Back to Journals » OncoTargets and Therapy » Volume 13
Propofol Inhibits the Migration and Invasion of Glioma Cells by Blocking the PI3K/AKT Pathway Through miR-206/ROCK1 Axis
Authors Wang D, Yang T, Liu J, Liu Y, Xing N, He J, Yang J, Ai Y
Received 26 September 2019
Accepted for publication 19 November 2019
Published 14 January 2020 Volume 2020:13 Pages 361—370
DOI https://doi.org/10.2147/OTT.S232601
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Yong Teng
Dongmei Wang, 1 Tao Yang, 2 Junqi Liu, 3 Yafei Liu, 4 Na Xing, 1 Juan He, 1 Jianjun Yang, 1 Yanqiu Ai 1
1Department of Anesthesiology, Pain and Perioperative Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, People’s Republic of China; 2Department of Anesthesiology, The Fifth Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, People’s Republic of China; 3Department of Radiotherapy, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, People’s Republic of China; 4Department of Neurosurgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, People’s Republic of China
Correspondence: Jianjun Yang; Yanqiu Ai
Department of Anesthesiology, Pain and Perioperative Medicine, The First Affiliated Hospital of Zhengzhou University, No.1, Building East Road, Zhengzhou, Henan 450052, People’s Republic of China
Tel +86 371 66278517; +86-0371-66278517
Email [email protected]; [email protected]
Background: Propofol has been identified to perform anti-tumor functions in glioma. However, the molecular mechanisms underlying propofol-induced prevention on migration and invasion of glioma cells remain unclear.
Methods: Cell proliferation, invasion and migration were measured by 3-(4,5)-dimethylthiahiazo(−z-y1)-3,5-di-phenytetrazoliumromide assay and transwell assay, respectively. The expression of microRNA (miR)-206 and Rho-associated coiled coil-containing protein kinase 1 (ROCK1) was detected by quantitative real-time polymerase chain reaction. Western blot was used to measure the activation of the PI3K/AKT pathway. The interaction between miR-206 and ROCK1 was analyzed using the dual-luciferase reporter assay, RNA immunoprecipitation assay, and pull-down assay.
Results: Propofol treatment inhibited the migration, invasion, and PI3K/AKT pathway activation in glioma cells. MiR-206 was decreased in glioma tissues and cells, while propofol exposure induced the upregulation of miR-206 in glioma cells. Besides that, we also found overexpressed miR-206 enhanced propofol-mediated inhibition on the migration, invasion, and PI3K/AKT pathway activation of glioma cells. Subsequently, ROCK1 was confirmed to be a target of miR-206. ROCK1 was elevated in glioma tissues and cells, but was reduced by propofol exposure in glioma cells. The rescue assay indicated that the miR-206/ROCK1 axis was involved in propofol-induced inhibition on the migration, invasion, and PI3K/AKT pathway activation in glioma cells.
Conclusion: Propofol inhibited the migration and invasion of glioma cells by blocking the PI3K/AKT pathway through the miR-206/ROCK1 axis, suggesting an effective clinical implication for the anesthetic to prevent the metastasis of glioma.
Keywords: propofol, miR-206, ROCK1, glioma, PI3K/AKT pathway
Introduction
Glioma is the most common primary intracranial tumor, accounting for 4050% of brain tumors, and is a lethal threat to human health.1 Currently, conventional treatments, including surgery, radiotherapy, and chemotherapy, have shown limited effectiveness for glioma therapy. Despite the improvement in multimodal therapy, the 5-year survival rate of glioma patients is consistently less than 5%.2 Thus, further investigations on molecular mechanisms of glioma pathogenesis are necessary to manage the survival of glioma patients. Propofol is a common and useful intravenous anesthetic in clinical surgery, characterized by rapid effect, short action, and few side effects.3,4 In addition to the advantages of anesthesia, emerging clinical evidence reveals that propofol paravertebral anesthesia in cancer patients undergoing tumor surgery can reduce the risk of recurrence and metastasis.5 In addition, recent studies have found that propofol exerts anticancer activity in many cancers, such as gastric cancer, esophageal squamous cell carcinoma, lung cancer, and hepatocellular carcinoma,6–9 via different molecular mechanisms. Emerging studies also identified the protective effects of propofol on glioma development.10,11 However, the molecular mechanisms by which propofol affects glioma cell migration and invasion remain vague.
MicroRNAs (miRNAs) are an endogenous group of small non-coding RNA molecules, which modulate gene expression by inducing translational inhibition or mRNA degradation.12,13 MiRNAs have been reported to be involved in various biological processes under physiological or pathological conditions, such as cell metabolism, apoptosis, proliferation, metastasis, tumorigenesis, and immune response,14 thereby regulating the development of many diseases. Among these miRNAs, miR-206 was identified to have protective effects on the development of coronary artery disease and cancers.15,16 In particular, studies revealed that miR-206 participated in the progression of glioma by functioning as a tumor inhibitor to regulate cellular biological processes, and decreased miR-206 expression was associated with poor prognosis in glioma.17–19 Further, miR-206 knockdown was shown to protect human embryonic stem cells (hESCs) against propofol-induced cell apoptosis, thereby inhibiting neurotoxicity.20 Rho-associated coiled coil-containing protein kinase 1 (ROCK1) is a protein serine/threonine kinase and a major regulator of the actomyosin cytoskeleton, thereby governing various cellular processes, including cell division, adhesion, contraction, migration, apoptosis, and proliferation.21 Accumulating studies have indicated that ROCK1 plays crucial roles in cancer development by modulating diverse vital cellular biological processes related to malignancy.22 Additionally, ROCK1 was also found to be involved in the regulation of glioma development.23,24 Thus, we further explored whether miR-206 or ROCK1 were implicated in propofol-mediated regulation on glioma cells.
This study aimed to investigate the effects of propofol on cell migration and invasion in glioma, uncover the relationship between propofol and miR-206, and identify the means by which it mediates ROCK1 to affect cell migration and invasion in glioma.
Materials and Methods
Clinical Specimens
Human glioma tissues from 28 surgical glioma patients and normal brain tissues from 28 brain trauma surgical patients were collected from the First Affiliated Hospital of Zhengzhou University. No patients had received any preoperative treatment. All specimens were frozen in liquid nitrogen until use. The study was permitted by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University and written informed consent has been collected from all patients.
Cell Culture, Transfection, and Propofol Exposure
Normal human astrocytes (NHAs) and human glioma cell lines (U251 and LN229) were purchased from the Chinese Academy of life Sciences (Shanghai, China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA, USA) with 10% fetal calf serum (Invitrogen) at 37°C with 5% CO2.
MiR-206 mimic (miR-206), miR-206 inhibitor (miR-206-I), their corresponding control (miR-NC or miR-NC-I), small interfering RNA (siRNA) targeting ROCK1 (si-ROCK1) and siRNA negative control (si-NC) were synthesized by Genepharma (Shanghai, Chinaexit ), and then these oligonucleotides or vectors were transfected into U251 and LN229 cells using Lipofectamine 2000 (Invitrogen) for 48 h before propofol treatment.
Propofol was obtained from Sigma (St Louis, MO, USA), following by being dissolved in DMSO in accordance with the protocol of the manufacturer. Cells were maintained in 96-well plates and treated with 5 µg/mL propofol for 6 h, and cells treated by the same volume of PBS were regarded as blank controls.
Cell Viability
Cells were cultured in a 96-well plate (5000 cells/well) and were incubated with 10 μL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (Sigma) for 4 h. After removing the supernatants, 150 μL DMSO (Sigma) was added to each cell. Finally, the absorbance was detected at 490 nm. The experiment was performed three times.
Transwell Assay
For migration assay, cells were seeded on the top of the transwell chambers with serum-free DMEM. Then, 500 μL DMEM mixed with 10% FCS was added into the lower chambers. After incubation for 24 h at 37°C, migrated cells were fixed and stained. For the invasion assay, the upper chamber membranes were pre-coated with Matrigel (BD Biosciences, San Jose, CA, USA); otherwise, the measurement was similar to the steps of cell migration. Finally, cells were counted with a microscope in five randomly selected fields. The same experiment was repeated three times, and the average was taken.
Western Blot
Proteins were isolated using RIPA lysis buffer (Beyotime, Shanghai, China ) and quantified by a bicinchoninic acid (BCA) method following the recommendations of the manufacturer. Then, extracted protein was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). Subsequently, the membranes were interacted with primary antibodies against phosphorylated (p-) PI3K (1:1000, ab182651, Abcam, Cambridge, MA, USA), PI3K (1:1000, ab40776, Abcam), p-AKT (1:1000, 9271, Cell Signaling Technology, Boston, MA, USA), AKT (1:1000, 9272, Cell Signaling Technology), and β-actin (1:1000, 4967, Cell Signaling Technology), and followed by incubation with HRP-conjugated secondary antibody (1:1000; ab9482; Abcam). Finally, protein signals were visualized using an ECL method. Experiments were performed three times.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated using Trizol reagent (Invitrogen) according to the standard introductions. Complementary DNA (cDNA) was synthesized using M-MLV reverse transcriptase (Promega, Shanghai, China ). Then, qPCR was carried out with SYBR Green PCR Master Mix (Takara, Dalian, China ). The relative expression was calculated using the 2−ΔΔCT method and normalized by U6 small nuclear B noncoding RNA (U6) or glyceraldehyde 3-phosphate dehydrogenase (GADPH). All experiments were repeated three times independently. The sequences of primers used in this study were listed as follows: miR-206, F 5ʹ-CGATGGAATGTAAGGAAGT-3ʹ, R, 5ʹ-GTGCAGGGTCCGAGGT-3ʹ; ROCK1, F, 5ʹ-AGGAAGGCGGACATATTAGTCCCT-3ʹ, R, 5ʹ-AGAGATAGTTGGGTCCCGGC-3ʹ; GAPDH F, 5ʹ-ATTCCATGGCACCGTCAAGGCTGA-3ʹ, R, 5ʹ-TTCTCCATGGTGGTGAAGACGCCA-3ʹ, U6, F, 5ʹ-CTCGCTTCGGCAGCACA-3ʹ, R 5ʹ-CGCTTCACGAATTTGCGT-3ʹ.
Dual-Luciferase Reporter Assay
The wild-type (WT) or mutant (MUT) ROCK1 3ʹUTR containing miR-206 sequences was synthesized and cloned into the pmiR-RB-Report (Promega). Subsequently, these constructed vectors were transfected into U251 and LN229 cells with miR-206 mimics, miR-NC, miR-206-I, or miR-NC-I. Finally, the relative luciferase activity was analyzed by a dual-luciferase reporter assay kit (Promega).
RNA Immunoprecipitation Assay
RNA immunoprecipitation (RIP) assay was carried out with the Magna RIP Kit (Millipore). Cells transfected with miR-NC or miR-206 were lysed, and then the lysate was incubated with magnetic beads coated with anti-Ago2 (Millipore) or IgG antibody (Abcam). Finally, the enrichment of ROCK1 was measured by qRT-PCR.
Pull-Down Assay
MiR-206 and miR-NC were biotinylated to generate Bio-miR-206, Bio-miR-206 MUT, and Bio-miR-NC by GenePharma Company (Shanghai, China ), and then these biotinylated oligonucleotides were transfected into the U251 and LN229 cells for 48 h. Subsequently, cells were collected and lysed, and the lysate was incubated with streptavidin-coated magnetic beads. After elution, the biotin-coupled RNA complex was pulled down, and then was analyzed by qRT-PCR.
Statistical Analysis
Statistical data from three independent experiments were presented as the mean ± standard deviation (SD) and. Significant differences were analyzed using one-way analysis of variance (ANOVA) or Student’s t-test on GraphPad Prism 7 software (GraphPad Inc., San Diego, CA, USA). P<0.05 suggested statistically significant differences.
Results
Propofol Suppresses Migration, Invasion, and PI3K/AKT Pathway Activation of Glioma Cells
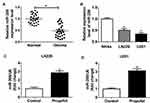
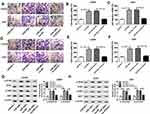
To explore the effects of propofol on cell tumorigenesis in glioma, U251 and LN229 cells were treated with or without propofol. After that, we found propofol treatment inhibited cell migration and invasion in glioma (Figure 1C and D). Propofol exposure also suppressed the activation of the PI3K/AKT pathway in U251 and LN229 cells, reflected by the reduction of p-PI3K and p-AKT expression (Figure 1E). However, treatment with propofol did not affect cell proliferation in glioma (Figure 1A and B). Therefore, these data indicated that propofol repressed the migration, invasion, and the activation of the PI3K/AKT pathway in glioma cells.
Propofol Promotes the Expression of miR-206 in Glioma Cells
To investigate whether the expression of miR-206 was affected by propofol in glioma cells, first, the expression of miR-206 was detected in glioma, and qRT-PCR analysis showed that miR-206 was decreased in glioma tissues and cell lines compared with the non-tumor tissues and NHAs (Figure 2A and B). After that, U251 and LN229 cells were treated with propofol and we found propofol significantly promoted miR-206 expression in U251 and LN229 cells (Figure 2C and D), indicating the potential regulatory role of miR-206 in propofol-induced glioma cells.
MiR-206 Enhances Propofol-Mediated Inhibition on the Migration, Invasion, and PI3K/AKT Pathway of Glioma Cells
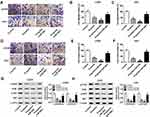
Based on the regulatory relationship between miR-206 and propofol, we further uncovered whether miR-206 was involved in propofol-mediated inhibition on glioma cell tumorigenesis. U251 and LN229 cells were transfected with miR-206 or miR-206-I prior to propofol exposure. The results showed that overexpressed miR-206 enhanced propofol-mediated inhibition on the migration, invasion (Figure 3A–F) and PI3K/AKT pathway activation (Figure 3G and H) of U251 and LN229 cells, while miR-206 inhibition reversed the anticancer activity of propofol on U251 and LN229 cells (Figure 3A–H). Therefore, we found that propofol inhibited cell tumorigenesis in glioma by regulating miR-206.
ROCK1 is a Target of miR-206
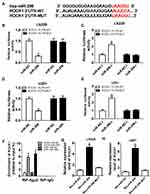
To elucidate the underlying mechanism of miR-206 in glioma progression, the potential targets of miR-206 were predicted according to the Starbase3.0 program and ROCK1 was found to have binding sites with miR-206 (Figure 4A). To verify this prediction, the dual-luciferase reporter assay was performed and we found that the miR-206 mimic significantly reduced the luciferase activity of the ROCK1-WT reporter vector but not the mutant reporter vector in U251 and LN229 cells (Figure 4B and D). The luciferase activity of the ROCK1-WT reporter was markedly promoted, but there was no obvious change in ROCK1-MUT reporter after miR-206 inhibition in U251 and LN229 cells (Figure 4C and E). The RIP assay indicated ROCK1 pull-down by Ago2 was significantly enriched in miR-206-transfected cells (Figure 4F). Meanwhile, RNA pull-down assay further showed bio-miR-206 could enrich more ROCK1 compared with the bio-miR-206 MUT and bio-miR-NC (Figure 4G and H). Taken together, miR-206 directly interacted with ROCK1 in glioma cells.
Propofol Represses ROCK1 Expression in Glioma Cells
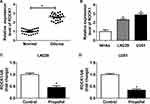
The expression of ROCK1 was detected and results showed that ROCK1 was upregulated in glioma tissues and cell lines compared with the non-tumor tissues and NHAs (Figure 5A and B). Afterwards, U251 and LN229 cells were treated with propofol and qRT-PCR analysis indicated that propofol decreased ROCK1 expression in U251 and LN229 cells (Figure 5C and D), suggesting that abnormal ROCK1 expression might be involved in propofol-mediated regulation on glioma cells.
Propofol Inhibits the Migration, Invasion, and PI3K/AKT Pathway Activation of Glioma Cells by miR-206/ROCK1 Axis
Based on the interaction between miR-206 and ROCK1, we further elaborated whether the miR-206/ROCK1 axis was responsible for propofol-mediated glioma cell tumorigenesis inhibition. U251 and LN229 cells were transfected with miR-NC-I, miR-206-I, miR-206-I + si-NC, or miR-206-I + si-ROCK1 prior to propofol treatment. Results suggested that miR-206 inhibition reversed propofol-induced suppression on the migration, invasion (Figure 6A–F), and PI3K/AKT pathway activation (Figure 6G and H) in U251 and LN229 cells, while these could be rescued by following ROCK1 deletion (Figure 6A–H). Altogether, these results suggested that propofol could regulate the migration, invasion, and PI3K/AKT pathway activation of glioma cells by the miR-206/ROCK1 axis.
Discussion
Glioma is still a serious problem due to its highly invasive and diffusive infiltrative nature. Even with optimal treatment, such as surgical resection combined with chemotherapy, radiotherapy, or other adjuvant therapies, the average survival of glioma patients is no more than 18 months.2,25 Propofol is commonly used in intravenous anesthetic medicines during clinical surgery. Increasing evidence has revealed that propofol also has neuroprotective, anti-anxiety, anti-oxidation and anti-cancer roles in pathogenic situations.8,26 The aim of this study was to identify the anti-tumor effects of propofol on the biological behavior of glioma cells and we observed that propofol treatment inhibited the migration and invasion of glioma cells, which was consistent with multiple previous findings. For example, Chen et al revealed propofol inhibited pancreatic cancer cell migration by interfering with NMDA receptor degradation.27 Yang et al indicated that treatment of propofol suppressed gastric cancer cell growth and survival via inhibiting ING3 expression.28 Thus, propofol may be a promising candidate for tumor surgery.
To date, mounting evidence has revealed that miRNAs play important roles in the pathogenesis of various cancers, including glioma.29 MiRNAs can regulate glioma cell tumorigenesis, proliferation, metastasis, apoptosis, and angiogenesis, thus affecting cancer maintenance and progression. For example, miR-155 functioned as an oncogene to induce glioma cell proliferation through directly interacting with CDX1.30 MiR-451 suppressed glioma cell proliferation and invasion by blocking the mTOR/HIF-1α/VEGF pathway through regulating CAB39 expression.31 Propofol has also been found to exert anti-tumor functions via regulating miRNAs in many types of tumors. For instance, Yu et al found propofol promoted cell apoptosis in breast cancer via downregulating miR-24.32 Propofol suppressed ovarian cancer cell invasion and growth by the regulation of the miR-9/NF-κB pathway.33 Therefore, we further investigated the underlying molecular mechanism of propofol in the migration and invasion of glioma cells. Previous studies revealed miR-206 served as a tumor suppressor to involve in the development of glioma.18,34 The present study exhibited that propofol treatment induced miR-206 upregulation in glioma cells. Moreover, neutralizing miR-206 with miR-206 inhibitor attenuated the inhibitory effects induced by propofol treatment on glioma cell migration and invasion, while increasing the level of miR-206 enhanced the anticancer activity of propofol on glioma cells. Thus, propofol restrained cell migration and invasion by inducing miR-206 up-regulation in glioma cells.
It is well recognized that miRNAs often function by modulating gene expression. Thus, we further explored the downstream genes of miR-206 in glioma cells and ROCK1 was validated to be a target of miR-206. In this study, we discovered that knockdown of ROCK1 could reverse miR-206 inhibition mediated promotion on cell migration and invasion in propofol-induced glioma cells. Thus, we knew propofol could suppress the migration and invasion of glioma cells by regulating the miR-206/ROCK1 axis. The PI3K/AKT pathway is an intracellular signaling pathway in regulating various cellular processes in different cell types, including migration and invasion.35 In the current study, our results demonstrated treatment with propofol led to an obvious downregulation of p-PI3K and p-AKT expression, but there were no notable changes of total PI3K and AKT expression in the glioma cells, indicating that propofol inactivated the PI3K/AKT pathway. Subsequently, we also found propofol treatment could inhibit the activation of the PI3K/AKT pathway through regulating the miR-206/ROCK1 axis.
Conclusion
Taken together, our data proved that propofol treatment increased the expression of miR-206, but decreased the level of ROCK1 in glioma cells. Propofol treatment could inhibit the migration and invasion of glioma cells by blocking the PI3K/AKT pathway through the miR-206/ROCK1 axis, providing an effective clinical implication for the anesthetic to inhibit the metastasis and improve patient outcomes for glioma.
Highlights
- Propofol suppresses migration, invasion, and PI3K/AKT pathway of glioma cells.
- Propofol promotes the expression of miR-206 in glioma cells.
- ROCK1 is a target of miR-206.
- Propofol represses ROCK1 expression in glioma cells.
- Propofol inhibits the migration, invasion, and PI3K/AKT pathway of glioma cells by miR-206/ROCK1 axis.
Funding
This work was supported by the National Natural Science Foundation of China (81703158).
Disclosure
The authors declare that they have no financial conflicts of interest.
References
1. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19(suppl_5):v1–v88. doi:10.1093/neuonc/nox158
2. Wang B, Wu ZS, Wu Q. CMIP promotes proliferation and metastasis in human glioma. Biomed Res Int. 2017;2017:5340160.
3. Chidambaran V, Costandi A, D’Mello A. Propofol: a review of its role in pediatric anesthesia and sedation. CNS Drugs. 2015;29(7):543–563. doi:10.1007/s40263-015-0259-6
4. Oren-Ziv A, Hoppenstein D, Shles A, et al. Sedation methods for intra-articular corticosteroid injections in Juvenile idiopathic arthritis: a review. Pediatr Rheumatol Online J. 2015;13(28). doi:10.1186/s12969-015-0021-0.
5. Chen X, Lu P, Chen L, et al. Perioperative propofol-paravertebral anesthesia decreases the metastasis and progression of breast cancer. Tumor Biol. 2015;36(11):8259–8266. doi:10.1007/s13277-015-4027-5
6. Zheng H, Fu Y, Yang T. Propofol inhibits proliferation, migration, and invasion of hepatocellular carcinoma cells by downregulating Twist. J Cell Biochem. 2019;120(8):12803–12809. doi:10.1002/jcb.v120.8
7. Sun H, Gao D. Propofol suppresses growth, migration and invasion of A549 cells by down-regulation of miR-372. BMC Cancer. 2018;18(1):1252. doi:10.1186/s12885-018-5175-y
8. Zhang W, Wang Y, Zhu Z, et al. Propofol inhibits proliferation, migration and invasion of gastric cancer cells by up-regulating microRNA-195. Int J Biol Macromol. 2018;120(Pt A):975–984. doi:10.1016/j.ijbiomac.2018.08.173
9. Zhou CL, Li JJ, Ji P. Propofol suppresses esophageal squamous cell carcinoma cell migration and invasion by down-regulation of sex-determining region Y-box 4 (SOX4). Med Sci Monit. 2017;23:419–427. doi:10.12659/MSM.899732
10. Wang XY, Li YL, Wang HY, et al. Propofol inhibits invasion and proliferation of C6 glioma cells by regulating the Ca(2+) permeable AMPA receptor-system xc(-) pathway. Toxicol in Vitro. 2017;44:57–65. doi:10.1016/j.tiv.2017.06.026
11. Xu J, Xu W, Zhu J. Propofol suppresses proliferation and invasion of glioma cells by upregulating microRNA-218 expression. Mol Med Rep. 2015;12(4):4815–4820. doi:10.3892/mmr.2015.4014
12. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi:10.1016/j.cell.2009.01.002
13. Shukla GC, Singh J, Barik S. MicroRNAs: processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol. 2011;3(3):83–92.
14. Kim J, Yao F, Xiao Z, et al. MicroRNAs and metastasis: small RNAs play big roles. Cancer Metastasis Rev. 2018;37(1):5–15. doi:10.1007/s10555-017-9712-y
15. Pan JY, Sun CC, Bi ZY, et al. miR-206/133b cluster: a weapon against lung cancer? Mol Ther Nucleic Acids. 2017;8:442–449. doi:10.1016/j.omtn.2017.06.002
16. Wang M, Ji Y, Cai S, et al. MiR-206 suppresses the progression of coronary artery disease by modulating vascular endothelial growth factor (VEGF) expression. Med Sci Monit. 2016;22:5011–5020. doi:10.12659/MSM.898883
17. Zhou F, Cao W, Xu R, et al. MicroRNA-206 attenuates glioma cell proliferation, migration, and invasion by blocking the WNT/beta-catenin pathway via direct targeting of Frizzled 7 mRNA. Am J Transl Res. 2019;11(7):4584–4601.
18. Hao W, Luo W, Bai M, et al. MicroRNA-206 inhibited the progression of glioblastoma through BCL-2. J Mol Neurosci. 2016;60(4):531–538. doi:10.1007/s12031-016-0824-6
19. Wang S, Lu S, Geng S, et al. Decreased expression of microRNA-206 correlates with poor clinical outcome in patients with malignant astrocytomas. Pathol Oncol Res. 2014;20(2):343–348. doi:10.1007/s12253-013-9701-6
20. Li Y, Jia C, Zhang D, et al. Propofol-induced neurotoxicity in hESCs involved in activation of miR-206/PUMA signal pathway. Cancer Biomark. 2017;20(2):175–181. doi:10.3233/CBM-170167
21. Priya R, Liang X, Teo JL, et al. ROCK1 but not ROCK2 contributes to RhoA signaling and NMIIA-mediated contractility at the epithelial zonula adherens. Mol Biol Cell. 2017;28(1):12–20. doi:10.1091/mbc.e16-04-0262
22. Wei L, Surma M, Shi S, et al. Novel Insights into the Roles of Rho Kinase in cancer. Arch Immunol Ther Exp (Warsz). 2016;64(4):259–278. doi:10.1007/s00005-015-0382-6
23. Wan X, Cheng Q, Peng R, et al. ROCK1, a novel target of miR-145, promotes glioma cell invasion. Mol Med Rep. 2014;9(5):1877–1882. doi:10.3892/mmr.2014.1982
24. Gao S, Chen J, Wang Y, et al. MiR-592 suppresses the development of glioma by regulating Rho-associated protein kinase. Neuroreport. 2018;29(16):1391–1399. doi:10.1097/WNR.0000000000001124
25. Jiang T, Zhao B, Li X, J. W. ARPP-19 promotes proliferation and metastasis of human glioma. Neuroreport. 2016;27(13):960–966. doi:10.1097/WNR.0000000000000638
26. Kurt M, Bilge SS, Kukula O, et al. Anxiolytic-like profile of propofol, a general anesthetic, in the plus-maze test in mice. Pol J Pharmacol. 2003;55(6):973–977.
27. Chen X, Wu Q, You L, et al. Propofol attenuates pancreatic cancer malignant potential via inhibition of NMDA receptor. Eur J Pharmacol. 2017;795:150–159. doi:10.1016/j.ejphar.2016.12.017
28. Zhang R, Liu R, Liu C, et al. A novel role for mir-520a-3p in regulating EGFR expression in colorectal cancer. Cell Physiol Biochem. 2017;42(4):1559–1574. doi:10.1159/000479397
29. Rolle K. miRNA Multiplayers in glioma. From bench to bedside. Acta Biochim Pol. 2015;62(3):353–365. doi:10.18388/abp.2015_1072
30. Yang L, Li C, Liang F, et al. MiRNA-155 promotes proliferation by targeting caudal-type homeobox 1 (CDX1) in glioma cells. Biomed Pharmacother. 2017;95:1759–1764. doi:10.1016/j.biopha.2017.08.088
31. Nan Y, Guo H, Guo L, et al. MiRNA-451 inhibits glioma cell proliferation and invasion through the mTOR/HIF-1alpha/VEGF signaling pathway by targeting CAB39. Hum Gene Ther Clin Dev. 2018;29(3):156–166. doi:10.1089/humc.2018.133
32. Yu B, Gao W, Zhou H, et al. Propofol induces apoptosis of breast cancer cells by downregulation of miR-24 signal pathway. Cancer Biomark. 2018;21(3):513–519. doi:10.3233/CBM-170234
33. Huang X, Teng Y, Yang H, et al. Propofol inhibits invasion and growth of ovarian cancer cells via regulating miR-9/NF-kappaB signal. Braz J Med Biol Res. 2016;49(12):e5717. doi:10.1590/1414-431x20165717
34. Yang X, Zhang C, Guo T, et al. Reduced expression of microRNA206 regulates cell proliferation via cyclinD2 in gliomas. Mol Med Rep. 2015;11(5):3295–3300. doi:10.3892/mmr.2015.3171
35. Martin-Orozco RM, Almaraz-Pro C, Rodriguez-Ubreva FJ, et al. EGF prevents the neuroendocrine differentiation of LNCaP cells induced by serum deprivation: the modulator role of PI3K/Akt. Neoplasia. 2007;9(8):614–624. doi:10.1593/neo.07337
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.