Back to Journals » International Journal of Nanomedicine » Volume 14
Prolonged retention of liposomes in the pleural cavity of normal mice and high tumor distribution in mice with malignant pleural effusion, after intrapleural injection
Authors Marazioti A, Papadia K, Giannou A, Stathopoulos GT , Antimisiaris SG
Received 22 January 2019
Accepted for publication 14 March 2019
Published 23 May 2019 Volume 2019:14 Pages 3773—3784
DOI https://doi.org/10.2147/IJN.S202568
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
A Marazioti,1 K Papadia,2 A Giannou,3 GT Stathopoulos,3,4 SG Antimisiaris1,2
1Foundation for Research and Technology Hellas, Institute of Chemical Engineering Sciences (FORTH/ICE-HT), Rio, Greece; 2Laboratory of Pharmaceutical Technology, Department of Pharmacy, University of Patras, Rio, Greece; 3Laboratory for Molecular Respiratory Carcinogenesis, Department of Physiology, Faculty of Medicine, University of Patras, Rio Greece; 4Comprehensive Pneumonology Center and Institute for Lung Biology and Disease, University Hospital, Ludwig-Maximilians University and Helmholtz Center Munich, Munich, Germany
Background: Intrapleural administration of compounds is a lung targeted, innovative therapeutic strategy for mesothelioma, which can be refined as a route for drug delivery that minimizes the potential for systemic toxicity. However, little is currently known about the retention of liposomal drugs at the site, after such topical administration.
Purpose: To evaluate the retention of liposomes in lungs following intrapleural injection, and how this might be modulated by liposome properties and disease progression.
Methods: DiR-incorporating liposomes with various lipid compositions and sizes were prepared, characterized (for size distribution and zeta potential) and injected intrapleurally in normal mice and mice with malignant pleural effusion (MPE). DiR retention in pleural cavity was followed by biofluorescence imaging.
Results: Experimental results demonstrate that liposome size and PEG-coating, have a significant effect on residence time in the pleural cavity; negative surface charge does not. More than 20% liposomal-DiR is retained 24 d post-injection (in some cases), indicating the high potential towards localized diseases. Ex-vivo liposomal-DiR signal in tumors of MPE mice was similar to signal in liver, suggesting high tumor targeting potential of intrapleurally injected liposomes. Finally, no difference was noticed in liposomal-DiR retention between tumor-inoculated (MPE) and healthy mice, indicating the stability of liposomes in the presence of effusion (in MPE mice).
Conclusion: The current study provides novel insights for using liposomes by intrapleural administration for the treatment of lung diseases.
Keywords: nanoparticles, sustained release, local administration, lung, cancer, mesothelioma
Introduction
Liposomes are well known for their potential applications as efficient carriers for drug delivery and targeting.1,2From the currently available liposomal drug products and the ones under clinical testing most are intended for intravenous administration.3–5 Little is known today about the therapeutic advantages of topically administered liposomes, and especially about their retention at the site following administration into confined body cavities, limiting their potential applications. One area for topical administration of liposomal drugs is the pleural cavity.
Intrapleural administration of compounds is a lung targeted, innovative therapeutic strategy for mesothelioma, which can be defined as a route for drug delivery that minimizes the potential for systemic toxicity. Indeed, direct pleural-cavity injection (PI) of small-molecule-drugs may have several therapeutic advantages.6 In addition to providing high concentrations of active compounds to target the pro-fibrogenic activities of pleural mesothelial cells (PMCs),7 it might also delay and/or decrease systemic absorption, minimizing systemic toxicity and increasing drug efficacy against mesothelioma and other malignancies.8,9 Indeed, intrapleural delivery has been demonstrated to realize higher and sustained drug concentrations in bronchoalveolar lavage and intrapleural fluids compared to serum,10,11 and also to widen the effective therapeutic index of drugs by improving patient tolerance .12 From a practical point, less invasive methods of accessing the pleural space such as tunneled pleural catheters could help to make intrapleural delivery a viable option for patients.13 However, PI is not absolutely toxicity-free since high doses of drugs in the pleural cavity were previously reported to cause pleural adhesion.9 Thereby, methodologies that would sustain the release of drugs in the pleural cavity, avoiding the presence of high and potentially toxic drug concentrations, may realize an optimal therapeutic outcome. A potential way to attain the later could be by topical injection of liposomal drugs.
The administration of liposomes or any other type of nanoparticles by intrapleural injection is not a common practice in therapeutics, and thereby little is known about their retention in the pleural cavity and their biodistribution. Biotin-tagged liposomes were injected to the pleural cavity;14 however no data about the retention of the liposomes in the pleural cavity were reported. Microparticles and nanoparticles of charcoal, polystyrene and poly(lactide-co-glycolide) (PLGA) were administered intrapleurally in rats,15 and it was found that the particles were cleared by regional lymphatic system, and lymphatic uptake occurred already 3 hrs post-injection. Microparticles with sizes between 0.7 and 2 µm had the best lymphatic distribution. Intrapleural fluorescein isothiocyanate-labeled PLGA nanoparticles decorated with a surface antibody to mesothelin, were found to migrate into the lung parenchyma with PMCs, supporting a potential role for pleural based therapies to modulate pleural mesothelial activation and parenchymal disease progression. The targeted PLGA nanoparticles were seen to localize to the pleural surface.16 In a phase-I clinical study,17 it was shown that intrapleural administration of liposomal cis-Bis-neodecanoato-trans-R,R-1,2-diaminocycl-ohexane platinum (II) (NDDP) is safe, and that the biological activity of liposomal-NDDP is confined to the pleural cavity. The mean tolerated dose of intrapleural liposomal-NDDP was demonstrated to be 50% higher than the corresponding i.v. dose, and the absorption of L-NDDP into the systemic circulation was much slower than that of the free cisplatin. By comparing urinary excretion in patients receiving systemic liposomal-NDDP and intrapleural liposomal-NDDP, authors concluded that drug was present in the cavity 24 hrs post-administration.18 In spite of its rapid absorption; intracavity cisplatin area under the curve (AUC) was significantly higher than plasma AUC, conferring the potential for an enhanced intracavity antitumor effect.17 Elsewhere, intrapleural administration of adeno-associated virus vector expressing an anti-VEGF-A antibody equivalent of bevacizumab resulted in sustained anti-VEGF-A localized expression within the lung and suppressed metastatic tumor growth.19 Intrapleural administration resulted in long-term expression of the anti-human VEGF-A antibody in the lung, demonstrating sustained and high-level anti-human VEGF titers in the lung epithelial lining fluid for 40 weeks. The in vivo therapeutic effect of a liposomal pemetrexed (PMX) formulation was also evaluated following PI.20 It was found that when PMX was encapsulated in cholesterol-free liposomes it drastically inhibited tumor growth in the pleural cavity, while free PMX and PMX encapsulated in cholesterol (Chol)-containing liposomes did not. The enhanced in vivo anti-tumor efficacy of the non-Chol PMX cationic liposomes was attributed to the prolongation of the retention of cationic liposomes in the pleural cavity via electrostatic interaction with the negatively charged membranes of the tumor cells, and also to higher drug release from the non-Chol liposomes. Recently, direct intrapleural injection of deltarasin to a mouse model of malignant pleural effusion (MPE) was attempted as a method to avoid potential toxicity after systemic administration;21 however, the free-drug was not effective probably due to rapid clearance from the site. On the other hand, a single intrapleural injection of one dose of liposomal-deltarasin exhibited equal efficacy with that observed after 14 repetitive (daily) intra-peritoneal doses of the free drug,22 proving the high therapeutic potential of the intrapleurally injected liposomal drug.
Prompted by all the previous results that indicate high therapeutic advantages of direct pleural administration of liposomal drugs, and the gap in the literature about the retention of liposomes at the site, and how this might be affected by liposome properties and disease progression, we conducted the current study. DiR-labeled liposomes in conjunction with a live animal imaging setup were used, in order to monitor the retention of different liposome types in the pleural cavity. Normal (FVB) mice and tumor inoculated-mice with accompanying MPE formation (as well as their corresponding wild-types) were used, in order to exploit the potential effect of the disease on the retention of the liposomes.
Material and methods
1, 2-Distearoyl-sn-glycerol-3-phosphatidylcholine (DSPC), 1,2-distearoyl-sn-glycero-3-phospho-(1’-rac-glycerol) (sodium salt) (DSPG) and 1,2-distearoyl-sn-glycerol-3-phosphoethanolamine-N-[methoxy(polyethyleneglycol)-2000] (DSPE-PEG2000 or PEG), were purchased from Avanti Polar Lipids. Cholesterol (99%) (Chol), Triton X-100, Sephadex G-50 and Sepharose CL-4B, were purchased from Sigma-Aldrich (Chemilab, Athens, Greece). Lipophilic tracer, 1,1-dioctadecyl-3,3,3,3-tetramethylindotricarbocyanine iodide (DiR) was used as liposome label for live animal imaging (Molecular Probes). All other chemicals were of analytical quality and were purchased by Sigma-Aldrich.
Liposome preparation
Multilamellar (MLV) and small unilamellar vesicle (SUV) liposomes were prepared by the thin film hydration method, as previously reported.23,24 In brief, appropriate amount of lipid or lipids for a concentration of 13mM of liposomes, were added in a 50 mL round bottom flask, as solutions in Chloroform/Methanol (2:1 v/v), and the organic solvents were evaporated by rotary evaporation (Buchi). DiR (0.2 mol% of total lipid) was also added in the lipid mixture before evaporation.25,26 After complete solvent evaporation a thin lipid film formed on the walls of the flask; solvent residues were remover by flashing the flask with N2. Hydration of the lipid film was then performed, by adding 1 mL of PBS pH 7.40 and applying intensive vortex and bath sonication, until a liposomal dispersion was obtained.
For SUV liposomes, MLV liposomes were subjected to probe sonication (Sonics & Materials) until formation of a clear dispersion, as previously described.23,25,26 DRV (dehydration-rehydration vesicles) liposomes were prepared from empty DiR-labelled SUVs; 1 mL of SUVs was mixed with 1 mL PBS pH 7.40, and the mixture was freeze-dried and then rehydrated.23,27 After re-hydration the liposomes were extruded 20 times through staked polycarbonate membranes with 400nm pore size, using a handheld extruder (Avestin).
Any amount of non-incorporated DiR was separated from DiR-liposomes by ultracentrifugation (2x60 min at 60,000 rpm; Sorvall WX90 Ultra).
Physicochemical properties of liposomes
The lipid concentration of liposomes was measured by the Stewart assay,28 and adjusted as required. All liposomes were characterized for their size distribution and zeta potential, as previously reported.23–27 The mean particle size was measured by dynamic light scattering (DLS) of vesicle dispersions (0.4 mg/mL) (Malvern Nano-Zs; Malvern Instrument, Malvern, UK) at 25°C and a 173°angle (to avoid back-scattering). Zeta potential of liposomes was measured in 10 mM PBS, pH 7.40 and at 25°C, utilizing the Doppler electrophoresis technique.
Cells
For the MPE experiments the colon adenocarcinoma cell line MC38 was used and was a gift from Dr. Barbara Fingleton (Vanderbilt University, Nashville, TN, USA); the use of the cell line was approved by the ethics committee of the Veterinary Administration, Prefecture of Western Greece (approval number: 118018/578). Additionally, the cell line was authenticated by STR profile (IDEXX BioResearch Case #50925–2015). Cells were cultured at 37°C in 5% CO2–95% air using DMEM containing 10% FBS, 2 mM L-glutamine, 1 mM pyruvate, 100 U/mL penicillin, and 100 mg/mL streptomycin. For in vivo injections, cells were harvested using trypsin, incubated with Trypan blue, counted in a hemocytometer, and 95% viable cells were injected intrapleurally.29–31
Mouse models and liposome treatments
All the procedures used for animal care and experiments were pre-approved by the Veterinary Administration Bureau of the Prefecture of Achaia, Greece, and by the University of Patras ethical review committee, and were conducted according to European Union Directive 86/609/EEC for animal experiments (
FVB mice chosen for their white skin and fur that permits enhanced light penetration, allowing easy and accurate imaging, were purchased from Hellenic Pasteur Institute (Athens, Greece) and bred at the Center for Animal Models of Disease, University of Patras (Rio, Greece). The mice used for the experiments were sex-, weight (20–25 g)-, and age (6–12 weeks)-matched. In FVB mice two sets of experiments were carried out in order to determine the maximum duration of accurate monitoring of the retention of liposomal-DiR in the pleural cavity, following intrapleural injection at two lipid doses. Intrapleural injections were done under direct stereoscopic vision via a small incision in the left anterolateral chest skin and fascia. For this, a 27 G needle was advanced to the pleural space at a 45° angle under direct contact with the superior rib and the tumor cell suspensions as well as the liposomal formulations were injected under direct visual inspection, as described previously.29,31
FVB mice were randomly allocated to treatment with one of each liposome type, via intrapleural injection (n=3 or 4 mice/group). In the first set, the retention of DiR liposomes was followed for up to 4.5 d post-injection of 150 µg of liposomal lipid/mouse. MLV and SUV liposomes of different lipid compositions were used in order to evaluate the effect of liposome size, negative charge and surface coating with PEG, on their retention in the cavity. Animals were imaged before and immediately after injection, as well as at 0.5 d, 2.5 d and 4.5 d post-injection.
In the second experiment, 2 mg (liposomal lipid) per animal, and two lipid compositions were used to evaluate the effect of adding negative charge in the liposome membrane, on the retention of liposomal-DiR in the cavity. Animals were imaged before (background) and immediately after injection, as well as 7 d, 12 d and 24 d post-injection.
For the MPE mice model, C57BL/6 (#000664) (Jackson Laboratory, Bar Harbor, ME, USA) mice were bred at the Center for Animal Models of Disease of the University of Patras. Male and female experimental mice and littermate controls were sex, weight (20–25 g), and age (6–12 weeks) matched. For MPE induction, mice received 150,000 MC38 cancer cells in 100 μL PBS intrapleurally, and were then allowed 4d for pleural tumor development. At this time tumor inoculated-mice, as well as healthy littermates were treated intrapleurally with one injection of liposomal-DiR (n=8). Mice were observed continuously till recovery and daily thereafter and were sacrificed when moribund (13–14 d post-tumor cell injection) for ex-vivo tissue imaging and pleural fluid analyses. All MPE-mice developed pleural tumors with accompanying pleural effusion formation. Injection, harvest, and sample handling are described analytically elsewhere .28–31 As mentioned above, liposome injections were initiated 4 d post-tumor cell inoculation. Each injection consisted of 100 μL containing 2 mg of liposomal lipid. Animals were imaged before (background) and immediately after injection, as well as at 1 d, 3 d, 6 d and 8 d post-injection. Longer monitoring periods were not possible in the case of the tumor inoculated mice (MPE) due to dramatic disease progression.
Biofluorescence – live animal imaging and ex-vivo studies
DiR was selected as an ideal liposome labeling fluorophore for the purpose of this study since it is known from the literature and was also verified by our groups, that free-DiR is rapidly eliminated from mice following injection and that the bio fluorescent signal of DiR is dramatically reduced (to background level) when the dye is released from the liposome membrane.25,32
Biofluorescence imaging of living mice was carried out on an IVIS Lumina II imager (Perkin Elmer, Santa Clara, CA, USA). After anesthetizing the mice with isoflurane, they were serially imaged at various time-points post-DiR-liposome injection, using DiR-specific EX and EM wavelengths (excitation: 710–760 nm; emission: 810–875 nm). Images were acquired and analyzed using Living Image v4.2 software (Perkin Elmer). In detail, specific regions of interests (ROIs), corresponding to the area of the injection site, were created and were superimposed over all images acquired in a uniform fashion. Subsequently, photon flux within these regions was measured and compared between mice receiving different treatments. In all cases, the background ROI measured for the specific animal prior to injection was subtracted from the signal of each time point acquired. In the case of the MPE-mice experiment, the BL/6 mice were shaved before imaging, when needed, in order to avoid possible reduction of the fluorescent signal due to their black fur.
Statistical analysis
All results are expressed as mean ± SD from at least three independent experiments. The significance of variability between results from various groups was determined by two-way ANOVA (for significance of interaction, time and liposome type) followed by Bonferroni tests for individual differences between groups.
Results
Liposome physicochemical properties
The physicochemical properties of the liposomes used in the in vivo studies, are presented in Table 1. As seen, the mean diameter of MLV liposomes is around 3.3 µm, for the SUV liposomes it ranges between 138 nm and 220 nm depending on the lipid composition, and for the DRV liposomes, it is around 400 nm (since they were extruded through 400 nm pore-size membranes). Liposomes of various sizes were prepared in order to evaluate the effect of the liposome size on their retention in the pleural cavity.
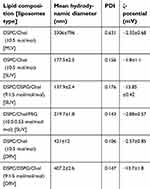 | Table 1 Compositions and physicochemical properties of the liposomes used. Each value is the mean from at least three different samples and SD of each mean is reported (mean ± SD) |
The polydispersity index values (PDI) are higher for the larger MLVs vesicles (compared to SUVs and DRVs) indicating that they are more polydisperse in size; while the DRVs which were extruded, have low polydispersity (Table 1). The high negative zeta-potential, confirms the incorporation of DSPG in the corresponding vesicle types.
FVB mice live animal imaging
As seen in Figure 1, the DiR signals (expressed as %Retention of liposomal-DiR normalized to the initial signal measured immediately after injection) in the animals injected with MLV and SUV liposomes remain high in the first half-day post injection, regardless of the lipid composition or liposomes size, and gradually decrease after 2.5 d and 4.5 d, for most liposome types. However, the PEG-coated SUV liposomal-DiR is practically retained in the pleural cavity for the full period monitored (4.5d) (Figure 1). The liposome size has a significant effect on the retention of liposomal-DiR in the pleural cavity during the time-period evaluated (P=0.043), regardless of the lipid composition; lower amounts were retained when MLV liposomes were injected compared to SUV liposomes. However, even in the case of the MLV liposomes, >20% of the liposomal-DiR is retained in the cavity 4.5d post-injection. For DSPC/Chol liposomes there was a significant difference between MLV and SUV liposomes concerning the levels of liposomal-DiR retained in the pleural cavity for the full time-period tested (P=0.0210). Additionally, significant differences were found between the liposomal-DiR retained when PEG-coated SUV liposomes were used, compared to the two other SUV lipid compositions evaluated (with DSPC/Chol liposomes, P=0.0154; and with DSPC/DSPG/Chol liposomes, P<0.0001). Oppositely, the addition of DSPG in the lipid membrane of SUV liposomes (DSPC/DSPG/Chol) did not confer a significant difference in the retention of liposomal-DiR in the pleural cavity (compared to the SUV liposomes without DSPG (DSPC/Chol), P>0.05) during the 4.5 d period.
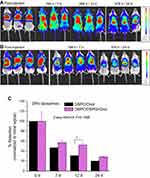
The last result was also verified by another experiment (Figures 2 and 3B), in which larger liposomes (DRV) and a higher lipid dose (2 mg/mouse) were used, and the retention was monitored for a much longer time period (24 d). Again, the effect of liposome zeta-potential on the retention of liposomal-DiR in the pleural cavity was not significant (P=0.1598).
In Figures 3A and B, the kinetics of the retention of liposomal-DiR from all the liposomes types evaluated in the FVB mice, following intrapleural injection of 0.150 mg/mouse (Figure 3A) or 2 mg/mouse (Figure 3B) are presented, and the graphs have the same axis values for easy comparison of the results. As seen, the kinetics of liposomal-DiR of the larger (compared to the SUV liposomes) DRV liposomes seem to be similar to those of the SUV liposomes with the same lipid compositions, although direct comparison of experimental results is not possible since different time points were used. From the results of the study conducted with the DRV liposomes, which was extended for up to 24 d, it is observed that after an initial more rapid clearance of liposomal-DiR from the cavity in the first 7 d, in the remaining time period the clearance is slower and almost linear; as a result, the liposomal-DiR levels retained after 24 d are >20% of the dose injected, for both lipid compositions tested.
The highest retention of the PEG-coated SUV liposomes compared to all the other liposome types evaluated in FVB mice is obvious. For this reason, we selected to use PEG-coated SUV liposomes in the MPE-mice experiment.
Finally, we did not observe any pleural adhesion during or after intrapleural injections of liposomes in FVB mice.
Tumor inoculated mice – live imaging
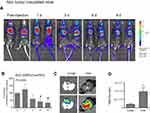
MPE mice and their healthy littermates were injected intrapleurally with DiR-labelled PEG-coated SUV liposomes (DSPC/Chol/PEG-lipid) at a dose of 2 mg/mouse and the retention of liposomal-DiR was followed until moribund (due to disease progression). We have not observed any pleural adhesion during or after the intrapleural injections of liposomes, in the tumor inoculated mice, and their littermates. The initial signals (Total Flux [p/s]) measured for liposomal-DiR immediately post-PI in the black BL/6 mice (after background subtraction) ranged between 2.07×1010–4.97×10,10 which are similar to the corresponding values measured in the white FVB mice following injection of the same lipid dose. We report this in order to stress that when the sample is localized at the injection site and the area is shaved right before imaging (in case of black mice), the DiR signals in both types of mice are similar; however we are not sure if the signals (and corresponding retention values of liposomal-DiR) measured at the other time points (when the sample is perhaps no longer localized at a small area and may be distributed in deeper tissues, and the area has not been shaved right before imaging) can be accurately compared between the two different mouse species. Assuming that such a comparison is possible, it is evident from Figure 3, that SUV liposomes are cleared much faster from BL/6 mice compared to FVB mice, suggesting a significant effect of animal species; however since we cannot be sure about the accuracy of the prior comparison, we will not discuss this more, and we will focus on the comparison between MPE and wild type mice.
As shown in Figure 3C the clearance of Liposomal-DiR from the pleural cavity follows an identical pattern in both types of mice (MPE and wild-type), suggesting that the development of the disease and the accumulated pleural fluid, does not significantly affect the stability or the clearance of the PEG-coated SUV liposomes at/from the site.
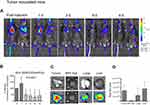
A gradual reduction of the liposomal-DiR signals in the pleural cavity was observed during the period evaluated (0 d–8 d) in both of the animal types tested, the healthy littermates (Figure 4A and B) and the mice with MPE (Figure 5A and B). In the case of the healthy mice, the ex-vivo DiR signal in the lungs, was about 5 times lower compared to that measured in the liver (Figure 4C and D), indicating that the largest fraction of DiR-liposomes was absorbed in blood and was finally taken up by the liver; however, the healthy littermates were harvested 24d post DiR-liposome injection, since they did not develop any disease. In the mice with MPE, in addition to liver and lungs, the MPE fluid and the tumors (that developed in the pleural cavity) were also imaged post-mortem (10 d post-injection of liposomal-DiR). Interestingly, as seen in Figure 5C and D, a very high liposomal-DiR signal was measured in the tumors developed in the cavity, indicating the high potential of the liposomes to be taken up by the tumor cells. Indeed, the liposomal-DiR signal in the tumor was similar to the signal measured in the liver (at this time point). The ex-vivo liposomal-DiR in lungs of mice with MPE was also significantly higher when compared to that found in the healthy mice, however, this is probably due to the deep invasion of the tumors into the lungs that could not be removed from the lung tissues before their ex-vivo imaging. The different times of harvesting between the two mouse-types (day 10 and day 24 post-injection of liposomal-DiR) may have additionally contributed to the latter observation.
In Figure 6 the Kaplan-Meier survival plot is shown (Figure 6A); one mouse died 13 days after the intrapleural injection of tumor cells due to progressed MPE disease, and signaled the mice harvesting in the following day (10 days post-PI of liposomes). Additionally, data summaries of effusion volume, pleural fluid cells and tumor weight are shown (Figure 6B), as well as representative images of effusions (dashed lines) and pleural tumors (Figure 6C). The effusion volume (0.69±0.29 mL) and pleural fluid cell counts (9.27±2.03) were, as expected, similar with the values reported earlier.29–31
Discussion
Herein we evaluated the potential of liposomes to sustain the retention of liposomal-DiR in the pleural cavity, following administration by direct intrapleural injection (PI). The current experimental results reveal that certain liposome preparative parameters, such as the liposome size and liposome surface-coating with PEG, significantly affect liposome retention in the pleural cavity, since SUVs and PEG-coated SUV liposomes are retained in the injection site at significantly higher amounts compared to MLVs, and non-coated SUV liposomes, respectively (Figures 1 and 3). The higher retention of the PEG-coated liposomes is most probably related to the well-documented higher integrity conferred to liposomes when they are coated with hydrophilic polymers, such as PEG.23,33 The lower retention of comparably large MLV liposomes may be attributed to their easier and faster interaction with macrophages which are present at the site, due to the lower curvature of MLVs (compared to the smaller SUV and DRV liposomes). In a previous report, microparticles injected intrapleurally in rats were found to be cleared by the regional lymphatic system and compared to nanoparticles, the larger particles with sizes between 0.7 and 2 µm had the best lymphatic distribution.15 Oppositely to the effect of PEG and size, the addition of a negatively charged lipid in the membrane of DiR-Liposomes did not have any effect on the retention of liposomal-DiR at the injection site; neutral and negatively charged liposomes demonstrated similar kinetics (Figures 2 and 3).
When the highest dose of 2 mg of liposomal lipid was injected in FVB mice, >20% of the injected liposomal-DiR was still at the site of injection after 24 d (Figure 3B), while the same lipid dose injected PI in non-tumor inoculated BL/6 mice (used as controls for mice with MPE) resulted in a significantly lower but still accurately detectable liposomal-DiR amount at the site (approximately 3.5% of the injected dose) (Figure 3C).
The current results confirm that liposomal-DiR is retained in the pleural cavity for prolonged time periods; thereby if liposomal drug formulations are stable (and the drug is not rapidly released from the liposomes) their retention in the cavity should also be substantially sustained. Nevertheless, the stability of liposomes in the pleural fluid of healthy mice, as the one applying in FVB mice, is probably a lot different from that in MPE, due to the known differences in their volumes as well as in their compositions (Table 2).29,30,34 Perhaps some liposomal drug formulations will be less stable and will be cleared faster from the pleural cavity when pleural malignancies are present. Additionally, MPE is characterized by increased vascular permeability, indicated by the leakage of Evans’ blue dye into the pleural space; 3.6±0.9 μg/mL in mice with MPE, compared to 0.04±0.02 μg/mL in healthy mice.29 Due to the striking differences between the different cases, we additionally monitored the pleural cavity-retention of liposomes in MPE-mice compared to littermate healthy mice. Interestingly, as clearly demonstrated in Figure 3C, Figure 4A and B, and Figure 5A and B, the time-frame of the retention of liposomal-DiR was similar in MPE-mice and healthy littermate mice, proving that at least in the case of PEG-coated SUV liposomes consisted of DSPC/Chol/PEG (and associated with the lipophilic tracer DiR), the presence of the pleural effusion does not affect the retention of liposomal-DiR in the cavity. Of course, when other types of liposomal drugs are considered, the retention of the drug in the liposomes is also a main factor that will highly determine the drug retention at the site.
 | Table 2 Comparison of the characteristics and compositions of pleural fluids between a normal mouse and a mouse model of malignant pleural effusion |
The current results fill the gap of the relevant bibliography, since the retention of liposomes or any other type of nanoparticles in that pleural cavity was never systematically studied up-to-date, although prolonged retention times have been suggested in several cases, in order to theoretically explain the enhanced therapeutic effects and/or lower toxicities observed by different types of intrapleurally administered nanocarrier-associated drugs (compared to other routes of administration).16–20 Only in one case, two types, neutral and cationic, DiR-loaded liposomes were injected intrapleurally in BALB/c nu/nu mice (1.5 mg lipid/mouse) and the DiR signal at the injection site (and in the whole body) was monitored, and reported to be equal to background 48 hr post-injection.20 The dramatic difference of those results with the current ones may be attributed to: (i) Different liposome compositions used (however the lipid composition was not mentioned in the previous report); and (ii) the mouse type used; immuno-deficient mice were used in the previous study while immunocompetent mice were used herein. Perhaps the composition of the fluids in the pleural cavity is drastically different in the two cases, and thus liposomes are less stable in the pleural fluids of immuno-deficient mice, compared to immunocompetent ones, conferring strikingly different liposome-clearance rates from the cavity, between the two types of mice.
Considering the safety of the proposed method of administration, it was recently shown that intrapleural injection of an adenoviral vector in healthy mice does not cause any pathologic changes in the histology of the pleural mesothelium. Additionally, it was proven that with this type of injection the lung tissue is not reached and thus not affected (Marazioti et al, unpublished results).
Finally, the very high accumulation of Liposomal-DiR in the tumors developed in the mice with MPE (Figure 5C and D), is especially important for chemotherapeutic drug delivery. Indeed, the high accumulation of the SUV liposomes in the localized tumors demonstrated herein, provides a concrete explanation for the previously reported high therapeutic effect of one dose of intrapleurally administered liposomal-deltarasin,22 and underlines the great potential of such locally injected liposomal drugs, for treatment of localized malignancies, such as mesothelioma.
Conclusion
The current results demonstrate the sustained retention of liposomes at the site of injection following PI, even in the case of MPE, as well as the high tumor targeting efficiency of intrapleurally injected liposomes. These results suggest a high potential for prolonged retention of liposomal drugs administered by PI, in the pleural cavity, as well as a high therapeutic potential for the treatment of localized diseases, providing that the drug release from the liposomes is appropriately controlled.
Acknowledgments
The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 260524. Partial support of this work by the projects: “Advanced Research Activities in Biomedical and Agro alimentary Technologies” (MIS 5002469) funded by the Operational Programme “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014-2020) and co-financed by Greece and the European Union (European Regional Development Fund), and “Advancing Young Researchers’ Human Capital in Cutting Edge Technologies in the Preservation of Cultural Heritage and the Tackling of Societal Challenges (ARCHERS)”, funded by the Stavros Niarchos Foundation, are acknowledged.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Antimisiaris SG, Kallinteri P, Fatouros DG. Liposomes and Drug Delivery Chapter 5.3 In Pharmaceutical Manufacturing Handbook: Production and Processes. Gad SC Editor. Hoboken, NJ: John Wiley & Sons, Inc. ISBN: 978-0-470-25958-0, 2008;443–550.
2. Antimisiaris S, Mourtas S, Papadia K. Targeted si-RNA with liposomes and exosomes (extracellular vesicles): how to unlock the potential. Inter J Pharm. 2017;525(2):293–312. doi:10.1016/j.ijpharm.2017.01.056
3. Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017;9(2):12. doi:10.3390/pharmaceutics9020012
4. Koudelka S, Turanek Knotigova P, Masek J, et al. Liposomal delivery systems for anti-cancer analogues of vitamin e. J Control Release. 2015;207:59–69. doi:10.1016/j.jconrel.2015.04.003
5. Fernandes E, Ferreira JA, Andreia P, et al. New trends in guided nanotherapies for digestive cancers: a systematic review. J Control Release. 2015;209:288–307. doi:10.1016/j.jconrel.2015.05.003
6. Jantz MA, Antony VB. Pleural fibrosis. Clin Chest Med. 2006;27:181–191. doi:10.1016/j.ccm.2005.12.003
7. Haber DA, Buckler AJ, Glaser T, et al. An internal deletion within an 11p13 zinc finger gene contributes to the development of Wilms’ tumor. Cell. 1990;61:1257–1269. doi:10.1016/0092-8674(90)90690-G
8. Park S, Schalling M, Bernard A, et al. The Wilms tumour gene WT1 is expressed in murine mesoderm-Derived tissues and mutated in a human mesothelioma. Nat Genet. 1993;4:415–420. doi:10.1038/ng0793-268
9. Tada Y, Hiroshima K, Shimada H, et al. An intrapleural administration of zoledronic acid for inoperable malignant mesothelioma patients: a phase I clinical study protocol. SpringerPlus. 2016;5:195. doi:10.1186/s40064-016-1893-2
10. Mubarak KK, Montes-Worboys A, Regev D, et al. Parenchymal trafficking of pleural mesothelial cells in idiopathic pulmonary fibrosis. Eur Respir J. 2012;39:133–140. doi:10.1183/09031936.00141010
11. Tohda Y, Iwanaga T, Takada M, et al. Intrapleural administration of cisplatin and etoposide to treat malignant pleural effusions in patients with non-small cell lung cancer. Chemotherapy. 1999;45(3):197–204. doi:10.1159/000007183
12. Greillier L, Monjanel-Mouterde S, Fraticelli A, et al. Intrapleural Administration of Pemetrexed. A Pharmacokinetic Study in an Animal Model. J Thorac Oncol. 2009;4:3. doi:10.1097/JTO.0b013e3181b6be12
13. Jomgeow T, Oji Y, Tsuji N, et al. Wilms’ tumor gene WT117AA(-)/KTS(-) isoform induces morphological changes and promotes cell migration and invasion in vitro. Cancer Sci. 2006;97:259–270. doi:10.1111/j.1349-7006.2006.00169.x
14. Medina LA, Calixto SM, Klipper R, Li Y, Phillips WT, Gions B. Mediastinal Node and Diaphragmatic Targeting after Intracavitary Injection of Avidin/99mTc-Blue-Biotin-Liposome System. J Pharml ScI. 2006;95:207–224.
15. Liu J, Wong HL, Moselhy J, Bowen B, Wu XY, Johnston MR. Targeting colloidal particulates to thoracic lymph nodes. Lung Cancer. 2006;51:377–386. doi:10.1016/j.lungcan.2005.11.006
16. Batra H, Antony VB. The pleural mesothelium in development and disease. Frontiers in Physiol Membr Physiol and Membr Biophys. 2014;5:Art 284.
17. Perez-Soler R, Shin DM, Siddik ZH, et al. Phase I clinical and pharmacological study of liposome-entrapped NDDP administered intrapleurally in patients with malignant pleural effusions. Clin Cancer Res. 1997;3(3):373–379.
18. Siddik ZH, Perez-Soler R, Khokhar AR, et al. Clinical pharmacokinetics of liposomal cis-bis-neodecanoato-trans-R,R-1,2-diaminocyclohexane platinum(II) (L-NDDP). Proc Annu Meet Am Assoc Cancer Res. 1991;32:176.
19. Watanabe M, Boyer JL, Crystal RG, Vrh AA. 10-mediated genetic delivery of bevacizumab to the pleura to provide local anti-VEGF to suppress growth of metastatic lung tumors. Gene Ther. 2010;17:1042–1051. doi:10.1038/gt.2010.87
20. Ando H, Kobayashi S, Lila ASA, et al. Advanced therapeutic approach for the treatment of malignant pleural mesothelioma via the intrapleural administration of liposomal pemetrexed. J Control Release. 2015;220:29–36. doi:10.1016/j.jconrel.2015.11.017
21. Agalioti T, Giannou AD, Krontira AC. Mutant KRAS promotes malignant pleural effusion formation. Nat Comms. 2017;8:15205. doi:10.1038/ncomms15205
22. Zimmermann G, Papke B, Ismail S, et al. Small molecule inhibition of the KRAS-PDEδ interaction impairs oncogenic KRAS signalling. Nature. 2013;497(7451):638–642. doi:10.1038/nature12205
23. Matloob AH, Mourtas S, Klepetsanis P, Antimisiaris SG. Increasing the stability of curcumin in serum with liposomes or hybrid drug-in-cyclodextrin-in-liposome systems: a comparative study. Inter J Pharm. 2014;476(1):108–115. doi:10.1016/j.ijpharm.2014.09.041
24. Tsotas VA, Mourtas S, Antimisiaris SG. Dexamethasone incorporating liposomes: effect of lipid composition on drug trapping efficiency and vesicle stability. Drug Deliv. 2007;14(7):441–445. doi:10.1080/10717540701603530
25. Markoutsa E, Papadia K, Giannou AD, et al. Mono and dually decorated nanoliposomes for brain targeting, in vitro and in vivo studies. Pharm Res. 2014;31(5):1275–1289. doi:10.1007/s11095-013-1249-3
26. Papadia K, Giannou AD, Markoutsa E, et al. Multifunctional LUV liposomes decorated for BBB and amyloid targeting – B. In vivo brain targeting potential in wild-type and APP/PS1 mice. Eur J Pharm Sci. 2017;102:180–187. doi:10.1016/j.ejps.2017.03.010
27. Antimisiaris SG. Preparation of DRV liposomes. Meth Mol Biol. 2017;1522:23–47.
28. Stewart JCM. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem. 1980;104:10–14. doi:10.1016/0003-2697(80)90269-9
29. Marazioti A, Kairi CA, Spella M, et al. Beneficial impact of CCL2 and CCL12 neutralization on experimental malignant pleural effusion. PLoS One. 2013;8(8):e71207. doi:10.1371/journal.pone.0071207
30. Giannou AD, Marazioti A, Spella M, et al. Mast cells mediate malignant pleural effusion formation. J Clin Invest. 2015;125:2317–2334. doi:10.1172/JCI80323
31. Marazioti A, Lilis I, Vreka M, et al. Myeloid-derived interleukin-1β drives oncogenic KRAS-NF-κΒ addiction in malignant pleural effusion. Nat Comms. 2018;9(1):Art num 672. doi:10.1038/s41467-018-03051-z
32. Xiang Y, Liang L, Wang X, Wang J, Zhang X, Zhang Q. Chloride channel-mediated brain glioma targeting of chlorotoxin-modified doxorubicine-loaded liposomes. J Control Release. 2011;152:402–410. doi:10.1016/j.jconrel.2011.03.014
33. Piperoudi S, Fatouros D, Ioannou PV, Frederik P, Antimisiaris SG. Incorporation of PEG-lipids in arsonoliposomes results in formation of highly stable arsenic-containing vesicles. Chem Phys Lipids. 2006;139(2):96–106. doi:10.1016/j.chemphyslip.2005.11.003
34. Stathopoulos GT, Zhu Z, Everhart MB, et al. Nuclear factor-kappaB affects tumor progression in a mouse model of malignant pleural effusion. Am J Respir Cell Mol Biol. 2006;34(2):142–150. doi:10.1165/rcmb.2005-0130OC
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.