Back to Journals » Patient Preference and Adherence » Volume 16
Promoting Adherence to Iron Chelation Treatment in Beta-Thalassemia Patients
Authors Eziefula C, Shah FT , Anie KA
Received 8 January 2022
Accepted for publication 29 March 2022
Published 7 June 2022 Volume 2022:16 Pages 1423—1437
DOI https://doi.org/10.2147/PPA.S269352
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Chinea Eziefula,1,2 Farrukh T Shah,3,4 Kofi A Anie5,6
1Camden & Islington NHS Foundation Trust, London, UK; 2Psychology Services, Whittington Health NHS Trust, London, UK; 3Department of Haematology, Whittington Health NHS Trust, London, UK; 4Medical Director for Transfusion, NHSBT, London, UK; 5London North West University Healthcare NHS Trust, London, UK; 6Imperial College London, London, UK
Correspondence: Farrukh T Shah, Department of Haematology, Whittington Health, Magdala Avenue, London, N19 5NF, Tel +44 0207 288 5144, Email [email protected]
Abstract: Thalassaemia is one of the commonest inherited genetic disorders world-wide with around 25,000 births of the most severely affected transfusion dependent children annually. Patients with transfusion dependent thalassaemia require regular blood transfusions to maintain life but because of this will develop iron overload. To remove the excess iron, patients are required to take iron chelation therapy (ICT). ICT requires lifelong adherence to treatment to prevent end organ damage from developing. Many of these preventable complications make adherence to therapy more complex for patients. In this review, we focus on two commonly encountered patient scenarios and discuss how different psychological models and a relational theory can be used to understand and support adherence to treatment.
Keywords: beta thalassaemia, iron chelation therapy, treatment, adherence, psychology
Introduction
The β-thalassemia syndromes are inherited blood disorders characterized by anemia due to either absent (β 0 mutations) or reduced synthesis (β + mutations) of the β globin chains of hemoglobin. Carriers have mutations in one β globin gene and patients with mutations in both β globin genes or additional disease modifiers, may either suffer from non-transfusion dependent thalassemia (NTDT) or transfusion dependent thalassemia (TDT) depending on the degree of imbalance between α/β-globin chains.1
Carriers are healthy individuals with a minimal or mild anemia and do not develop iron overload (IOL). Patients with NTDT and TDT suffer from a mild to severe degree of anemia in the absence of transfusion, which may result in the development of hepatosplenomegaly, bone marrow expansion and extramedullary hematopoiesis resulting in the classical boney deformities seen in thalassemia syndromes.2,3 The severity of the anemia suppresses the iron regulatory peptide hepcidin, which results in increased iron absorption from the gastrointestinal (GI) tract and consequently iron overload.4 In non-transfused patients this is the primary mechanism for the development of iron overload. However, the use of blood transfusion is associated with a more severe and rapid onset of iron overload which on average is 40 fold faster (0.4 mg/kg/day)5 than from GI iron absorption in NTDT (0.01mg/kg/day).6 Transfusion therapy is initiated in patients with NTDT for intermittent severe anemia or to manage complications arising due to chronic anemia.7 In TDT, transfusion is generally initiated for failure to thrive in early childhood.8,9 Both the intensity of the transfusion frequency as well as the duration of high levels of tissue iron will impact on the development of complications.5,10
Iron is initially deposited in the liver, spleen and bone marrow and subsequently in endocrine organs once the liver iron is consistently above 7mg/g/dw. This can result in endocrine complications developing such as hypogonadotropic hypogonadism, hypothyroidism, hypoparathyroidism, adrenal and growth hormone insufficiency. Once the liver iron exceeds 15 mg/g/dw there is an increasing risk of cardiac iron deposition which may lead to the development of heart failure if chelation therapy is not intensified.10–14 Late sequelae such as pancreatic exocrine insufficiency and chronic or paroxysmal atrial fibrillation, liver cirrhosis and hepatocellular carcinoma may occur many years after tissue iron has been controlled.15–18
Management of Patients with Thalassaemia
Children may be diagnosed as part of antenatal screening or at some time after birth as part of screening programmes.19 In some patients with NTDT a diagnosis may not be made until well into adulthood.20 For TDT patients, regular transfusion is initiated when there is failure to thrive or a hemoglobin below 70g/l.21 NTDT patients may continue to grow and thrive with a moderately severe anaemia and occasionally need blood transfusion during intercurrent illness.22
Transfusions once initiated for TDT are given on a 3 to 4 weekly basis with the aim to maintain the pretransfusion hemoglobin level above 95g/l. Patients start iron chelation therapy (ICT) after 10–20 transfusions episodes or when ferritin levels are above 1000ug/l on least 2 occasions. Patients with TDT should have started ICT within 2 years of starting regular transfusion. IOL is often diagnosed late in NTDT patients because they slowly accumulate iron and are monitored less frequently.23
Monitoring of Iron Overload
Effectiveness of treatment (both transfusion and chelation) is assessed by routine monitoring. Pre transfusion haemoglobin levels provide assurance that the transfusion regime is providing sufficient suppression of the bone marrow to prevent hepatosplenomegaly and extramedullary haemopoiesis. Routine monitoring of iron is undertaken using serum ferritin levels.24 Historically quantification of iron burden was undertaken by liver biopsy and specialist analysis to measure iron in the biopsy sample.25 Since the early 2000ʹs magnetic resonance imaging techniques (MRI) have been used to quantify iron.26,27 This has allowed a greater understanding of how iron is distributed across organs and how iron is cleared from various organs.28–30
Iron Chelation Regimes
Three drugs are licensed for treatment of IOL. Their mechanism of action and indications are well described in the literature, and we recommend readers refer to these guidelines for further information.9,31 All the drugs can be used as a combination of two agents.
Deferoxamine
Deferoxamine was identified as a chelation agent in the early 1960s. Initially used intramuscularly, important studies in the 1970s showed that it was more effective as a subcutaneous infusion administered over 8 to 24 hours depending on the goal of chelation.32–35 If the aim is to maintain stable iron burden, then treatment is 8–12 hours subcutaneously and if the aim is to reduce iron burden particularly in the heart, then treatment is 24 hours intravenously.36,37 Subcutaneous infusions require a needle to be inserted into subcutaneous tissue on the abdomen or legs and are associated with side effects such as pain and swelling at the site of the infusion. Intravenous infusions require a central venous access device such as a port-a-cath or PiCC line.
Deferasirox
This is administered orally once daily and can be used as monotherapy or in combination with either of the drugs. This was initially available in dispersible formulation from 2006 but since 2016 has been available in film coated tablet formulation.38–40 Common side effects are nausea, vomiting and diarrhoea and abdominal pain.38,41 These are less frequent with the film coated preparation.42,43
Deferiprone
This is administered three times daily and is available in tablet and syrup formulations. Common side effects include gastrointestinal side effects such as nausea, vomiting, and diarrhoea, arthralgias and a low white cell count.44,45 The risk of a low neutrophil count requires weekly monitoring upon initiation and for the first year.
Patients are started on ICT generally after a year of regular transfusions. In children below the age of 2 years, chelation is initiated with deferoxamine and in older children with deferasirox. Deferiprone is offered second line in patients unable to comply with deferoxamine or deferasirox or in those with cardiac IOL. Clinical guidelines on ICT have been modified over the years to support a less prescriptive approach.8,21,46
Adherence to Treatment
The adherence literature is not specific to thalassaemia populations and further targeted research is required to understand the challenges of adherence in these patients. Nonetheless, adherence is a significant issue due to the lifelong nature of treatment and it is possible to draw upon literature from chronic medical conditions with notable disease burden when examining adherence in this population.
Adherence broadly refers to how closely a patient follows a prescribed treatment regimen. This includes their willingness to start treatment and ability to take medications exactly as directed. One in four patients do not adhere well to prescribed drug treatment.47 Patients who are non-adherent are more likely to experience worsening medical conditions, unnecessary complications, and higher rates of morbidity and mortality. However, non‑adherence is not the sole responsibility of a patient and represents a fundamental limitation in the delivery of Healthcare.48
The incidence of non-adherence in chronic conditions is greater with the introduction of new medications49 mainly resulting from patients’ experiences of problems when they start new medication, including side effects, concerns and practical aspects. There are two possible factors in non-adherence;49 first, healthcare professionals may provide insufficient information about medication and often make inappropriate judgements about patients’ expectations. Communication between doctors and patients can be poor leading to recall difficulties, low satisfaction and ultimately reduced adherence. Second, after patients start to use new medication, they will have experienced side effects, and may have beliefs, concerns or uncertainties that need to be addressed. Consequently, patients may sometimes be let down by either unresponsive healthcare services or conflicting advice from primary and secondary care physicians and therefore may consult with friends or relatives who may offer incorrect information or propagate false beliefs that encourage non-adherence.
Adherence to medication is a multidimensional phenomenon that is affected by patient-related, therapy-related, condition-related and demographic factors. Patient-related factors include patient knowledge, patient/caregiver education, psychological factors, attitudes, beliefs, perceptions of severity of disease and expectations from a treatment. Therapy-related factors include factors such as frequency of dosage and complexity of regimen.
Patient adherence to a chelation regimen could differ depending on the influence of modifying factors that have been shown to correlate with adherence across a range of health conditions such as underlying psychological problems including depression, and the negative beliefs and assumptions that can result.50–52 Additional factors that impact adherence include relational processes, such as the patient-clinician alliance53 and positive, effective communication.54 Adherence interventions can actively target these modifying factors, which can be easily overlooked in clinical practice due to service-related constraints. For instance, conducting psychological wellbeing screening, exploring idiosyncratic attitudes and beliefs with patients, and engaging in active attempts to address any challenges to developing and sustaining trusting and supportive patient-clinician partnerships.
Adherence can be observed in 3 stages: initiation, implementation, and discontinuation. Initiation is where a patient takes the first dose of medication; implementation is the extent to which a patient follows the treatment regimen; discontinuation is when a patient stops taking medication.55,56 The multidimensional adherence phenomenon demonstrated in thalassemia studies also includes difficulties in treatment administration as related to demographic, patient-related and therapy-related factors. Largely, better adherence has been reported with deferasirox compared to deferoxamine with pain at the site of injection being stated as the most common reason for non-adherence with deferoxamine.57–62 In terms of TDT demographics, older age was associated with lower levels of chelation adherence.63 Improved knowledge and education had a positive outcome on adherence.64–66 Important psychological factors that influence non-adherence include poor quality of life and social stigma.67,68
Psychological theory can be utilised to help understand adherence and offer interventions. Studies of interventions intended to improve adherence and clinical outcomes across various health conditions have been found to be complex and with limited effectiveness.69 Conversely, a meta-analysis by Holmes and colleagues,70 found that multiple and extended models of adherence improved predictions of adherence and concluded that “no single theory should be used to inform the development of adherence enhancing interventions”. In addition, the recognition of the multidimensional adherence phenomenon and distal factors, such as general beliefs about self-efficacy, perceived health locus of control as well as personality traits, have been identified as playing a significant role in influencing treatment adherence.70,71 Thus, clinical approaches that aim to address adherence should include interventions with multiple components that are informed by theoretical, and evidence-based models. Additionally, such interventions should pay particular attention to components from health psychology models of adherence that go beyond practical, therapy-related factors. We consider three psychological models and a relational theory.
Adherence Models
Medication Adherence Model
This model explains two types of non-adherence, and how these contribute to inconsistent medication use. That is, intentional decisions to omit medications, and unintentional interruptions that cause medications not to be taken as prescribed.72 Furthermore, three core concepts are defined in this model, namely Purposeful Action, Patterned Behaviour, and Feedback.72 Purposeful action is based on the patient’s perceived need, effectiveness, and safety. For example: the need for a particular type of ICT; whether it reduces IOL in an individual; and its side-effect profile. Patterned behaviour is required for the patient to establish medication-taking patterns through availability, routines (eg at breakfast), and ability to remember (eg use of a dosette box or phone alarm). Feedback for the patient is important and tends to influence purposeful action and patterned behaviour. This should be given through information, prompts, and during occasions when treatment evaluation takes place.
Health Belief Model
The Health Belief Model,73,74 offers a psychological framework to explain and predict health behaviours by focusing on the attitudes and beliefs of individuals (Figure 1).75 Applied to thalassaemia, this model is based on the understanding that a patient will have a health-related behaviour (use of ICT); if that patient feels that a harmful health condition (IOL) can be avoided (using ICT will be effective in avoiding IOL) and believes that they can successfully take a recommended health action (can use ICT comfortably and with confidence).
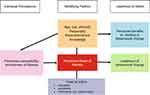 |
Figure 1 Health Belief Model. Notes: Adapted with permission from John Wiley and Sons. Glanz K, Rimer BK, Lewis FM. Health Behavior and Health Education. Theory, Research and Practice. San Francisco: Jossey-Bass; 2002; permission conveyed through Copyright Clearance Center, Inc.75 |
Within the context of this model, common-sense models of illness have been referred to as illness cognitions, illness representations, or prototypes. These have been studied in a variety of chronic illnesses.76–78
Beliefs regarding the seriousness of the condition may also act to encourage or discourage healthy behaviours. For example, if a patient does not accept that they have a serious problem with IOL, they may not be inclined to take appropriate action to attenuate the progress of the disease. However, the likelihood of engaging in these behaviours is also potentially influenced by beliefs that they can affect their health, and that they have the resources to deal with the problem ie, personal control and self-efficacy belief. Therefore, the notion of being able to exert control over the course of an individual’s illness is an important cognition.
Taken together, these demonstrate the importance of psychological factors in addition to clinical variables when the long-term effects of adherence to ICT are considered.
Three-Factor Interventional Model
This model comprises three important clinical actions.71 First, ensuring that patients have the correct information and ability to enable them to adhere to treatment. This includes listening to patients’ concerns, encouraging their participation and relationship in decision-making, building Trust and empathy, and enhancing recall. Second, helping patients’ believe in their treatment and become motivated to commit to it. That is, addressing their reasoning, together with social, cultural norms and circumstantial factors (eg, stigma) that affect patients’ beliefs, attitudes, and motivation. Third, assisting patients to overcome practical barriers to treatment adherence and develop a workable strategy for long-term disease management. This includes assessing and enhancing patients’ social support, identifying and establishing treatment plans to treat or manage any mental health problems (eg, anxiety, depression, psychosis, personality disorders) and helping patients overcome cost-related treatment barriers (eg, unaffordable prescription charges or lack of health insurance).
Attachment Theory
Effective communication is a key component of a good patient-clinician alliance.53 Patient reported trust in the relationship with clinicians79,80 can play an important role in determining treatment outcomes such as satisfaction with treatment, psychosocial wellbeing, quality of life indicators and treatment adherence.81 If a patient is unable to share concerns openly with their healthcare professional, then practical and behavioural goals involved in disease management between patient and clinician may become misaligned and pose a risk to shared decision-making and, ultimately, to adherence.
Attachment theory offers an opportunity to assess, understand and improve patient-clinician relationship quality.82,83 It provides a standardised way of understanding interpersonal relationship patterns that are commonly explored in patient-therapist relationships in psychological therapies. Attachment theory posits that all individuals derive their first “internal working model” of relationships with the self and others based on early caregiving relationships. Learning occurs within this model, predominantly learning ways to reduce distress and create a sense of safety in relationships. The internal working model goes on to inform our adult relationship patterns and has been extensively researched across the lifespan to identify four prototypes of adult attachment styles (secure, insecure dismissive-avoidant, insecure preoccupied, insecure fearful-avoidant). These sit along two continua which are the view of self and view of others, ranging from positive to negative.84–86
A secure attachment style is characterised by a positive model of oneself and others, suggesting that such individuals have confidence in themselves (self-esteem), are trusting of others and are open to intimacy and closeness in relationships. The three insecure attachment styles have some degree of negative models of either self or others. A dismissive-avoidant style suggests individuals have a positive model of oneself but a negative model of others leading to avoidance of closeness and intimacy and high levels of self-reliance. A preoccupied style is indicative of a positive model of others but a negative model of oneself resulting in a strong desire for closeness and intimacy but a fear or lack of trust that this will be available. A fearful-avoidant style reflects individuals with a negative model of themselves and of others; such individuals have a conflict between a strong desire for intimacy and closeness and simultaneously an intense fear of intimacy. This is compounded by a lack of trust that this will be available, and thus avoidance of attachments. A clear strategy for coping with negative relational models can be identified among individuals with dismissive-avoidant and preoccupied attachment styles, however for fearful-avoidant styles there is an absence of strategy to cope with dual negative relational models.87
Although there can be a predominant attachment style, individuals can vary in how they categorise themselves and may have different attachment relationships with different people. Thus, a dimensional (rather than categorical) approach is a preferable way to view attachment styles whereby there is a focus on low or high scores for measures of dependence (self-models that map onto relational anxiety) and degree intimacy/ closeness to others (other-models that map onto relational avoidance).87 Attachment relationship patterns can also change over time and there is evidence of “attachment reorganisation”, for those with insecure attachment styles, through the establishment of supportive relationships and changes in social roles that can happen at times of transition.85,88
When applied to the patient-clinician relationship, the attachment model can be used to adequately identify and support patients with relational challenges. For instance, Ciechanowski and colleagues discovered that, after adjusting for demographic and clinical variables, diabetes patients with dismissive attachment styles showed poorer adherence than those with other attachment styles, based on measures of HBA1c, use of oral hypoglycaemic medications and glucose-monitoring.89 Additionally, adherence was poorer among those with dismissive attachment styles when the quality of patient-provider communication was also accounted for. They also found that those with secure attachment styles showed better self-management and outcomes.90 Evidence from literature relating to patients with health conditions such as diabetes,89–91 HIV92 functional somatic disorders93 and systemic lupus erythematosus81 demonstrate the powerful role that attachment relationships can play in health management and adherence.
Patients’ attachment styles (and attempted coping strategies) can be triggered by relational processes, such as perceived threats to a sense of safety in self. Other-relational models may impact on attachment styles as well, such as threats to comfortable levels of dependence and intimacy which rely upon support from and contact with carers, family members, friends and/ or healthcare clinicians.
There are simple self-report measures that can be used to establish attachment styles; the simple Relationship Questionnaire85 which can be used in a healthcare settings to allow for guided discussions and the 30-item Relationship Scales Questionnaire.94 Both items can map onto the 4-prototype, 2-continua model to derive categorical or continuum scores.95
Modifications to the patient-clinician partnership as proposed in Table 1 can support adherence through active attempts to understand and attend to attachment style and the patient-clinician partnership.
 |
Table 1 Attachment Styles, Attempted Coping (Behaviour) and Strategies for Health Care Professionals |
Attachment Styles, Attempted Coping (Behaviour) and Strategies for Health Care Professionals
We will now consider the clinical application of attachment theory and these psychological models of adherence as applied to two hypothetical patient case studies. Both studies include commonly encountered scenarios.
Psychological Models and Theory in Clinical Practice
Illustrative Case Study 1
This young man was from an Asian family with significant stigma and shame related to the diagnosis of thalassaemia resulting in non-disclosure to the wider family. His mother had limited understanding of the disorder and struggled emotionally with the burden of care required. He started ICT at 18 months with deferoxamine, however the family really struggled with needle insertion and administration. As a teenager the absence of self-efficacy to treatment resulted in severe IOL because he lacked core knowledge about the importance of treatment due to suboptimal communication by his parents and healthcare professionals. He has an episode of heart failure and a port-a cath was inserted and he was initiated on combination ICT. He developed insulin dependent diabetes and had several episodes of recurrent line infections requiring port-a-caths changes and developed severe hypoparathyroidism and hypothyroidism. His IOL, diabetes and endocrinopathies remained very poorly controlled due to poor adherence and he continued deferoxamine with deferiprone until deferasirox was licenced in 2006. He was started on deferasirox, but adherence was poor as he did not like the taste of the dispersible formulation and was switched back to deferoxamine and deferiprone combination. He then had several admissions with heart failure, diabetic ketoacidosis and profound depression. His family relationships deteriorated severely which correlated with escalating depressive symptoms with deeply established negative thoughts about his thalassaemia and future health. He refused clinical psychological intervention apart from during inpatient admissions.
Over the years, he received multiple educational sessions with his doctor and specialist nurse in the adult setting. However, this had limited effect and he was only clinically optimised when he was on a 6-month inpatient admission and received direct help with health professionals administering his treatments.
Understanding and Altering Patterns of Behaviour
Medication Adherence Model
There is evidence of unintentional interruptions to chelation adherence in early life, with low adherence in teenage years (intentional interruptions).
Factors influencing such behaviour may include patient perceptions and depressive beliefs (eg, negative predictions, hopelessness and helplessness) about the need for ICT, with limited opportunity for balanced and open feedback about its effectiveness and drawbacks facilitated by professionals trained to work with depressive illness narratives. Additionally, family and health service factors seem to have contributed to medication initiation with a lack of implementation of routine, patterned behaviour early on in life.
With patients presenting in this way, possible methods for enhancing adherence include offering evaluative discussions about treatment effectiveness, including feedback from periods when treatment is and is not optimised, and introducing simple routines that maximise remembering to take medications and minimise practical issues related to access to medications.
Health Belief and Three Factor Interventional Models
The patient and family perceptions of the severity and life-threatening complications of thalassaemia seem to be unvoiced or there is no evidence of a shared understanding of this. These models highlight the importance of creating opportunities for open dialogue to explore and establish shared understandings about disease severity as linked to different chelation therapies and the perceived benefits or likelihood of changes in disease severity. Education is a feature which was offered to this patient as an adult by a haematologist and specialist nurse. This could have been maximised with additional and repeated non-judgemental discussions with the patient and supportive family members. If this intervention had been undertaken early on in paediatric settings (ideally as early as late childhood, pre-adolescence) then this may have bolstered self-efficacy (through influencing illness beliefs such as perceived severity, modifying treatment beliefs and attitudes and encouraging commitment to making changes based on this information). This may have mitigated the development of negative perceptions about the thalassaemia diagnosis. For patients with this presentation, such discussions can focus on progression of symptoms and severe complications, in particular understandings of mechanisms and advantages and disadvantages to making changes to adherence behaviours (in the style of motivational interviewing). The aim is to target the removal of potential barriers to change, once one has some clarity about what these barriers are.
An additional step could be to introduce and explore the pros and cons of referring the patient for individualised psychological therapy, as well as considering this in relation to other forms of social and therapeutic support, such as community activities and support groups, to address mood problems in the absence of formal therapy.
Attachment Theory
A notable feature of this presentation indicates negative models of self (depression, low self-esteem, low self-efficacy, and confidence regarding treatment initiation and maintenance). Passive receipt of help with chelation from caregivers and loved ones is also suggestive of positive models of others. This is indicative of a high intimacy/ low relational avoidance and high dependence/ high relational anxiety attachment pattern (insecure-preoccupied style) which may benefit from consistent and regular contact, actively seeking opportunities for engagement with peer or social communities and opportunities for success, reward or other forms of positive reinforcement related to health management, alongside other valued life areas.
Illustrative Case Study 2
This young man developed cardiac failure at a young age requiring admission and intensive combination ICT. He was one of a large family of siblings with one other thalassaemia major sibling. The family was socially very active with a large community network. His thalassaemia has never been hidden and his parents had a positive approach to supporting their children. However, the family’s social activities probably resulted in unintentional adherence issues with deferoxamine infusions in early and late childhood. The cardiac failure reversed, and he continued intravenous chelation through the port-a-cath at home. His liver iron became well controlled but myocardial iron remained severe. His adherence as a teenager was poor despite efforts by family and friends to support him. His psychological shift during adolescence was towards being exactly like his siblings and friends with a busy social life and playing sports. He was switched to dual oral combination to help adherence, but this remained suboptimal despite extensive support from community counsellors, healthcare providers as well as his wider social network. From the age of 20 his adherence improved and was associated with his older TDT sibling having a child which impacted on his understanding of the reality of an almost normal life. His focus shifted to having a family of his own. A better understanding of risks of IOL and the use of oral ICT altered to suit his work and routine have helped improve adherence. His marriage made a very positive impact on his adherence to treatment.
Understanding and Altering Patterns of Behaviour
Medication Adherence Model
In this case, there is evidence of both intentional and unintentional interruptions to chelation adherence in early life with notable family lifestyle factors impacting on the establishment of medication adherence patterns and routines.
Factors influencing behaviour may include family and patient perceptions about the importance, effectiveness and need for regular and routine chelation and minimising of the risks associated with adherence which may have been influenced by feedback from the patient’s only other sibling with thalassaemia, who did not progress to develop cardiac failure.
For patients illustrated with this case, early introduction of methods to minimise unintentional interruptions to medication use can be an important intervention. An additional focus on information provision and feedback relating to treatment optimisation effects, and implications may also have an impact on adherence.
Health Belief Model and Three Factor Interventional Model
Furthermore, patient and family perceptions of severity and life-threatening complications from thalassaemia are targets for adherence-related behaviour change along with the perceived severity of thalassaemia and lifestyle factors that impact upon barriers to recommended treatment regimens.
For patients with this presentation, adherence may be influenced via a focus on exploring, evaluating, and altering treatment beliefs and attitudes as well as increasing motivation and commitment to treatment. This took place in this example, whereby the patient’s desire and goal to start a family became a key motivation for adherence, and crucial to behavioural change. In addition, adherence may be influenced through addressing barriers to treatment through simplifying treatment regimens, enhancing social support along with recall and memory cues for promoting adherence. Patient collaboration with open dialogue, exploration of the benefits and barriers to treatments and written action plans are simple ways to invite and seek to elaborate on existing beliefs and attitudes towards adherence-related changes.
Attachment Theory
There is some evidence of positive models of others in this patient’s presentation, with acceptance of support from both his health team and his partner suggesting high intimacy/low relational avoidance. There is also some evidence of this coupled with a negative model of self (high dependency/ high relational anxiety) due to a possible focus on the desire not to appear different from his healthy siblings and friends; if this relational anxiety is notably high for patients with this presentation, then this combined with a positive model of others (high intimacy/low relational avoidance) may be indicative of an insecure-preoccupied style of attachment. Similar to the patient in Case Study 1, helpful interventions for this patient may include: consistent and regular contact with opportunities for developing self-efficacy and confidence with health management (eg positive reinforcement) and actively seeking opportunities for engagement with peer or social communities; particularly where there is contact with role models who focus on routine thalassaemia treatment adherence as well as having values in line with the patient (eg in this case having active social and sporty lifestyles or starting families).
If this patient presentation combined a positive model of others (high intimacy/ low relational avoidance) with a positive model of self (low dependence/ low relational anxiety), then the practical interventions, focused on simplifying treatment regimens, reducing barriers to treatment associated with lifestyle and addressing treatment beliefs and motivations, may be helpful adherence-related targets.
Additional Considerations
Psychological difficulties can range from common mental health problems such as mood problems and anxiety right the way through to severe mental illness such as psychosis and personality disorders. The prevalence of mental health problems among thalassaemia populations has been documented by several studies worldwide.96–100 As illustrated in the example of Case Study 1, untreated mental health problems can indirectly influence adherence and result in increased healthcare utilisation and costs.97 Facilitating access to psychological interventions and/or treatment is an important consideration when attempting to improve adherence. This may require discussing any possible hesitation, benefits, or barriers to engaging with psychological approaches with patients. It may also require making referrals to appropriate psychological therapies services and/or seeking consultation and advice from psychological therapists and mental health services.
Working with patients with complexity in their disease presentations and related treatment adherence may include work with patients with disease- and transfusion-related complications, comorbid mental health problems, personality traits or anxious and avoidant relational patterns. Such work can be emotionally and cognitively demanding as well as require time, thoughtfulness, and patience. Our own relational patterns can become triggered, our own personality traits or emotional needs may inadvertently become challenges to the effective application of methods to enhance adherence for patients. Taking time with colleagues to engage in regular reflective practice facilitated by a psychological therapist or taking up opportunities for individual reflection on our own thoughts, emotional reactions and actions is essential to offering comprehensive adherence-related support to patients.
Conclusion
Thalassaemia is a complex lifelong disorder and successful therapy involves adherence to complex regimes which are associated with side effects. IOL complications create more complexity of treatment and that then further impacts on adherence both for the pre-existing thalassaemia and from the treatment of the complications. Understanding the factors that impact on adherence to treatment for individuals is critical in ensuring good outcomes. Working in a multi-professional team aids communication and different approaches to be used for patients to help improve their self-efficacy. Psychological support for families from the time of diagnosis to empower them to provide care for their child has a major impact in ensuring a good foundation for lifelong adherence to treatment.
Disclosure
Dr Farrukh T Shah reports personal fees from Celgene International, Bluebird bio Inc, Novartis Pharma AG, Vertex Pharmaceuticals, Chiesi Ltd, Bristol Myers Squibb Pharmaceuticals Ltd, Silence Therapeutics plc, and Agios Pharmaceuticals Ltd, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Taher AT, Saliba AN. Iron overload in thalassemia: different organs at different rates. Hematology. 2017;2017(1):265–271. doi:10.1182/asheducation-2017.1.265
2. Piomelli SKM, Arzanian M, Zamani M, et al. Hypertransfusion regimen in patients with Cooley’s anemia. Ann N Y Acad Sci. 1974;232:186–192. doi:10.1111/j.1749-6632.1974.tb20584.x
3. Cazzola M, De Stefano P, Ponchio L, et al. Relationship between transfusion regimen and suppression of erythropoiesis in beta-thalassaemia major. Br J Haematol. 1995;89(3):473–478. doi:10.1111/j.1365-2141.1995.tb08351.x
4. Gardenghi S, Marongiu MF, Ramos P, et al. Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood. 2007;109(11):5027–5035. doi:10.1182/blood-2006-09-048868
5. Cohen AR, Glimm E, Porter JB. Effect of transfusional iron intake on response to chelation therapy in beta-thalassemia major. Blood. 2008;111(2):583–587. doi:10.1182/blood-2007-08-109306
6. Taher AT, Porter J, Viprakasit V, et al. Deferasirox reduces iron overload significantly in nontransfusion-dependent thalassemia: 1-year results from a prospective, randomized, double-blind, placebo-controlled study. Blood. 2012;120(5):970–977. doi:10.1182/blood-2012-02-412692
7. Taher AT, Musallam KM, Cappellini MD. Thalassaemia intermedia: an update. Mediterr J Hematol Infect Dis. 2009;1(1):e2009004. doi:10.4084/MJHID.2009.004
8. Yardumian A, Telfer PT, Darbyshire PJ. Standards for the Clinical Care of Children and Adults with Thalassaemia in Th UK.
9. Cappellini DM, Cohen A, Porter J, Taher A, Viprakasit V. Guidelines for the Management of Transfusion Dependent Thalassaemia (TDT). In: Federation TI, editor. Guidelines for the Management of Transfusion Dependent Thalassaemia (TDT). Nicosia (CY): Thalassaemia International Federation (c) 2014 Thalassaemia International Federation; 2014.
10. Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997;89(3):739–761.
11. Jensen CE, Tuck SM, Old J, et al. Incidence of endocrine complications and clinical disease severity related to genotype analysis and iron overload in patients with beta-thalassaemia. Eur J Haematol. 1997;59(2):76–81. doi:10.1111/j.1600-0609.1997.tb00729.x
12. Toumba M, Sergis A, Kanaris C, Skordis N. Endocrine complications in patients with Thalassaemia Major. Pediatric Endocrinol Rev. 2007;5(2):642–648.
13. Belhoul KM, Bakir ML, Kadhim AM, Dewedar HE, Eldin MS, Alkhaja FA. Prevalence of iron overload complications among patients with b-thalassemia major treated at Dubai Thalassemia Centre. Ann Saudi Med. 2013;33(1):18–21. doi:10.5144/0256-4947.2013.18
14. Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22(23):2171–2179. doi:10.1053/euhj.2001.2822
15. Moukhadder HM, Halawi R, Cappellini MD, Taher AT. Hepatocellular carcinoma as an emerging morbidity in the thalassemia syndromes: a comprehensive review. Cancer. 2016;123(5):751–758. doi:10.1002/cncr.30462
16. Yamamura J, Grosse R, Jarisch A, et al. Pancreatic exocrine function and cardiac iron in patients with iron overload and with thalassemia. Pediatr Blood Cancer. 2011;57(4):674–676. doi:10.1002/pbc.22990
17. Soliman A, De Sanctis V, Elsedfy H, et al. Growth hormone deficiency in adults with thalassemia: an overview and the I-CET recommendations. Georgian Med News. 2013;3(222):79–88.
18. Walker JM. Thalassaemia major and the heart: a toxic cardiomyopathy tamed? Heart. 2013;99(12):827–834. doi:10.1136/heartjnl-2012-302857
19. Ryan K, Bain BJ, Worthington D, et al. Significant haemoglobinopathies: guidelines for screening and diagnosis. Br J Haematol. 2010;149(1):35–49. doi:10.1111/j.1365-2141.2009.08054.x
20. Premawardhena A, Fisher CA, Olivieri NF, et al. Haemoglobin E beta thalassaemia in Sri Lanka. Lancet. 2005;366(9495):1467–1470. doi:10.1016/S0140-6736(05)67396-5
21. Yardumian A, Telfer PT, Shah F, et al. Standards for the Clinical Care of Children and Adults with Thalassaemia in Th UK.
22. Taher AT, Musallam KM, Cappellini MD, Weatherall DJ. Optimal management of beta thalassaemia intermedia. Br J Haematol. 2011;152(5):512–523. doi:10.1111/j.1365-2141.2010.08486.x
23. Taher AT, Viprakasit V, Musallam KM, Cappellini MD. Treating iron overload in patients with non-transfusion-dependent thalassemia. Am J Hematol. 2013;88(5):409–415. doi:10.1002/ajh.23405
24. Olivieri NF, Koren G, Matsui D, et al. Reduction of tissue iron stores and normalization of serum ferritin during treatment with the oral iron chelator L1 in thalassemia intermedia. Blood. 1992;79(10):2741–2748. doi:10.1182/blood.V79.10.2741.bloodjournal79102741
25. Barry M. Liver Iron Concentration, Stainable Iron, and Total Body Iron Storage Iron. Gut. 1974;15:411–415. doi:10.1136/gut.15.5.411
26. Chan YL, Li CK, Lam CWK, et al. Liver Iron Estimation in [beta]-thalassaemia: comparison of MRI Biochemical Assay and Histological Grading. Clin Radiol. 2001;56(11):911–916. doi:10.1053/crad.2001.0777
27. Macarini L, Marini S, Pietrapertosa A, Scardapane A, Ettorre GC. Non cardiopatic and cardiopatic beta thalassemic patients: quantitative and qualitative cardiac iron deposition evaluation with MRI. Radiol Med. 2005;109(1–2):77–90.
28. Anderson LJ, Westwood MA, Holden S, et al. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br J Haematol. 2004;127(3):348–355. doi:10.1111/j.1365-2141.2004.05202.x
29. Lai ME, Grady RW, Vacquer S, et al. Increased survival and reversion of iron-induced cardiac disease in patients with thalassemia major receiving intensive combined chelation therapy as compared to desferoxamine alone. Blood Cells Mol Dis. 2010;45(2):136–139. doi:10.1016/j.bcmd.2010.05.005
30. Bonifazi F, Conte R, Baiardi P, et al. Pattern of complications and burden of disease in patients affected by beta thalassemia major. Curr Med Res Opin. 2017;33(8):1525–1533. doi:10.1080/03007995.2017.1326890
31. Porter JB, Shah FT. Iron overload in thalassemia and related conditions: therapeutic goals and assessment of response to chelation therapies. Hematol Oncol Clin North Am. 2010;24(6):1109–1130. doi:10.1016/j.hoc.2010.08.015
32. Letsky EA, Barry M. The effect of treatment with long-term chelating agents on iron overload in thalassaemia. Br J Haematol. 1973;25(2):285.
33. Barry MFD, Letsky EA, Risdon RA. Long-term chelation therapy in thalassaemia major: effect on liver iron concentration, liver histology, and clinical progress. Br Med J. 1974;2:16–20. doi:10.1136/bmj.2.5909.16
34. Cohen A, Schwartz E. Iron chelation therapy with deferoxamine in Cooley anemia. J Pediatr. 1978;92(4):643–647. doi:10.1016/S0022-3476(78)80314-X
35. Carlos AG, Soares AD. The Use of Desferrioxamine in the Treatment of Hemochromatosis. J Med. 1964;53:1021–1032.
36. Davis BA, O’Sullivan C, Jarritt PH, Porter JB. Value of sequential monitoring of left ventricular ejection fraction in the management of thalassemia major. Blood. 2004;104(1):263–269. doi:10.1182/blood-2003-08-2841
37. Davis BA, Porter JB. Results of long term iron chelation treatment with deferoxamine. Adv Exp Med Biol. 2002;509:91–125.
38. Cappellini MD, Cohen A, Piga A, et al. A Phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood. 2006;107(9):3455–3462. doi:10.1182/blood-2005-08-3430
39. Vichinsky E, Onyekwere O, Porter J, et al. A randomised comparison of deferasirox versus deferoxamine for the treatment of transfusional iron overload in sickle cell disease. Br J Haematol. 2007;136(3):501–508. doi:10.1111/j.1365-2141.2006.06455.x
40. Taher AT, Origa R, Perrotta S, et al. New film-coated tablet formulation of deferasirox is well tolerated in patients with thalassemia or lower-risk MDS: results of the randomized, Phase II ECLIPSE study. Am J Hematol. 2017;92(5):420–428. doi:10.1002/ajh.24668
41. Taher AT, Temraz S, Cappellini MD. Deferasirox for the treatment of iron overload in non-transfusion-dependent thalassemia. Expert Rev Hematol. 2013;6(5):495–509. doi:10.1586/17474086.2013.827411
42. Tartaglione I, Origa R, Kattamis A, et al. Two-year long safety and efficacy of deferasirox film-coated tablets in patients with thalassemia or lower/intermediate risk MDS: phase 3 results from a subset of patients previously treated with deferasirox in the ECLIPSE study. Exp Hematol Oncol. 2020;9:20. doi:10.1186/s40164-020-00174-2
43. Taher AT, Origa R, Perrotta S, et al. Patient-reported outcomes from a randomized phase II study of the deferasirox film-coated tablet in patients with transfusion-dependent anemias. Health Qual Life Outcomes. 2018;16(1):216. doi:10.1186/s12955-018-1041-5
44. Cohen A, Galanello R, Piga A, Vullo C, Tricta F. A multi-center safety trial of the oral iron chelator deferiprone. Ann N Y Acad Sci. 1998;850:223–226. doi:10.1111/j.1749-6632.1998.tb10478.x
45. Ceci A, Baiardi P, Felisi M, et al. The safety and effectiveness of deferiprone in a large-scale, 3-year study in Italian patients. Br J Haematol. 2002;118(1):330–336. doi:10.1046/j.1365-2141.2002.03554.x
46. Shah FT, Porter JB, Sadasivam N, et al. Guidelines for the monitoring and management of iron overload in patients with haemoglobinopathies and rare anaemias. Br J Haematol. 2021;196(2):336–350. doi:10.1111/bjh.17839
47. DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. doi:10.1097/01.mlr.0000114908.90348.f9
48. NICE. Medicines Adherence: Involving Patients in Decisions About Prescribed Medicines and Supporting Adherence [NICE Guideline No CG76]. NICE; 2009.
49. Barber N, Parsons J, Clifford S, Darracott R, Horne R. Patients’ problems with new medication for chronic conditions. Qual Saf Health Care. 2004;13(3):172–175. doi:10.1136/qshc.2003.005926
50. Chao J, Nau DP, Aikens JE, Taylor SD. The mediating role of health beliefs in the relationship between depressive symptoms and medication adherence in persons with diabetes. Res Social Adm Pharm. 2005;1(4):508–525. doi:10.1016/j.sapharm.2005.09.002
51. Manning M, Bettencourt BA. Depression and medication adherence among breast cancer survivors: bridging the gap with the theory of planned behaviour. Psychol Health. 2011;26(9):1173–1187. doi:10.1080/08870446.2010.542815
52. Tang HY, Sayers SL, Weissinger G, Riegel B. The role of depression in medication adherence among heart failure patients. Clin Nurs Res. 2014;23(3):231–244. doi:10.1177/1054773813481801
53. Trevino KM, Fasciano K, Prigerson HG. Patient-oncologist alliance, psychosocial well-being, and treatment adherence among young adults with advanced cancer. J Clin Oncol. 2013;31(13):1683–1689. doi:10.1200/JCO.2012.46.7993
54. Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47(8):826–834. doi:10.1097/MLR.0b013e31819a5acc
55. Blaschke TF, Osterberg L, Vrijens B, Urquhart J. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annu Rev Pharmacol Toxicol. 2012;52:275–301. doi:10.1146/annurev-pharmtox-011711-113247
56. Vrijens B, Urquhart J. Methods for measuring, enhancing, and accounting for medication adherence in clinical trials. Clin Pharmacol Ther. 2014;95(6):617–626. doi:10.1038/clpt.2014.59
57. Delea TE, Edelsberg J, Sofrygin O, et al. Consequences and costs of noncompliance with iron chelation therapy in patients with transfusion-dependent thalassemia: a literature review. Transfusion. 2007;47(10):1919–1929. doi:10.1111/j.1537-2995.2007.01416.x
58. Trachtenberg F, Vichinsky E, Haines D, et al. Iron chelation adherence to deferoxamine and deferasirox in thalassemia. Am J Hematol. 2011;86(5):433–436. doi:10.1002/ajh.21993
59. Goulas V, Kourakli-Symeonidis A, Camoutsis C. Comparative effects of three iron chelation therapies on the quality of life of Greek patients with homozygous transfusion-dependent Beta-thalassemia. ISRN Hematol. 2012;2012:139862. doi:10.5402/2012/139862
60. Porter J, Bowden DK, Economou M, et al. Health-Related Quality of Life, Treatment Satisfaction, Adherence and Persistence in β-Thalassemia and Myelodysplastic Syndrome Patients with Iron Overload Receiving Deferasirox: results from the EPIC Clinical Trial. Anemia. 2012;2012:297641. doi:10.1155/2012/297641
61. Rashid M, Karimi M. Compliance of deferoxamine injection in beta-thalassaemia major patients in Iran. Transfus Med. 2012;22(2):104–107. doi:10.1111/j.1365-3148.2011.01130.x
62. Haghpanah S, Zarei T, Zahedi Z, Karimi M. Compliance and satisfaction with deferasirox (Exjade®) compared with deferoxamine in patients with transfusion-dependent beta-thalassemia. Hematology. 2014;19(4):187–191. doi:10.1179/1607845413Y.0000000121
63. Evangeli M, Mughal K, Porter JB. Which psychosocial factors are related to chelation adherence in thalassemia? A systematic review. Hemoglobin. 2010;34(3):305–321. doi:10.3109/03630269.2010.485080
64. Lee YL, Lin DT, Tsai SF. Disease knowledge and treatment adherence among patients with thalassemia major and their mothers in Taiwan. J Clin Nurs. 2009;18(4):529–538. doi:10.1111/j.1365-2702.2007.02150.x
65. Trachtenberg FL, Mednick L, Kwiatkowski JL, et al. Beliefs about chelation among thalassemia patients. Health Qual Life Outcomes. 2012;10:148. doi:10.1186/1477-7525-10-148
66. Jeesh YAA, Yousif ME, Al-Haboub MA. The Effects of Patients’ and Care-Givers’ Knowledge, Attitude, & Practice (KAP) on Quality of Life Among Thalassemia Major Patients’ in Damascus-Syrian Arab Republic. Eur Sci J. 2018;14:12. doi:10.19044/esj.2018.v14n12p308
67. Trachtenberg FL, Gerstenberger E, Xu Y, et al. Relationship among chelator adherence, change in chelators, and quality of life in thalassemia. Qual Life Res. 2014;23(8):2277–2288. doi:10.1007/s11136-014-0671-2
68. Vosper J, Evangeli M, Porter JB, Shah F. Psychological Factors Associated with Episodic Chelation Adherence in Thalassemia. Hemoglobin. 2018;42(1):30–36. doi:10.1080/03630269.2018.1433686
69. Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Sys Rev. 2014;2014(11):Cd000011.
70. Holmes EA, Hughes DA, Morrison VL. Predicting adherence to medications using health psychology theories: a systematic review of 20 years of empirical research. Value Health. 2014;17(8):863–876. doi:10.1016/j.jval.2014.08.2671
71. DiMatteo MR, Haskard-Zolnierek KB, Martin LR. Improving patient adherence: a three-factor model to guide practice. Health Psychol Rev. 2012;6(1):74–91. doi:10.1080/17437199.2010.537592
72. Johnson MJ. The Medication Adherence Model: a guide for assessing medication taking. Res Theory Nurs Pract. 2002;16(3):179–192. doi:10.1891/rtnp.16.3.179.53008
73. Becker MH. The Health Belief Model and Sick Role Behavior. Health Educ Monogr. 1974;2(4):409–419. doi:10.1177/109019817400200407
74. Rosenstock IM. The Health Belief Model and Preventive Health Behavior. Health Educ Monogr. 1974;2(4):354–386. doi:10.1177/109019817400200405
75. Glanz K, Rimer BK, Lewis FM. Health Behavior and Health Education. Theory, Research and Practice. San Francisco: Jossey-Bass/Wiley and Sons; 2002.
76. Becker MH, Radius SM, Rosenstock IM, Drachman RH, Schuberth KC, Teets KC. Compliance with a medical regimen for asthma: a test of the health belief model. Public Health Rep. 1978;93(3):268–277.
77. Rodin J, Salovey P. Health psychology. Annu Rev Psychol. 1989;40:533–579. doi:10.1146/annurev.ps.40.020189.002533
78. Bender BG. Risk taking, depression, adherence, and symptom control in adolescents and young adults with asthma. Am J Respir Crit Care Med. 2006;173(9):953–957. doi:10.1164/rccm.200511-1706PP
79. Hillen MA, de Haes HC, Stalpers LJ, et al. How attachment style and locus of control influence patients’ trust in their oncologist. J Psychosom Res. 2014;76(3):221–226. doi:10.1016/j.jpsychores.2013.11.014
80. Birkhäuer J, Gaab J, Kossowsky J, et al. Trust in the health care professional and health outcome: a meta-analysis. PLoS One. 2017;12(2):e0170988. doi:10.1371/journal.pone.0170988
81. Bennett JK, Fuertes JN, Keitel M, Phillips R. The role of patient attachment and working alliance on patient adherence, satisfaction, and health-related quality of life in lupus treatment. Patient Educ Couns. 2011;85(1):53–59. doi:10.1016/j.pec.2010.08.005
82. Bowlby J. Attachment and Loss: Volume II: Separation, Anxiety and Anger. London: The Hogarth press and the institute of psycho-analysis; 1973:1–429.
83. Bowlby J. Attachment and loss: retrospect and prospect. Am j Orthopsychiatry. 1982;52(4):664. doi:10.1111/j.1939-0025.1982.tb01456.x
84. Ainsworth MD. Attachments beyond infancy. Am Psychol. 1989;44(4):709–716. doi:10.1037/0003-066X.44.4.709
85. Bartholomew K, Horowitz LM. Attachment styles among young adults: a test of a four-category model. J Pers Soc Psychol. 1991;61(2):226–244. doi:10.1037/0022-3514.61.2.226
86. Cassidy J, Shaver PR. Handbook of Attachment: Theory, Research, and Clinical Applications.
87. Stein H, Koontz AD, Fonagy P, et al. Adult attachment: what are the underlying dimensions? Psychol Psychother. 2002;75(Pt 1):77–91. doi:10.1348/147608302169562
88. Iyengar U, Rajhans P, Fonagy P, Strathearn L, Kim S. Unresolved Trauma and Reorganization in Mothers: attachment and Neuroscience Perspectives. Front Psychol. 2019;10:110. doi:10.3389/fpsyg.2019.00110
89. Ciechanowski PS, Katon WJ, Russo JE, Walker EA. The patient-provider relationship: attachment theory and adherence to treatment in diabetes. Am J Psychiatry. 2001;158(1):29–35. doi:10.1176/appi.ajp.158.1.29
90. Ciechanowski P, Russo J, Katon W, et al. Influence of patient attachment style on self-care and outcomes in diabetes. Psychosom Med. 2004;66(5):720–728. doi:10.1097/01.psy.0000138125.59122.23
91. Costa-Cordella S, Luyten P, Cohen D, Mena F, Fonagy P. Mentalizing in mothers and children with type 1 diabetes. Dev Psychopathol. 2021;33(1):216–225. doi:10.1017/S0954579419001706
92. Warner SRS. Traumatic beginnings, complicated lives: attachment styles, relationships and HIV care. In: Croston MRS, editor. Psychological Perspectives in HIV Care: An Inter-Professional Approach. London: Routledge; 2020.
93. Luyten P, Lemma A, Target M, Fonagy P. A mentalization-based approach to the understanding and treatment of functional somatic disorders. Psychoanal Psychother. 2012;26:121–140. doi:10.1080/02668734.2012.678061
94. Griffin DW, Bartholomew K. Models of the self and other: fundamental dimensions underlying measures of adult attachment. J Pers Soc Psychol. 1994;67(3):430–445. doi:10.1037/0022-3514.67.3.430
95. Bartholomew K. Self-Report Attachment Measures. 2021.
96. Maheri A, Sadeghi R, Shojaeizadeh D, Tol A, Yaseri M, Rohban A. Depression, Anxiety, and Perceived Social Support among Adults with Beta-Thalassemia Major: cross-Sectional Study. Korean J Fam Med. 2018;39(2):101–107. doi:10.4082/kjfm.2018.39.2.101
97. Patel K, Bhivandkar S, Desai R, Antin T. The burden of psychiatric illnesses in adult patients with beta-thalassemia: a 5-year nationwide inpatient evaluation in the United States. Ann Hematol. 2019;98(4):851–860. doi:10.1007/s00277-018-3557-5
98. Betts M, Flight PA, Paramore LC, Tian L, Milenković D, Sheth S. Systematic Literature Review of the Burden of Disease and Treatment for Transfusion-dependent β-Thalassemia. Clin Ther. 2020;42(2):322–337.e322. doi:10.1016/j.clinthera.2019.12.003
99. Nisha S, Alam SS, Rahman MN, Islam K. A Strategy to Assess Morbidity Pattern, Mental Health of Patients with Thalassemia: physiological and Mental Health Conditions. Am J Med Case Rep. 2021;9(1):4–8. doi:10.12691/ajmcr-9-1-2
100. Jaafari Z, Sadidi N, Abdolahinia Z, Shahesmaeili A. Prevalence of Depression among Iranian Patients with Beta-Thalassemia Major: a Systematic Review and Meta-analysis. Iran J Med Sci. 2022;47(1):15–24. doi:10.30476/ijms.2020.85941.1557
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
