Back to Journals » Cancer Management and Research » Volume 12
Propofol Inhibits Proliferation and Invasion of Stomach Cancer Cells by Regulating miR-205/YAP1 Axis
Authors Xian XS, Wang YT, Jiang XM
Received 20 July 2020
Accepted for publication 4 September 2020
Published 29 October 2020 Volume 2020:12 Pages 10771—10779
DOI https://doi.org/10.2147/CMAR.S270344
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ahmet Emre Eşkazan
Xiang-Shu Xian,1 Yu-Tie Wang,2 Xiao-Meng Jiang3
1Department of Gastroenterology, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Qingdao 264000, People’s Republic of China; 2Department of Rheumatology and Immunology, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Qingdao 264000, People’s Republic of China; 3Department of Digestive, Sir Run Run Hospital, Nanjing Medical University, Nanjing, Jiangsu, 211166, People’s Republic of China
Correspondence: Xiao-Meng Jiang
Department of Digestive, Sir Run Run Hospital, Nanjing Medical University, Nanjing Jiangsu Province 211166, People’s Republic of China
Email [email protected]
Background: Propofol is a common clinical intravenous anesthetic. In the last few years, studies have revealed that propofol not only has good anesthetic effect but also has certain anticancer effect. However, its role in stomach cancer (SC) and related mechanisms are still under investigation.
Objective: This study was designed to determine the effect of propofol on SC and its related mechanisms.
Methods: Purchased SC cells were treated with propofol at different concentrations (5, 10, and 20 μg/mL), miR-205 overexpression, and YAP1 inhibition. Then, the Cell Counting Kit-8 (CCK8), Transwell, and flow cytometry were carried out to determine the biological behavior changes of treated cells and the expression of miR-205 and YAP1 after treatment.
Results: Propofol (10 μg/mL and 20 μg/mL) inhibited the growth of SC cells and promoted their apoptosis, and overexpressing miR-205 or inhibiting YAP1 can exert the same effects. In addition, propofol (10μg/mL and 20μg/mL) up-regulated miR-205 in SC cells. The dual-luciferase reporter assay revealed that YAP1 could be targeted and regulated by miR-205, and the rescue assay revealed that inhibiting miR-205 or overexpressing YAP1 could weaken the effect of propofol on the biological behaviors of SC cells.
Conclusion: Propofol can strongly suppress the proliferation and invasion of SC cells and induce their apoptosis via the miR-205/YAP1 axis.
Keywords: propofol, stomach cancer, miR-205, YAP1, cell biology
Introduction
Stomach cancer (SC) is a common malignant tumor in the gastroenterology department, and also the third primary cause of malignant tumor-related death worldwide.1 According to statistics, there were more than 1 million new patients with SC worldwide in 2018, while the number of dead patients was close to 800,000, posing a serious threat to human life and health.2 Currently, SC is mainly comprehensively treated by surgery and chemotherapy, but the prognosis of patients is unsatisfactory, and the 5-year overall survival rate of the patients dose not even reach 30%.3 Therefore, it is necessary to find novel anticancer agents to improve the prognosis of patients with SC.
Propofol, also known as disoprofol, is a widely used intravenous anesthetic. In recent years, propofol was found to have a good anesthetic effect and an anticancer effect in various cancers. For example, propofol inhibits the proliferation and metastasis of pancreatic cancer cells by regulating the microRNA (miR)-328/ADAM8 axis, thus playing an anti-cancer role.4 It also suppresses the activity of lung cancer cells and induces their apoptosis by regulating miR-486.5 In addition, propofol inhibits the growth of endometrial carcinoma cells, and accelerates their apoptosis by regulating Sox4.6 There are also related reports on propofol in SC. Protocol is regarded as an SC suppressor of, because it can inhibit the proliferation, migration, and invasion of SC cells and induce their apoptosis. However, the mechanism of propofol in inhibiting SC progression is largely unknown, and more research is still needed to further study the mechanism. MiR is a short-chain non-coding RNA involved in biological events such as cell apoptosis, proliferation, and differentiation.8,9 MiR is a key regulator of gene expression. Increasing studies have verified that miR can participate in tumor growth. Therefore, miR is considered as an essential target for cancer treatment.10,11 MiR-205 is an important miR, which is down-regulated in SC and can prevent SC from progressing.12 Therefore, we suspected that propofol can play an anticancer role in SC by regulating miR-205.
In this study, we mainly explored the effect of propofol at different concentrations on the biological behaviors of SC cells and analyze the relationship between propofol and miR-205 in SC, with the goal of further investigating the potential development mechanism of SC.
Data and Methods
Cell Processing
(1) Cell source and culturing: SC cell strains (SGC-7901 and HGC-27) from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) were cultured in dulbecco’s modified eagle medium (DMEM) (Gibco, USA) supplemented with 10% fetal bovine serum and penicillin-streptomycin solution under 5% CO2 at 37°C. Upon reaching 80–90% confluency in adherent growing, the cells were digested with 25% trypsin for passage.
(2) Propofol treatment: For the purpose of determining the influence of propofol on SC, SGC-7901, as well as HGC-27 cells cultured overnight in an incubator were collected, added with 0, 5, 10 or 20 µM propofol (Sigma, USA), and continuously cultured in the incubator.
(3) Cell transfection: The cells were transferred to a 6-well plate at 2*105 cells/well, and incubated in an incubator at 37°C overnight. MiR-205 mimics (miR-205-mimics) and YAP1 inhibitor (si-YAP1), YAP1 mimics (sh-YAP1), miR-205-mimics were constructed using pcDNA3.1 vectors, and miR-NC was adopted as a control for miR, and si-NC as a control for si-YAP1. The vectors were transfected into SGC-7901 as well as HGC-27 cells by a Lipofectamine 2000 Kit (Invitrogen, USA) under the kit instructions, and then transferred to DMEM with 10% FBS, and cultured for 6 h after transfection.
(4) Co-treatment of miR-205, YAP1, and propofol: SC cells were randomly assigned to a control group (without any treatment), propofol group (cultured through 10 μg/mL propofol for 48 h), miR-205+propofol group (cultured through 10 μg/mL propofol for 48 h after transfection of miR-205-inhibit), and YAP1+propofol group (transfected with sh-YAP1).
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Assay
Total RNA was extracted from cells with a TRIzol Kit (Invitrogen Company, USA), and its concentration, purity, as well as integrity were detected via an ultraviolet spectrophotometer and agarose gel electrophoresis. Subsequently, reverse transcription was carried out on the total RNA through a Reverse Transcription Kit (Invitrogen, USA), and amplification was conducted by SYBR_Premix ExTaq II (Takara, Dalian, China) under the amplification system consisting of 20 μL total reaction volume with 10 μL SYBR Premix Ex Taq II (2X), 2 μL cDNA, 0.8 μL upstream and downstream primers, respectively, as well as sterile-purified water added to adjust the volume. The amplification conditions: 95°C for 30 s, followed by 40 cycles of 95°C for 5 s, and 60°C for 30 s. U6 was adopted as an internal reference for miR-205. All primers were purchased from Shanghai Genechem Co., Ltd. (Shanghai, China). The forward primer of miR-205: 5ʹ-GGCGTGAGGCTGAGGCTA-3ʹ; the reverse primer of it: ATGGCTGAGCGAAATTGCGGAC-3ʹ. The forward primer of U6: 5ʹ-CATCACCATCAGGAGAGTCG-3ʹ; the reverse primer of it: 5ʹ-TGACGCTTGCCCACAGCCTT-3ʹ. 2−ΔΔct was adopted to analyze data in this study.13
Western Blot Assay
Cultured cells of each group were harvested, and the total protein was extracted using the RIPA lysis method. The protein concentration was detected by the bicinchoninic acid (BCA) method, and adjusted to 4μg/μL. Then, the total protein was isolated through 12% SDS-PAGE, and transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was dyed with Ponceau’s stain liquid, washed with phosphate buffer saline with Tween (PBST) for 5 min, immersed in 5% skim milk for 2 h, and then added with YAP1, caspase 3, Bcl-2, Bax, and β-catenin (1:1000 each) primary antibody (Abcam, USA), and sealed at 4°C all night long. The membrane was cleaned to remove the primary antibody, and then it was added with goat anti-rabbit secondary antibody (1:5000, Abcam, USA), cultured at 37°C for 1 h, washed with PBS 3 times, 5 min/time, and developed in the dark. The liquid on the membrane was removed by a filter paper, and the membrane was made to be luminescent through electrochemiluminescence (ECL) for development. The protein band was scanned, and the gray value was evaluated through Quantity One software to calculate the protein expression. The relative expression of protein = the gray value of target protein band/the gray value of β-Actin protein band.
Cell Counting Kit-8 (CCK8) Assay
CCK-8 (Beyotime Biotechnology Co., Ltd., China) was used for cell proliferation detection according to the kit instructions as follows: The cells transfected for 24 h were cultured in a 96-well plate at 2.5×103 cells/well. At 24, 48, 72, and 96 h after culturing, 10 μL CCK-8 solution was added into every well, and the plate was incubated at indoor temperature for 2 h. Subsequently, the optical density of each well at 490 nm was detected by a microplate reader (Molecular Devices, USA), and corresponding survival curves were drawn.
Cell Invasion Detection
A Transwell Kit (Gibco Company, USA) was adopted for invasion detection as follows: Cells in each group were transferred to a 6-well plate at 5*104 cells/well, and washed with PBS twice. The upper and lower compartments were added with 200 μL DMEM and 500mL DMEM containing 20% FBS, respectively. The plate was incubated for 48 h at 37°C, and the substrates and cells that did not penetrate the membrane in the upper compartment were wiped off. The plate was cleaned with PBS three times, immobilized with paraformaldehyde for 10 min, and cleaned and dried in the air, and it was then stained with 0.5% crystal violet. Finally, cell invasion in the plate was analyzed using a microscope.
Cell Apoptosis Detection
The transfected cells were collected and trypsinized with0.25% trypsin, and then prepared into 1*106 cells/mL suspension. The suspension was added with AnnexinV-FITC/PI (Yeasen Biotechnology Co., Ltd., Shanghai, China) in order, cultured at indoor temperature in the dark for 5 min, and finally determined using the FC500MCL flow cytometer system.
Dual Luciferase Reporter (DLR) Assay
TargetScan 7.2 (www.targetscan.org) was adopted to predict target genes of miR-205, and the Lipofectamine™ 2000 reagent kit was utilized for construction ofYAP1-3ʹUTR wild type (Wt) and YAP1-3ʹUTR mutant type (Mut). The two were transfected into the downstream of the luciferase reporter genes to sequence and identify the constructed plasmids. Correctly sequenced plasmids along with miR-205-mimics or miR-NC were co-transfected into SGC-7901 cells. A DLR Gene Determination Kit (Solarbio Company, Beijing, China) was used to determine luciferase activity.
Statistical Analyses
In this study, the obtained data were analyzed statistically using SPSS 21.0 (IBM Corp, Armonk, NY, USA), and visualized into required figures using GraphPad 7. Inter-group comparison was carried out by the independent t-test, and multi-group comparison was carried out by the one-way ANOVA, and verified by the Tukey HSD method. Comparison in expression at different time points was conducted through the repeated measures analysis of variance, and analyzed through the Bonferroni post hoc test. P< 0.05 indicates a significant difference.
Results
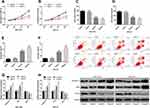
Effect of Propofol at Different Concentrations on the Biological Behaviors of SC Cells
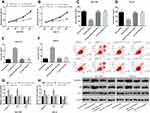
In order to determine the effect of propofol on the biological behaviors of SC cells, propofol at different concentrations was cultured with SC cells, separately. It was found through CCK-8, Transwell, and flow cytometry assays that compared with 0 μg/mL propofol, 5 μg/mL propofol had no significant effect on the biological behaviors of SGC-7901 and HGC-27 cells, while 10 μg/mL and 20 μg/mL propofol inhibited the cell growth, and promoted apoptosis, and also up-regulated caspase-3 and Bax and down-regulated Bcl-2, implying that propofol can inhibit SC progression. Figure 1.
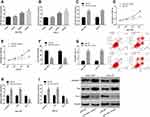
Effect of miR-205 on the Biological Events of SC Cells
It was found through the detection of changes of miR-205 expression in cells from each group that compared with 0 μg/mL propofol, 5 μg/mL propofol exerted no effect on miR-205 in SGC-7901 and HGC-27 cells, but 10 μg/mL and 20 μg/mL propofol up-regulated miR-205 in the cells. The results suggest that propofol may affect the biological behaviors of SC cells by up-regulating miR-205. With the aim of investigating the influence of miR-205 on the biological behaviors of SC cells, miR-205 in SGC-7901 and HGC-27 cells was overexpressed, and the changes in cell biological behaviors were detected after treatment. It was found that SGC-7901 and HGC-27 cells showed up-regulated miR-205, intensified apoptosis, weakened proliferation and invasion, increased caspase-3 and Bax expression and decreased Bcl-2 expression after being transfected with miR-205-mimics, which indicates that miR-205 plays the role as a tumor suppressor gene in SC. Figure 2.
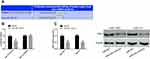
Relationship Between miR-205 and YAP1
It is well known that miR can exert biological effects by regulating downstream target genes. For the purpose of further understanding the action mechanism of miR-205 in SC, we predicted a targeted binding site between miR-205 and YAP1 based on online biological prediction software Targetscan7.2. To explore the correlation between miR-205 and YAP1, we carried out a DLR assay, finding that transfection of miR-205-mimics inhibited the luciferase activity of YAP1-3ʹUTR Wt, but exerted no influence on that of YAP1-3ʹUTR Mut. It was also found that after being transfected with miR-205-mimics, SGC-7901 and HGC-27 cells showed down-regulated YAP1. Figure 3.
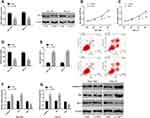
Effect of YAP1 on the Biological Behaviors of SC Cells
According to the results of 2.3, miR-205 can target and regulate YAP1, but it was still unclear that whether miR-205 can play the role as a tumor suppressor gene in SC through YAP1. Therefore, we analyzed the influence of inhibiting YAP1 on the biological behaviors of SC cells, finding that after being transfected with si-YAP1, SGC-7901 and HGC-27 cells showed down-regulated YAP1 and Bcl-2, weakened proliferation and invasion, intensified apoptosis, and up-regulated caspase-3 and Bax. Figure 4.
Effect of Inhibiting miR-205 or Overexpressing YAP1 on Reversing the Effect of Propofol on Biological Behaviors of SC Cells
In order to further investigate the relationship between propofol and miR-205/YAP1 axis, SGC-7901 and HGC-27 cells were treated with miR-205+ propofol and over-expressed YAP1+ propofol, separately, and the effects of these two treatments on the biological events of the cells were evaluated. It came out that both inhibition of miR-205 and overexpression of YAP1 reversed the influence of propofol on the biological behaviors of SC cells and apoptosis-related proteins, which implies that propofol can exert anticancer effect by regulating the miR-205/YAP1 axis. Figure 5.
Discussion
SC is one of the major causes of human death. Although an enormous progress has been achieved in its treatment, the clinical treatment outcomes are still poor due to SC’s characteristics of high metastasis and recurrence rates.14 Therefore, it is urgent to improve the current treatment strategy. Propofol is a safe and effective anesthetic with quick response and short acting time.15 Previous studies have revealed that propofol can inhibit the growth and invasion of SC cells and induce cell apoptosis.7,16 In the present study, we explored the effect of propofol on SC, finding that propofol inhibited SC progression by inhibiting the growth of SC cells and inducing cell apoptosis, which is similar to previous research results. However, it is still unclear how propofol exerts anti-cancer effect in SC.
MiR regulates 1/3 of human genes, and its abnormal expression is believed to be the cause of many human diseases.17 In terms of tumors, increasing studies have confirmed that miR can be used as a regulator in tumor progression, so it is considered as an essential target for developing new anticancer drugs.18,19 MiR-205 is a highly conservative miR in various species and is involved in the development of various cancers. For example, miR-205 is expressed in a high level in cases of ovarian cancer and promotes the progression of the disease by targeting TCF21,20 and it plays an anti-cancer role in colon cancer by inhibiting the PTK7 expression.21 In addition, miR-205 can suppress the growth and metastasis of hepatic carcinoma cells by targeting vascular endothelial growth factor (VEGF).22 In our study, SC cells showed weakened proliferation and invasion, intensified apoptosis, elevated caspase-3 and Bax expression, as well as lowered Bcl-2 expression after being transfected with miR-205-mimics, which indicates that miR-205 serves as a tumor suppressor in SC. We explored the association between miR-205 and propofol, finding that inhibition of miR-205 could weaken the effect of propofol on the biological events of SC cells, which suggests that propofol can act as an anticancer agent in SC by regulating miR-205.
It is universally known that miR participates in biological events by regulating target genes.23 For the purpose of further exploring the action mechanism of propofol in SC, we predicted the target genes downstream of miR-205 based on the biological prediction website Targetscan7.2, and found that there was a targeted binding site between miR-205 and YAP1. YAP1, located in chromosome 11q22, is a transcription regulator of Hippo signal and can participate in various cellular biological behaviors, including cell growth, apoptosis and invasion.24 YAP1, considered as a carcinogenic factor in tumors, can promote the progression of various cancers including SC.25–27 Previous studies have uncovered that various miRs can affect tumorigenesis through targeted regulation on YAP1. For example, miR-506 can inhibit the growth of SC cells through targeted inhibition on YAP1 expression.28 MiR-345 can also play an anti-cancer role in hepatic carcinoma by inhibiting YAP1 expression.29 Furthermore, miR-195 can inhibit the proliferation and accelerate the apoptosis of esophageal cancer cells through YAP1.30 In this study, the DLR assay revealed that YAP1 can be targetedly regulated by miR-205, and inhibition of YAP1 in SC cells caused the same influence on the changes of cell biological events and apoptosis-related proteins as those produced by overexpression of miR-205, which indicates that miR-205 can affect SC by regulating YAP1. Subsequently, we analyzed the relationship between YAP1 and propofol, finding that overexpression of YAP1 could weaken the influence of propofol on the biological events of SC cells, which suggests that propofol can act as an anticancer agent in SC by regulating the miR-205/YAP1 axis.
In the present study, we have investigated the role of propofol in SC and analyzed its mechanism, finding that propofol can inhibit the growth of SC cells and accelerate their apoptosis, and it was related to regulation of propofol on the miR-205/YAP1 axis. However, there are some limitations in this study. First, no nude mouse tumorigenesis assay has been conducted to explore the effect of propofol and miR-205/YAP1 axis on tumor growth. Secondly, no clinical experiment has been designed to explore the clinical value of miR-205 and YAP1. For these limitations, we will conduct more experiments to supplement the results of this study in the future.
To sum up, propofol can act as an anti-cancer agent by changing the biological behaviors of SC cells by regulating the miR-205/YAP1 axis.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet. 2016;388(10060):2654–2664. doi:10.1016/S0140-6736(16)30354-3
2. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
3. Sharp SK, Jianfeng L, Wang D, Xu XY. Prognostic value of circulating CD133(+) cells in patients with gastric cancer. Cell Prolif. 2015;48:311–317. doi:10.1111/cpr.12175
4. Yu X, Gao Y, Zhang F. Propofol inhibits pancreatic cancer proliferation and metastasis by up‐regulating miR‐328 and down‐regulating ADAM8[J]. Basic Clin Pharmacol Toxicol. 2019;125(3):271–278. doi:10.1111/bcpt.13224
5. Yang N, Liang Y, Yang P, et al. Propofol inhibits lung cancer cell viability and induces cell apoptosis by upregulating microRNA-486 expression. Brazilian J Med Biol Res. 2017;50:1. doi:10.1590/1414-431x20165794
6. Du Q, Liu J, Zhang X, et al. Propofol inhibits proliferation, migration, and invasion but promotes apoptosis by regulation of Sox4 in endometrial cancer cells. Brazilian J Med Biol Res. 2018;51:4. doi:10.1590/1414-431x20176803
7. Zhu F, Li Q, Yang Y, et al. Propofol suppresses proliferation, migration, invasion and promotes apoptosis by upregulating microRNA-140-5p in gastric cancer cells. Onco Targets Ther. 2019;12:10129. doi:10.2147/OTT.S225360
8. Agarwal V, Bell GW, Nam JW, et al. Predicting effective microRNA target sites in mammalian mRNAs. elife. 2015;4:e05005. doi:10.7554/eLife.05005
9. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203.
10. Wei CH, Wu G, Cai Q, et al. MicroRNA-330-3p promotes invasion and metastasis of non-small cell lung cancer cells via GRIA3 by activating the MAPK/ERK signaling pathway. J Hematol Oncol. 2017;10(1):125. doi:10.1186/s13045-017-0493-0
11. Hannafon BN, Trigoso YD, Calloway CL, et al. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016;18(1):90. doi:10.1186/s13058-016-0753-x
12. Yin WZ, Li F, Zhang L, et al. Down-regulation of microRNA-205 promotes gastric cancer cell proliferation. Eur Rev Med Pharmacol Sci. 2014;18(7):1027–1032.
13. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. methods. 2001;25(4):402–408.
14. Liu Y, Feng Y, Gao Y, et al. Clinical benefits of combined chemotherapy with S-1, oxaliplatin, and docetaxel in advanced gastric cancer patients with palliative surgery. Onco Targets Ther. 2016;9:1269.
15. Bosnjak ZJ, Logan S, Liu Y, et al. Recent insights into molecular mechanisms of propofol-induced developmental neurotoxicity: implications for the protective strategies. Anesth Analg. 2016;123(5):1286. doi:10.1213/ANE.0000000000001544
16. Yang C, Gao J, Yan N, et al. Propofol inhibits the growth and survival of gastric cancer cells in vitro through the upregulation of ING3. Oncol Rep. 2017;37(1):587–593. doi:10.3892/or.2016.5218
17. Li G, Luo J, Xiao Q, et al. Predicting microRNA-disease associations using label propagation based on linear neighborhood similarity. J Biomed Inform. 2018;82:169–177. doi:10.1016/j.jbi.2018.05.005
18. Fu F, Jiang W, Zhou L, et al. Circulating exosomal miR-17-5p and miR-92a-3p predict pathologic stage and grade of colorectal cancer. Transl Oncol. 2018;11(2):221–232. doi:10.1016/j.tranon.2017.12.012
19. Qiao F, Gong P, Song Y, et al. Downregulated PITX1 modulated by MiR-19a-3p promotes cell malignancy and predicts a poor prognosis of gastric cancer by affecting transcriptionally activated PDCD5. Cellular Physiol Biochem. 2018;46(6):2215–2231.
20. Wei J, Zhang L, Li J, et al. MicroRNA-205 promotes cell invasion by repressing TCF21 in human ovarian cancer. J Ovarian Res. 2017;10(1):33. doi:10.1186/s13048-017-0328-1
21. Chen S, Wang Y, Su Y, et al. miR-205-5p/PTK7 axis is involved in the proliferation, migration and invasion of colorectal cancer cells. Mol Med Rep. 2018;17(5):6253–6260.
22. Zhao X, Zhou S, Wang D, et al. MicroRNA-205 is downregulated in hepatocellular carcinoma and inhibits cell growth and metastasis via directly targeting vascular endothelial growth factor A. Oncol Lett. 2018;16(2):2207–2214.
23. Sanders SJ, He X, Willsey AJ, et al. Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron. 2015;87(6):1215–1233.2. doi:10.1016/j.neuron.2015.09.016
24. Kapoor A, Yao W, Ying H, et al. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 2014;158(1):185–197. doi:10.1016/j.cell.2014.06.003
25. Huang C, Ma R, Yue J, et al. MiR-497 suppresses YAP1 and inhibits tumor growth in non-small cell lung cancer. Cellular Physiol Biochem. 2015;37(1):342–352. doi:10.1159/000430358
26. Sun D, Li X, He Y, et al. YAP1 enhances cell proliferation, migration, and invasion of gastric cancer in vitro and in vivo. Oncotarget. 2016;7(49):81062. doi:10.18632/oncotarget.13188
27. Ou C, Sun Z, Li X, et al. MiR-590-5p, a density-sensitive microRNA, inhibits tumorigenesis by targeting YAP1 in colorectal cancer. Cancer Lett. 2017;399:53–63. doi:10.1016/j.canlet.2017.04.011
28. Deng J, Lei W, Xiang X, et al. MicroRNA-506 inhibits gastric cancer proliferation and invasion by directly targeting Yap1. Tumor Biol. 2015;36(9):6823–6831. doi:10.1007/s13277-015-3364-8
29. Zhang H, Liu H, Bi H. MicroRNA-345 inhibits hepatocellular carcinoma metastasis by inhibiting YAP1. Oncol Rep. 2017;38(2):843–849. doi:10.3892/or.2017.5772
30. Gao X, Lu M, Xu W, et al. miR-195 inhibits esophageal cancer cell proliferation and promotes apoptosis by downregulating YAP1. Int J Clin Exp Pathol. 2019;12(1):275.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.