Back to Journals » Journal of Pain Research » Volume 7
Proinflammatory cytokines and DHEA-S in women with fibromyalgia: impact of psychological distress and menopausal status
Authors Sturgeon J, Darnall B , Zwickey H, Wood L, Hanes D, Zava D, Mackey S
Received 18 July 2014
Accepted for publication 27 August 2014
Published 4 December 2014 Volume 2014:7 Pages 707—716
DOI https://doi.org/10.2147/JPR.S71344
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Michael Schatman
John A Sturgeon,1 Beth D Darnall,1 Heather L Zwickey,2 Lisa J Wood,3 Douglas A Hanes,2 David T Zava,4 Sean C Mackey1
1Stanford University School of Medicine, Department of Anesthesiology, Perioperative and Pain Medicine, Palo Alto, CA, USA; 2Helfgott Research Institute, National College of Natural Medicine, Portland, OR, USA; 3MGH Institute of Health Professions, Boston, MA, USA; 4ZRT Laboratories, Beaverton, OR, USA
Abstract: Though fibromyalgia is not traditionally considered an inflammatory disorder, evidence for elevated inflammatory processes has been noted in this disorder in multiple studies. Support for inflammatory markers in fibromyalgia has been somewhat equivocal to date, potentially due to inattention to salient patient characteristics that may affect inflammation, such as psychiatric distress and aging milestones like menopause. The current study examined the relationships between proinflammatory cytokines and hormone levels, pain intensity, and psychological distress in a sample of 34 premenopausal and postmenopausal women with fibromyalgia. Our results indicated significant relationships between interleukin-8 and ratings of pain catastrophizing (r=0.555, P<0.05), pain anxiety (r=0.559, P<0.05), and depression (r=0.551, P<0.05) for postmenopausal women but not premenopausal women (r<0.20 in all cases). Consistent with previous studies, ratios of interleukin-6 to interleukin-10 were significantly lower in individuals with greater levels of depressive symptoms (r=−0.239, P<0.05). Contrary to previous research, however, dehydroepiandrosterone sulfate did not correlate with pain intensity or psychological or biological variables. The results of the current study highlight the importance of psychological functioning and milestones of aging in the examination of inflammatory processes in fibromyalgia.
Keywords: fibromyalgia, cytokines, psychological distress, inflammation
Introduction
Fibromyalgia (FM) is a chronic pain disorder that is characterized by widespread bodily pain with a prevalence of 2% of the US population.1 FM is characterized by widespread bodily pain for at least 3 months that is accompanied by significant complaints of fatigue, sleep disturbance, and cognitive symptoms,2 and also shows comorbidity with other somatic complaints, including headaches and gastrointestinal pain.3 As these symptoms overlap with other somatic disorders like chronic fatigue syndrome and irritable bowel syndrome, it has been suggested that FM should be defined more broadly as a somatic distress disorder consisting of multiple physical symptoms, rather than strictly a chronic pain disorder.4
Inflammatory processes in FM
Given the diverse nature of FM symptoms, evidence suggests a complex etiology underlying FM. Researchers have proposed several potential mechanisms of FM symptoms, including central nervous system sensitization,5 sleep disturbance,6 affective dysregulation,7 and genetic abnormalities.8 Although FM is not commonly defined as an inflammatory disorder, studies have identified potential abnormalities in inflammatory processes that may be contributory factors to FM.9 Indeed, at a minimum, these findings suggest that there are likely to be subclinical elevations in inflammation in this syndrome.10 Typically, proinflammatory cytokines are released as part of an acute-phase response to an inflammatory stimulus and are centrally involved as mediators of immune responses.11 Elevated levels of circulating inflammatory cytokines and chemokines, small cytokines involved in chemotaxis, have been reported by various researchers in studies of individuals with FM, including interleukin (IL)-6, IL-8, IL-10, and tumor necrosis factor-α (TNF-α).
IL-6, a proinflammatory cytokine related to major depression and various symptoms characteristic of FM (including hyperalgesia, fatigue, and depression), has been correlated with pain levels in some pain populations.12 IL-8 is a proinflammatory chemokine involved in increased nociceptive sensitivity and promotion of sympathetic pain,13,14 as well as major depression.15 Results from a recent meta-analysis16 suggest that proinflammatory IL-6 and IL-8 are cytokines most commonly elevated in individuals with FM. The proinflammatory cytokine TNF-α, which is typically involved in acute-phase inflammation, has also been found in higher serum levels in individuals with FM.17 Additionally, elevations of IL-10, a cytokine involved in the regulation of inflammatory processes that is typically closely associated with IL-6, have been reported in some individuals with FM without accompanying elevations in IL-6 in FM.18 Evidence regarding IL-10 and TNF-α in FM is somewhat less robust, though the presence of these markers in the literature warrants their inclusion in future studies.
In addition to cytokine dysregulation, there may be hormonal differences that underlie inflammatory processes in FM. Dysregulation of dehydroepiandrosterone sulfate (DHEA-S), an endogenous steroid hormone, may play an etiologic role in the maintenance of FM symptomatology, as it modulates inflammatory responses through direct inhibition of IL-6 and TNF-α activity19 and indirectly through promotion of IL-10 release.20 The normative decline of DHEA-S levels with age has been theoretically linked with the onset of FM symptomatology across the life span.21
Psychiatric distress and FM
High levels of psychiatric distress are common in FM; some estimates suggest a lifetime prevalence of major depressive disorder in this population as high as 68%, as well as elevated rates of anxiety.22 The psychological distress common in FM is an important consideration when profiling cytokine activity, as this distress has implications for inflammatory processes. Researchers have identified a link between major depression and inflammatory markers like IL-615 and TNF-α,12 as well as less robust relationships with IL-812,23 and DHEA-S.14,24,25 Further, these cytokines may have overlapping contributions to inflammatory profiles; Dhabhar et al26 reported that individuals with major depression also demonstrate an increased ratio of serum IL-6 levels to serum IL-10 levels, which may be an etiological contributor to depression-related inflammatory dysregulation. There is also evidence of increased levels of IL-6,27 IL-10, and TNF-α in certain anxiety disorders. Similarly, an inverse relationship between DHEA-S and anxiety has been demonstrated in some populations,28 though the evidence for this relationship is largely correlational. Further, the relationship between DHEA-S and anxiety may vary significantly by sex28 and by age.29 To date, few studies have examined inflammatory processes in FM with specific consideration for psychiatric distress, but psychological factors are likely a key factor in further characterizing the relationship between FM and cytokine levels.
Effects of aging on FM symptomatology
Cytokine and chemokine differences underlying FM must also be examined within the framework of human aging, as the prevalence of FM increases in older populations,30 and inflammatory responses change as a function of the normal aging process.16,17 It is thought that age-related changes in inflammation are mediated by changes in immune function.31 Although aging is well established as a predictor of inflammatory changes in healthy populations, there is a paucity of research examining age-related differences in cytokine activity in FM. Studies suggest that IL-6, IL-8, and TNF-α increase with age,32–34 particularly when accompanied by significant life stress.32,33 Similarly, DHEA-S levels have been found to decline as a normal function of aging.35 The extant literature also suggests the presence of sex differences in cytokine production, attributable in part to hormonal differences between men and women.36 As previous studies have found differential rates of production of cytokines from human T-cells and monocytes that vary according to both age and sex,37 these factors likely have coexisting effects on immune function and cytokine production and must both be acknowledged.
One salient factor that is relevant to female-specific aging and cytokine production is menopause. Menopause is a key biologic milestone that may underlie age-related cytokine changes in women with FM, as researchers have noted an increased rate of early menopause and hysterectomies in women with FM and have suggested that these factors may contribute to the development of FM.38 Others have noted that FM symptomatology may initiate or worsen at the onset of menopause.8 The mechanisms by which menopause may cause or exacerbate FM symptoms have not been clearly demonstrated. However, postmenopausal women with FM have demonstrated decreased circulating levels of some hormones implicated in the regulation of inflammatory responses, which have been associated with greater serum levels of IL-6 and IL-8 in these individuals.39 There is also evidence of a decline in DHEA-S levels in the years leading up to menopause,40,41 suggesting a contribution of aging factors that are unique to women. As menopausal status and psychiatric distress may contribute differentially to inflammatory processes in FM, the current study aimed to provide preliminary evidence regarding contributions of these variables to inflammatory markers and FM-related pain.
Hypotheses of the current study
The current study proposed to characterize cytokine activity in FM with a greater focus on the potential psychological and biologic contributors underlying this disorder. The current study utilized a subsample of women with FM from a larger study of cytokine reactivity in FM and chronic musculoskeletal pain. We sampled baseline serum cytokine levels (IL-6, IL-8, IL-10, and TNF-α), as well as DHEA-S levels, in pre- and postmenopausal women diagnosed with FM. We aimed to characterize all serum analytes and hypothesized that 1) cytokine and DHEA-S levels would vary significantly as a function of menopausal status; 2) there would be significant relationships between measures of depressive symptoms, pain-specific anxiety, and pain catastrophizing and all serum analytes; and 3) there would be a specific effect of depression on the ratio of serum IL-6/serum IL-10 levels.
Methods
Participants
Thirty-four women were recruited through the Oregon Health and Science University, Portland, OR, USA, as part of an experimental study of cytokine responses to a negative imaginal focus induction. The current manuscript describes only the basal profiles of the FM participants. Twenty-one women reported having completed menopause and 13 reported being premenopause. Due to aberrantly high levels of IL-6, one postmenopausal participant was identified as an outlier and was excluded from analyses involving IL-6. The overall sample was a mean age of 50.3 years (standard deviation [SD] =12.1); premenopausal participants were a mean age of 37.8 years (SD =6.4), and postmenopausal women were a mean age of 58.3 years (SD =6.2). The sample was predominantly Caucasian (N=30). All premenopausal participants were in the follicular stage of their menstrual cycle when their lab visit was conducted. FM diagnosis was confirmed through review of copies of participants’ medical charts. All postmenopausal women in the current sample underwent natural menopause, rather than surgically induced menopause. Menopausal status was determined by participants’ self-reports regarding the cessation of their menstrual cycles; participants were asked how long they had been postmenopausal for, and only those women who reported being postmenopausal for at least 1 year were included in the study. Further, we supplemented participant self-report of menopausal status with baseline sex steroid testing (data not shown). Inclusionary and exclusionary criteria can be found in Table 1. The Beck Depression Inventory-Second Version (BDI-II)42 was used to identify participants with current and severe major depression at the screening session. Participants with active suicidality or a BDI-II score >35 were excluded from the current study.
  | Table 1 Inclusionary/exclusionary criteria |
Measures
Depressive symptoms
The Center for Epidemiologic Studies Scale-Depression (CES-D) is a commonly used self-report scale that is used to assess subjective symptoms of depression in research studies.43 The CES-D contains 20 items, coded 0 (“rarely or none of the time, less than 1 day per week”) to 4 (“most or all of the time, 5–7 days per week”), with higher numerical responses corresponding to the greatest intensity of each depressive symptom. Participant responses are summed, up to a maximum total of 80. A cutoff score of either 1944 or 2045 has been suggested to reflect clinically significant depression in chronic pain populations.
Pain-related anxiety
Participants were administered the Pain Anxiety Symptoms Scale 40-Item Version (PASS-40).46 This questionnaire assesses domains of cognitive anxiety, pain-related fear, escape and avoidance responses, and physiological anxiety using 20 items on a 6-point scale, from 0 (“never”) to 5 (“always”).47 The PASS-40 has demonstrated adequate psychometric properties in clinical pain populations.47
Pain catastrophizing
Participants were administered the Pain Catastrophizing Scale (PCS),48 which assesses trait levels of catastrophic thoughts about pain and has demonstrated a reliable factor structure in a variety of studies.49–51 The PCS contains 13 items, coded from 0 (“not at all”) to 4 (“all the time”), and summed for a total score from 0 to 52.
Pain intensity
Pain intensity was assessed using an 11-point visual analog scale (VAS), with scores from 0 (“no pain at all”) to 10 (“worst pain imaginable”). VAS scales are a common method of assessment for pain intensity in chronic pain populations and have demonstrated validity for this purpose.52
Procedure
Study visit scheduling
To minimize hormonal variability, premenopausal participants called the study coordinator at onset of menses, and the study visit was scheduled to occur during the follicular phase, within days 3–9 of their cycle. The follicular phase was chosen because it follows the menstrual period and is easy to predict in our sample of normally menstruating women by using a calendar. Study visits were scheduled ad libitum for postmenopausal participants.
Study visit procedures
Participants in the current study received $150 compensation for their participation in a larger study that involved 6 hours in the laboratory and a stress experiment. All participants arrived at the study site at 8 am, fasting since dinner the night before, having consumed only water and no caffeine that morning and having taken medications as prescribed. Participants completed study questionnaires and were measured for height, weight, and blood pressure. A topical anesthetic was offered prior to the placement of an intravenous catheter (22-gauge or 20-gauge) in the nondominant arm or hand. Following a 25-minute rest period, blood was drawn into two 10 mL Vacutainers (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Whole blood was immediately spun and aliquotted, and serum was stored at −80°C until the assays were performed. Serum levels of TNF-α, IL-6, IL-8, and IL-10 were measured in duplicate using a bead-based, high-sensitivity immunofluorescence assay according to the manufacturer’s instructions (Millipore Inc.). Data were collected and analyzed using the Luminex 200 system Version 2.3 (Luminex, Austin, TX, USA). A four-parameter regression formula was used to calculate the sample concentrations from the standard curves. The threshold for detection for all analytes was 0.13 pg/mL.
DHEA-S levels were sampled using an enzyme-linked immunosorbent assay (ELISA).53 At baseline, whole blood was collected in a sodium heparin tube, and an L1000 pipette set at 75 μL was used to pipette blood spots onto filter paper. After drying, blood-spotted filter paper was individually stored in tightly sealed plastic bags with several desiccant packets at −80°C. DHEA-S was measured by a serum-based solid-phase ELISA according to the manufacturer (DRG EIA-1562) and modified for dried blood spots as described.53 Blood spot levels for DHEA-S correlate well with matched serum (r=0.89). Intra-assay coefficients of variation for the dried blood spot DHEA-S assay are 11.2% at 38 μg/dL, 5.0% at 126 μg/dL, and 12.9% at 244 μg/dL. Interassay coefficients of variation are 10.8% at 58 μg/dL, 4.4% at 113 μg/dL, and 7.8% at 260 μg/dL. The mean recovery is 104%. The ZRT Laboratory assay range (20–80 percentile) for women aged 15–80 years is 40–290 μg/dL and is age dependent, with higher values at younger ages and progressively tapering with age.
Statistical analysis
Means and SDs were calculated for demographic variables (age, body mass index), psychological variables (PCS, PASS-40, CES-D), cytokines (IL-6, IL-8, IL-10, TNF-α), and DHEA-S. All cytokine variables and DHEA-S were log transformed to address significant non-normality in their distributions. Demographic differences and psychological variables can be found in Table 2, and descriptive statistics can be found in Table 3. Differences between premenopausal and postmenopausal participants were calculated using between-group t-tests, and relationships between study variables were tested using Pearson r correlations. Correlations between study variables in pre-menopausal women can be found in Table 4, and correlations between study variables in post-menopausal women can be found in Table 5. Moderation of relationships between psychological and cytokine measures was tested, when appropriate, by the interaction between menopausal status and the psychological predictor (eg, CES-D scores) in an analysis of covariance model. All analyses were conducted using SPSS Version 20 (IBM Corporation, Armonk, NY, USA). The correlations reported in the results section are presented with probability values that are not adjusted for multiple comparisons between variables. Given the small sample size and the prevailing goal adopted by the current study of identifying associations for future study, this was deemed an appropriate step for characterizing the potential relationships of substantive interest.
Results
Study demographics
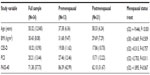
Participants in the current study showed comparable levels of pain anxiety as previous studies of healthy women (mean [M] =54.82, SD =27.53 in the Osman et al54 study) and individuals with FM and chronic low back pain (mean scores ranging from 68.4 to 84.7 in the Roelofs et al55 study). Participants in the current study showed higher scores on the CES-D than healthy women in a community sample (M=10.4, SD =10.3 in the Frerichs et al56 study) but comparable CES-D scores were also comparable with previous samples of individuals with FM (M=17.29, SD =9.39 in the Giesecke et al57 study). PCS scores in the current sample were also generally comparable with scores in healthy women from a community sample (M=15.68, SD =10.93 in the Osman et al50 study) and in samples of women with chronic pain (M=24.29, SD =8.75 in the Osman et al50 study). Notably, however, PCS scores were significantly higher in premenopausal than postmenopausal participants in our study (t[32] =2.703, P=0.011).
Menopausal status and biological variables
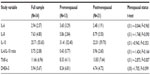
DHEA-S levels did not differ significantly according to menopausal status. Regarding cytokine and chemokine differences, some statistically significant differences were noted according to menopausal status. TNF-α was significantly higher in postmenopausal women (t[31] =−2.873, P=0.007). Serum IL-8 levels did not significantly differ by menopausal status, though a trend toward higher IL-8 levels in postmenopausal women was detected (t[31] =−1.75, P=0.090). There were no menopausal status-based differences noted in levels of IL-6, IL-10, or IL-6/IL-10 ratios that reached statistical significance (P>0.10 in all cases).
Relationships between biologic variables and psychological variables
IL-8 correlated significantly with CES-D scores in the overall sample (r=0.363, P=0.038), but not with PCS (r=0.200, P=0.264) or PASS-40 scores (r=0.238, P=0.183). Significant and positive correlations were found between IL-8 and all of the psychological measures for postmenopausal women only: CES-D (r=0.551, P=0.012), PCS (r=0.555, P=0.011), and PASS-40 (r=0.559, P=0.010). IL-8 did not correlate with CES-D (r=0.042, P=0.893), PASS-40 (r=−0.068, P=0.824), or PCS scores (r=−0.290, P=0.336) in the premenopausal group. IL-6 and IL-10 levels were uncorrelated with PCS, PASS-40, or CES-D scores and when examined separately by menopausal status. Similarly, TNF-α levels were uncorrelated with PCS, PASS-40, and CES-D scores in the overall participant sample and when tested separately by menopausal group. DHEA-S levels did not correlate with serum cytokine or chemokine levels, pain intensity, or PCS, PASS-40, or CES-D scores in the overall sample or in any subgroup of participants.
Depression and IL-6/IL-10 ratios
A significant correlation was noted between IL-6/IL-10 ratios and CES-D scores in the overall sample (r=−0.409, P=0.018), though this relationship did not reach statistical significance in either the premenopausal group (r=−0.490, P=0.089) or the postmenopausal group (r=−0.337, P=0.146). Menopausal status did not moderate the relationship between CES-D and IL-6/IL-10 ratios (P>0.50).
Discussion
The current study aimed to characterize basal cytokine profiles in pre- and postmenopausal women with FM through examination of serum concentrations of cytokines, chemokines, and inflammation-regulating hormones. Further, we sought to provide additional context to these findings through the examination of other factors that are likely to be contributory to inflammatory processes, namely menopausal status and psychological distress. Our findings revealed interesting differences in inflammatory processes when the menopausal status and psychological status of participants were taken into account.
Mean scores on psychological variables in the current study were comparable with previous samples of chronic pain using the PCS, CES-D, and PASS-40 scores. Premenopausal and postmenopausal participants reported similar levels of depressive symptoms and pain anxiety. Although premenopausal participants did report significantly higher levels of pain catastrophizing, the average scores on the PCS for these participants were similar to previous samples of individuals with FM.58 As noted previously, however, participants in our study showed somewhat elevated levels of depressive symptoms compared with community samples of healthy women, though no obvious differences in pain anxiety or pain catastrophizing were noted.
Our results suggested that IL-8 was correlated with depressive symptoms, pain catastrophizing, and pain-related anxiety for postmenopausal women but not for premenopausal women. These findings suggest a possible contributing role for IL-8 in psychological functioning in chronic pain, which is contrary to some previous findings.14,18,59 While previous work has largely focused on describing inflammatory factors in isolation, our findings underscore the importance of considering menopausal status and psychological functioning in future studies that examine inflammatory processes that may underlie FM. It is particularly important to acknowledge the relative scarcity of attention paid to the role of menopause and other age-related factors in FM, despite evidence that the prevalence of FM increases as individuals progress further into old age,30 and that there appear to be changes in brain structure and function that have age-dependent implications for individuals with FM.60
Our results suggested no relationship between IL-6 and measures of psychological distress. While these findings stand at odds with the broader literature, largely comprising studies describing healthy samples,10,12 they support previous findings for FM. A study by Bazzichi et al18 reported no differences in IL-6 and IL-8 plasma levels between those with FM and a diagnosed psychiatric disorder like depression and anxiety and those with FM and no psychiatric diagnoses. The reason for this divergence is unclear, though these findings may suggest a unique immune characteristic of FM. Our results do not allow for inferences regarding the specificity of this effect in FM versus a general effect of the experience of chronic pain, but the relationship between IL-6 and depression in other chronic pain disorders warrants further study in the future. Further, our finding of a significant relationship between depressive symptoms and IL-6/IL-10 ratios replicates previous research that suggests dysregulation of cytokine activity in depression.26 We expected that menopausal status would modify the salience of IL-6/IL-10 ratios in the context of psychological functioning, but this was not supported by our findings. This null finding may have been attributable to a small sample size, however, which will be discussed later. Additional research of this relationship is therefore needed in larger samples.
It is notable that DHEA-S levels did not correlate with pain intensity or cytokine or chemokine levels in our study, which were somewhat unexpected findings. However, it is conceivable that DHEA-S plays a more dynamic role in the regulation of inflammatory responses, as to an acute stressor, but does not have a detectable relationship in the basal state with these immune factors. It is also possible that the regulatory effects of DHEA-S are too complex to be adequately characterized using correlations. Previous evidence suggests that the effects of DHEA-S may be best described in their relation to cortisol61 and testosterone levels,62 both of which have implications for immune competence and markers of inflammation like IL-6 and IL-8, and may be affected by other underlying medical factors like hypothyroidism63 or low estrogen.64
The overall literature regarding inflammatory processes in FM is inconsistent and commonly includes conflicting results.65 The inconsistency of these effects may be attributed to variability in measurement and analytic methods, sample size, and lack of sufficient adjustment for relevant confounding variables such as physical activity, medical status, and psychiatric comorbidities.16 Such methodological and statistical issues limit the interpretability of the extant literature and highlight the need for greater methodological rigor and a greater consideration for relevant third variables in order to characterize inflammatory processes in FM in the correct context. The current study was intended as a preliminary attempt to identify salient contributing factors to inflammatory processes in FM, thereby highlighting the need to account for these factors in future studies.
Limitations
A primary limitation of the current study is the size of our sample. As a result, the current study should be considered a pilot study that was exploratory in nature and was conceived as an attempt to detect novel associations between the study variables that could be more stringently examined in future studies. The limited size of our sample means that our findings require replication in larger samples to ensure that they are reliable. Our results are similarly limited due to the unbalanced size of the groups; conclusions regarding our findings should be made with caution and with the understanding that there was a comparatively larger number of postmenopausal women, which may explain some degree of the difference in significant relationships between variables in the pre- and postmenopausal groups. Further, our presented findings do not provide adjusted significance values that account for the multiple comparisons posed by our analyses. We deemed this approach necessary, given the small size of our sample and our intention to identify novel effects, but the lack of adjustment to the presented probability values is nevertheless a significant limitation. Consequently, our findings should be interpreted with this caution in mind. Additionally, some recent evidence suggests that measuring reactivity, rather than basal levels, of IL-6 may constitute a more reliable measurement approach in individuals with FM.66 As the current study provided only basal values of IL-6 and other biological variables, examination of these relationships with regard to cytokine reactivity is warranted in future studies.
We determined the presence of FM by relying on participants’ medical records, rather than performing diagnostic testing to confirm participant diagnoses during the study. This approach relies on diagnosis from previous medical providers, which may be variable with regard to adherence to a strict set of diagnostic criteria for FM. Though we have no reason to suspect inaccurate FM diagnoses in our study sample, our results should be interpreted with this caution in mind. Similarly, the current study did not specifically target individuals with clinical mood disorders. It is possible that specific examination of individuals with diagnosed mood disorders such as major depressive disorder may demonstrate stronger relationships between distress and cytokine production if these relationships are examined according to sex- and age-specific factors, as in the current study. Due to differences in the processing of sampling assays compared with previous studies, we were also unable to provide comparisons with cytokine levels in healthy pre- and postmenopausal women, which could serve to provide additional context to our findings and would provide a clearer illustration of the processes of FM. Although FM is predominantly found in women, comparison of our findings with similarly aged men, both with and without FM, would also be beneficial to delineate the unique aspects of sex, aging, and inflammatory processes in FM. As a result, we urge attention to these issues in future studies. Despite these limitations, we propose that our findings regarding differential implications of psychological and menopausal status for IL-6, IL-8, and IL-10 activity should be interpreted as preliminary findings that warrant further examination with additional sampling in a larger study in the future.
Conclusion
The current study sought to characterize differences in basal proinflammatory cytokine profiles in women with FM. While data are preliminary, the current study is among the first to present serum cytokine levels in women with FM, and the first to do so with specific attention paid to age-related and psychological factors. Our findings regarding the role of menopause are particularly important, as they highlight the potential importance of aging factors in understanding the development of FM in women. However, replication of our results in larger samples of women with FM is necessary. Our findings highlight an avenue for future research that may provide additional utility in characterization of the complex etiology and clinical presentation of FM.
Disclosure
Dr Zava is the owner and president of ZRT Laboratories. The authors report no other conflicts of interest in this work.
References
Wolfe F, Brähler E, Hinz A, Häuser W. Fibromyalgia prevalence, somatic symptom reporting, and the dimensionality of polysymptomatic distress: results from a survey of the general population. Arthritis Care Res (Hoboken). 2013;65(5):777–785. | |
Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010;62(5):600–610. | |
Wolfe F, Clauw DJ, Fitzcharles M-A, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38(6):1113–1122. | |
Wolfe F, Walitt BT, Häuser W. What is fibromyalgia, how is it diagnosed and what does it really mean? Arthritis Care Res (Hoboken). 2014;66(7):969–971. | |
Clauw DJ. Fibromyalgia: more than just a musculoskeletal disease. Am Fam Physician. 1995;52(3):843–851, 853–844. | |
Nicassio PM, Moxham EG, Schuman CE, Gevirtz RN. The contribution of pain, reported sleep quality, and depressive symptoms to fatigue in fibromyalgia. Pain. 2002;100(3):271–279. | |
Finan PH, Zautra AJ, Davis MC, Lemery-Chalfant K, Covault J, Tennen H. Genetic influences on the dynamics of pain and affect in fibromyalgia. Health Psychol. 2010;29(2):134. | |
Buskila D, Sarzi-Puttini P, Ablin JN. The genetics of fibromyalgia syndrome. Pharmacogenomics. 2007;8(1):67–74. | |
Wallace D, Linker-Israeli M, Hallegua D, Silverman S, Silver D, Weisman M. Cytokines play an aetiopathogenetic role in fibromyalgia: a hypothesis and pilot study. Rheumatology (Oxford). 2001;40(7):743–749. | |
Giacomelli C, Talarico R, Bombardieri S, Bazzichi L. The interaction between autoimmune diseases and fibromyalgia: risk, disease course and management. Expert Rev Clin Immunol. 2013;9(11):1069–1076. | |
Watkins LR, Maier SF, Goehler LE. Immune activation: the role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain. 1995;63(3):289–302. | |
Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. | |
Di Franco M, Iannuccelli C, Valesini G. Neuroendocrine immunology of fibromyalgia. Ann N Y Acad Sci. 2010;1193(1):84–90. | |
Gür A, Karakoç M, Nas K, Denli A, Saraç J. Cytokines and depression in cases with fibromyalgia. J Rheumatol. 2002;29(2):358–361. | |
Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. | |
üçeyler N, Häuser W, Sommer C. Systematic review with meta-analysis: cytokines in fibromyalgia syndrome. BMC Musculoskelet Disord. 2011;12(1):245. | |
Wang H, Moser M, Schiltenwolf M, Buchner M. Circulating cytokine levels compared to pain in patients with fibromyalgia: a prospective longitudinal study over 6 months. The J Rheumatol. 2008;35(7):1366–1370. | |
Bazzichi L, Rossi A, Massimetti G, et al. Cytokine patterns in fibromyalgia and their correlation with clinical manifestations. Clin Exp Rheumatol. 2007;25(2):225. | |
Straub R, Schuld A, Mullington J, Haack M, Scholmerich J, Pollmacher T. The endotoxin-induced increase of cytokines is followed by an increase of cortisol relative to dehydroepiandrosterone (DHEA) in healthy male subjects. J Endocrinol. 2002;175(2):467–474. | |
Spencer NF, Norton SD, Harrison LL, Li G-Z, Daynes RA. Dysregulation of IL-10 production with aging: possible linkage to the age-associated decline in DHEA and its sulfated derivative. Exp Gerontol. 1996;31(3):393–408. | |
Mikkelsson M. One year outcome of preadolescents with fibromyalgia. J Rheumatol. 1999;26(3):674–682. | |
Epstein SA, Kay G, Clauw D, et al. Psychiatric disorders in patients with fibromyalgia: a multicenter investigation. Psychosomatics. 1999;40(1):57–63. | |
Simon N, McNamara K, Chow C, et al. A detailed examination of cytokine abnormalities in major depressive disorder. Eur Neuropsychopharmacol. 2008;18(3):230–233. | |
Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol. 2009;30(1):65–91. | |
Takebayashi M, Kagaya A, Uchitomi Y, et al. Plasma dehydroepiandrosterone sulfate in unipolar major depression. J Neural Transm. 1998;105(4–5):537–542. | |
Dhabhar FS, Burke HM, Epel ES, et al. Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. J Psychiatr Res. 2009;43(11):962–969. | |
O’Donovan A, Hughes BM, Slavich GM, et al. Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion–biologyrelationships. Brain Behav Immun. 2010;24(7):1074–1077. | |
Dell’Osso L, Carmassi C, Massimetti E, et al. P01-143-gender differences in the correlations between cortisol levels or DHEA-S/cortisol ratio and panic-agoraphobic dimensions in healthy subjects. Eur Psychiatry. 2011;26:143. | |
Loerbroks A, Thomas GN, Engeland CG, Hollands MA, Fischer JE, Bosch JA. Age-dependent and-independent associations between depression, anxiety, DHEAS, and cortisol: from the MIPH Industrial Cohort Studies (MICS). Psychoneuroendocrinology. 2012;37(7):929–936. | |
Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38(1):19–28. | |
Licastro F, Candore G, Lio D, et al. Innate immunity and inflammation in ageing: a key for understanding age-related diseases. Immun Ageing. 2005;2(8):1–14. | |
Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003;100(15):9090–9095. | |
Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhøj P, Pedersen BK. A high plasma concentration of TNF-α is associated with dementia in centenarians. J Gerontol A Biol Sci Med Sci. 1999;54(7):M357–M364. | |
Burns EA, Goodwin JS. Immunodeficiency of aging. Drugs Aging. 1997;11(5):374–397. | |
Rozenberg S, Bosson D, Peretz A, Caufriez A, Robyn C. Serum levels of gonadotrophins and steroid hormones in the post-menopause and later life. Maturitas. 1988;10(3):215–224. | |
Rohleder N, Schommer NC, Hellhammer DH, Engel R, Kirschbaum C. Sex differences in glucocorticoid sensitivity of proinflammatory cytokine production after psychosocial stress. Psychosom Med. 2001; 63(6):966–972. | |
Pietschmann P, Gollob E, Brosch S, et al. The effect of age and gender on cytokine production by human peripheral blood mononuclear cells and markers of bone metabolism. Exp Gerontol. 2003;38(10):1119–1127. | |
Pamuk öN, Dönmez S, çakir N. Increased frequencies of hysterectomy and early menopause in fibromyalgia patients: a comparative study. Clin Rheumatol. 2009;28(5):561–564. | |
Ross RL, Jones KD, Bennett RM, Ward RL, Druker BJ, Wood LJ. Preliminary evidence of increased pain and elevated cytokines in fibromyalgia patients with defective growth hormone response to exercise. Open Immunol J. 2010;3:9. | |
Davis SR, Burger HG. Clinical review 82: androgens and the postmenopausal woman. J Clin Endocrinol Metab. 1996;81(8):2759–2763. | |
Overlie I, Moen M, Morkrid L, Skjæraasen J, Holte A. The endocrine transition around menopause: a five years prospective study with profiles of gonadotropines, estrogens, androgens and SHBG among healthy women. Acta Obstet Gynecol Scand. 1999;78(7):642–647. | |
Beck A, Steer R, Brown G. Manual for the BDI-II. San Antonio, TX: Psychological Corporation; 1996. | |
Beekman AT, Deeg D, Van Limbeek J, Braam A, De Vries M, Van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol Med. 1997;27(1):231–236. | |
Turk DC, Okifuji A. Detecting depression in chronic pain patients: adequacy of self-reports. Behav Res Ther. 1994;32(1):9–16. | |
Magni G, Moreschi C, Rigatti-Luchini S, Merskey H. Prospective study on the relationship between depressive symptoms and chronic musculoskeletal pain. Pain. 1994;56(3):289–297. | |
McCracken LM, Zayfert C, Gross RT. The Pain Anxiety Symptoms Scale: development and validation of a scale to measure fear of pain. Pain. 1992;50(1):67–73. | |
McCracken LM, Gross RT. Does anxiety affect coping with chronic pain? Clin J Pain. 1993;9(4):253–259. | |
Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7(4):524–532. | |
Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O’Neill E. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med. 1997;20(6):589–605. | |
Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med. 2000;23(4):351–365. | |
Van Damme S, Crombez G, Bijttebier P, Goubert L, Van Houdenhove B. A confirmatory factor analysis of the Pain Catastrophizing Scale: invariant factor structure across clinical and non-clinical populations. Pain. 2002;96(3):319–324. | |
Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. | |
Du JY, Sanchez P, Kim L, Azen CG, Zava DT, Stanczyk FZ. Percutaneous progesterone delivery via cream or gel application in postmenopausal women: a randomized cross-over study of progesterone levels in serum, whole blood, saliva, and capillary blood. Menopause. 2013;20(11):1169–1175. | |
Osman A, Barrios FX, Osman JR, Schneekloth R, Troutman JA. The Pain Anxiety Symptoms Scale: psychometric properties in a community sample. J Behav Med. 1994;17(5):511–522. | |
Roelofs J, McCracken L, Peters ML, Crombez G, van Breukelen G, Vlaeyen JW. Psychometric evaluation of the Pain Anxiety Symptoms Scale (PASS) in chronic pain patients. J Behav Med. 2004;27(2):167–183. | |
Frerichs RR, Aneshensel CS, Clark VA. Prevalence of depression in Los Angeles county. Am J Epidemiol. 1981;113(6):691–699. | |
Giesecke T, Williams DA, Harris RE, et al. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis Rheum. 2003;48(10):2916–2922. | |
Crombez G, Eccleston C, Van den Broeck A, Goubert L, Van Houdenhove B. Hypervigilance to pain in fibromyalgia: the mediating role of pain intensity and catastrophic thinking about pain. Clin J Pain. 2004;20(2):98–102. | |
Ang DC, Moore MN, Hilligoss J, Tabbey R. MCP-1 and IL-8 as pain biomarkers in fibromyalgia: a pilot study. Pain Med. 2011;12(8):1154–1161. | |
Ceko M, Bushnell MC, Fitzcharles M-A, Schweinhardt P. Fibromyalgia interacts with age to change the brain. Neuroimage Clin. 2013;3:249–260. | |
Patacchioli FR, Monnazzi P, Simeoni S, et al. Salivary cortisol, dehydroepiandrosteronesulphate (DHEA-S) and testosterone in women with chronic migraine. J Headache Pain. 2006;7(2):90–94. | |
Finset A, øverlie I, Holte A. Musculo-skeletal pain, psychological distress, and hormones during the menopausal transition. Psychoneuroendocrinology. 2004;29(1):49–64. | |
Gerwin RD. A review of myofascial pain and fibromyalgia: factors that promote their persistence. Acupunct Med. 2005;23(3):121–134. | |
Craft RM. Modulation of pain by estrogens. Pain. 2007;132:S3–S12. | |
Raison V. Neurobiology of depression, fibromyalgia and neuropathic pain. Front Biosci. 2009;14:5291–5338. | |
Geiss A, Rohleder N, Anton F. Evidence for an association between an enhanced reactivity of interleukin-6 levels and reduced glucocorticoid sensitivity in patients with fibromyalgia. Psychoneuroendocrinology. 2012;37(5):671–684. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.