethiopia. The total proportion of ATR was 5.2%. The allergic reaction was the commonest reaction observed with a proportion of 65% while FNHTR presented with a proportion of 30%. The rest 5% was alloantibody other than ABO incompatibility. A statistically significant relationship was observed between ATRs and previous transfusion history, abortion history, storage time of blood component and a number of units transfused. Patients with the previous history of transfusion and history of abortion should be closely monitored because they are at risk for ATR. It is also recommended that patients’ transfused blood component stored ≥ 14 days, and patients’ transfused more than two units are also at high risk for transfusion reaction and should be closely monitored. None of the ATRs were reported to blood transfusion service centre. Therefore, establishing a hemovigilance system of monitoring, collecting, and analyzing data on the ATR both locally and nationally should be strengthened. In a given study, the sample size was small compared to most studies conducted in other countries. The low sample size also causes inadequate elder patients/participants proportionally, which have an effect on some type of ATRs. The association between the explanatory variables and types of ATR were not analyzed, because the number of participants who develop the outcome of interest are small (n=20). Therefore, we recommended researchers to do further studies with large sample size, which can solve these limitations.
Abbreviations
AGH, antihuman globulin; AHTR, acute hemolytic transfusion reaction; AOR, adjusted odd ratio; ATR, acute transfusion reaction; BS, blood sepsis; CBC, complete blood count; COR, crude odd ratio; DAT, direct human anti-globulin test; FHCRH, Felege Hiwot Comprehensive Referral Hospital; FNHTR, febrile non-hemolytic transfusion reaction; Hb, hemoglobin; RBC, red blood cell; TACO, transfusion-associated cardiac overload; TRALI, transfusion-related acute lung injury.
Data Sharing Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Acknowledgments
We like to put our great full appreciation to the study participants and organizations: Amhara Regional State Health Bureau, Bahir Dar Blood Bank Service, Amhara Public Health Institute, Felege Hiwot Comprehensive Referral Hospital.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors have declared that no competing interests exist.
References
1. WHO. The clinical use of blood in medicine, obstetrics, paediatrics, surgery and anaesthesia, trauma and burns. World Health Organization; 2001. Available from: http://apps.who.int/iris/bitstream/handle/10665/42397/a72894.pdf?sequence=1&isAllowed=y.
2. Tamene M, Tsegaye A, Birhanu A, Taye B, Birhaneselassie M. Assessment of transfusion utilization and patient outcomes at the largest referral and university hospital in Addis Ababa, Ethiopia. ISBT Sci Ser. 2016;11(1):7–13.
3. Bayanzay K, Alzoebie L. Reducing the iron burden and improving survival in transfusion-dependent thalassemia patients: current perspectives. J Blood Med. 2016;7:159–169. doi:10.2147/JBM.S61540
4. Qari MH, Wali Y, Albagshi MH, et al. Regional consensus opinion for the management of beta thalassemia major in the Arabian Gulf area. Orphanet J Rare Dis. 2013;8(1):1–12. doi:10.1186/1750-1172-8-143
5. Man J. A comparison of blood transfusion practice guidelines: what quality of evidence is being utilized to develop transfusion guideline recommendations? Yale Med Thesis Digital Lib. 2011;1574:1–64.
6. Belayneh T, Messele G, Abdissa Z, Tegene B. Blood requisition and utilization practice in surgical patients at University of Gondar Hospital, northwest Ethiopia. J Blood Transfus. 2013;2013:1–5. doi:10.1155/2013/758910
7. Taye Makuria A, Gebremichael D, Demoz H, et al. Obstetric hemorrhage and safe blood for transfusion in Ethiopia: the challenges of bridging the gap. Transfusion. 2017;57(10):2526–2531. doi:10.1111/trf.14219
8. Chakravarty-Vartak U, Shewale R, Vartak S, Faizal F, Majethia N. Adverse reactions of blood transfusion: a study in a tertiary care Hospital. Int J Sci Study. 2016;4(2):90–94.
9. Ribed-Sánchez B, González-Gaya C, Varea-Díaz S, Corbacho-Fabregat C, Bule-Farto I, de-Oteyza JP. Analysis of economic and social costs of adverse events associated with blood transfusions in Spain. Gac Sanit. 2018;32(3):269–274. doi:10.1016/j.gaceta.2017.10.021
10. Pedrosa AK, Pinto FJ, Lins LD, Deus GM. Blood transfusion reactions in children: associated factors. J Pediatr (Rio J). 2013;89(4):400–406. doi:10.1016/j.jped.2012.12.009
11. Apriastini NKT, Ariawati K. Risk factors of acute blood transfusion reactions in pediatric patients in Sanglah General Hospital, Bali-Indonesia. transfusion. 2017;3(5):534–538.
12. Kato H, Uruma M, Okuyama Y, et al. Incidence of transfusion-related adverse reactions per patient reflects the potential risk of transfusion therapy in Japan. Am J Clin Pathol. 2013;140(2):219–224. doi:10.1309/AJCP6SBPOX0UWHEK
13. Sahu S, Hemlata AV. Adverse events related to blood transfusion. Indian J Anaesth. 2014;58(5):543. doi:10.4103/0019-5049.144650
14. Oakley FD, Woods M, Arnold S, Young PP. Transfusion reactions in pediatric compared with adult patients: a look at rate, reaction type, and associated products. Transfusion. 2015;55(3):563–570. doi:10.1111/trf.12827
15. Sharma S, Sharma P, Tyler LN. Transfusion of blood and blood products: indications and complications. Am Fam Physician. 2011;83(6):719–724.
16. WHO, Global consultation on haemovigilance. Jointly organized by WHO HQ/Geneva, Sharjah Blood Transfusion and Research Center, the Government of the United Arab Emirates, in collaboration with International Haemovigilance network, International Society of Blood Transfusion. 20–22 November 2012, Dubai, United Arab Emirates. Geneva: WHO; 2013.[
17. Arewa OP. Evaluation of transfusion pyrexia: a review of differential diagnosis and management. ISRN Hematol. 2012;2012:1–7.
18. Organization WH. Clinical transfusion practice: guidelines for medical interns. Clin Transfus Pract Guidel Med Interns. 2012:1–42.
19. Australian Red Cross Blood Service. Factors contributing to transfusion-related adverse events. Australian Red Cross Blood Service; 2018. Available from: https://transfusion.com.au/adverse_events/risks.
20. WHO, Global status report on blood safety and availability. World Health Organization; 2016. Available from: http://apps.who.int/iris/bitstream/handle/10665/254987/9789241565431-eng.pdf?sequence=1.
21. Mafirakureva N, Khoza S, Mvere DA, Chitiyo ME, Postma MJ, van Hulst M. Incidence and pattern of 12 years of reported transfusion adverse events in Zimbabwe: a retrospective analysis. Blood Transfus. 2014;12(3):362–367. doi:10.2450/2014.0156-13
22. Belay A. 17th International haemovigilance seminar. 9–11 march, Paris – France. National Blood Bank Service of Ethiopia; 2016. Available from: http://ihs-seminar.org/content/uploads/Abiyi-Belay-Ambaye-Ethiopia.pdf.
23. MOH. National Guidelines for Appropriate Clinical Use of Blood. Addis Ababa, Ethiopia: Ministery Of Health; 2015.
24. Goyal M, Bindal J, Jain B. Prevalence of adverse events related to blood transfusion at tertiary care center of central India. IOSR J Dent Med Sci. 2017;16(10):21–28.
25. Madhushree D, Metgud MC, Patil K. Retrospective analysis of all patients undergoing blood transfusion in obstetrics at a Tertiary Care Hospital, Belgaum: a cross-sectional study. Indian J Health Sci Biomed Res. 2018;11(2):116–120. doi:10.4103/kleuhsj.kleuhsj_233_17
26. Gwaram BA, Borodo MM, Dutse AI, Kuliya-Gwarzo A. Pattern of acute blood transfusion reactions in Kano, North-Western Nigeria. Nig J Basic Clin Sci. 2012;9(1):27–32. doi:10.4103/0331-8540.102110
27. Lubart E, Segal R, Tryhub N, Sigler E, Leibovitz A. Blood transfusion reactions in elderly patients hospitalized in a multilevel geriatric hospital. J Aging Res. 2014;2014:1–3. doi:10.1155/2014/178298
28. Ibrahim N, Garba N, Tilde I. Acute blood transfusion reactions in pregnancy, an observational study from North Eastern Nigeria. J Blood Disord Transfusion. 2013;4(3):145.
29. Eder A, Dy B, Herron R, Strupp A, Dodd R, Benjamin R. Effective reduction of TRALI risk with plasma collected predominantly from male donors. Transfusion. 2010;50(3):1732–1742. doi:10.1111/j.1537-2995.2010.02652.x
30. Hatayama Y, Matsumoto S, Hamada E, et al. Analysis of acute transfusion reactions and their occurrence times. Yonago Acta Med. 2018;61(1):87–90. doi:10.33160/yam.2018.03.013
31. Sandler SG, Sandler D. Transfusion reactions: medscape reference; 2004. [
32. Gosset P, Tillie‐Leblond I, Malaquin F, Durieu J, Wallaert B, TONNEL AB. Interleukin‐8 secretion in patients with allergic rhinitis after an allergen challenge: interleukin‐8 is not the main chemotactic factor present in nasal lavages. Clin Exp Allergy. 1997;27(4):379–388. doi:10.1111/j.1365-2222.1997.tb00722.x
33. Chang -C-C, Lee T-C, Su M-J, et al. Transfusion-associated adverse reactions (TAARs) and cytokine accumulations in the stored blood components: the impact of prestorage versus poststorage leukoreduction. Oncotarget. 2018;9(4):4385–4394. doi:10.18632/oncotarget.23136
34. Kumar P, Thapliyal R, Coshic P, Chatterjee K. Retrospective evaluation of adverse transfusion reactions following blood product transfusion from a tertiary care hospital: a preliminary step towards hemovigilance. Asian J Transfus Sci. 2013;7(2):109–115. doi:10.4103/0973-6247.115564
35. Haslina M, Fakhri M, Saw T, Salamah A. An audit on acute transfusion reaction in North Eastern Malaysia. Scholar J Med. 2012;2(5):60–62.
36. Khalid S, Usman M, Khurshid M. Acute transfusion reactions encountered in patients at a tertiary care center. J Pak Med Assoc. 2010;60(10):832–836.
37. Arewa O, Akinola N, Salawu L. Blood transfusion reactions; evaluation of 462 transfusions at a tertiary hospital in Nigeria. Afr J Med Med Sci. 2009;38(2):143–148.
38. Mukherjee S, Bhattacharya P. Severe anaphylactic reaction in IgA deficient patient following transfusion of whole blood. Asian J Transfus Sci. 2011;5(2):177. doi:10.4103/0973-6247.83248
39. Kim H, Choi J, Park KU, et al. Anaphylactic transfusion reaction in a patient with anhaptoglobinemia: the first case in Korea. Ann Lab Med. 2012;32(4):304–306. doi:10.3343/alm.2012.32.4.304
40. Hirayama F. Current understanding of allergic transfusion reactions: incidence, pathogenesis, laboratory tests, prevention and treatment. Br J Haematol. 2013;160(4):434–444. doi:10.1111/bjh.12150
41. Reyhaneh K, Ahmad G, Gharib K, Vida V, Raheleh K, Mehdi TN. Frequency & specificity of RBC alloantibodies in patients due for surgery in Iran. Indian J Med Res. 2013;138(2):252–256.
42. Dajak S, Čulić S, Stefanović V, Lukačević J. Relationship between previous maternal transfusions and haemolytic disease of the foetus and newborn mediated by non-RhD antibodies. Blood Transfus. 2013;11(4):528–532. doi:10.2450/2013.0193-12
43. Suddock JT, Crookston KP. Transfusion reactions. StatPearls [Internet]. StatPearls Publishing; 2019. [
44. Menis M, Forshee R, Anderson S, et al. Febrile non‐haemolytic transfusion reaction occurrence and potential risk factors among the US elderly transfused in the inpatient setting, as recorded in M edicare databases during 2011–2012. Vox Sang. 2015;108(3):251–261. doi:10.1111/vox.12215
45. Triulzi DJ, Kleinman S, Kakaiya RM, et al. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: implications for a transfusion‐related acute lung injury risk reduction strategy. Transfusion. 2009;49(9):1825–1835. doi:10.1111/j.1537-2995.2009.02206.x
46. Heddle NM, Cook RJ, Arnold DM, et al. Effect of short-term vs. long-term blood storage on mortality after transfusion. N Engl J Med. 2016;375(20):1937–1945. doi:10.1056/NEJMoa1609014
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
