Back to Journals » OncoTargets and Therapy » Volume 12
Progression of the role of CRYAB in signaling pathways and cancers
Authors Zhang JF , Liu J, Wu JL, Li WF, Chen ZW, Yang LS
Received 16 January 2019
Accepted for publication 7 April 2019
Published 30 May 2019 Volume 2019:12 Pages 4129—4139
DOI https://doi.org/10.2147/OTT.S201799
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yao Dai
JunFei Zhang, Jia Liu, JiaLi Wu, WenFeng Li, ZhongWei Chen, LiShan Yang
Department of Emergency Medical, General Hospital of Ningxia Medical University, Yinchuan, Ningxia 750000, People’s Republic of China
Abstract: CRYAB is a member of the small heat shock protein family, first discovered in the lens of the eye, and involved in various diseases, such as eye and heart diseases and even cancers, for example, breast cancer, lung cancer, prostate cancer, and ovarian cancer. In addition, CRYAB proteins are involved in a variety of signaling pathways including apoptosis, inflammation, and oxidative stress. This review summarizes the recent progress concerning the role of CRYAB in signaling pathways and diseases. Therefore, the role of CRYAB in signaling pathways and cancers is urgently needed. This article reviews the regulation of CRYAB in the apoptotic inflammatory signaling pathway and its role in cancers progression and as a key role in anti-cancer therapy targeting CRYAB in an effort to improve outcomes for patients with metastatic disease.
Keywords: CRYAB, signaling pathways, cancers
Introduction
Small heat shock proteins (sHsps or HspBs) form a large and evolutionary ancient family, whose members have been found in viruses, archaea, bacteria, plants, and animals.1 The human genome contains 10 genes encoding sHsps.2 Some sHsps (HspB1, HspB5, HspB6, and HspB8) are expressed ubiquitously, while others (HspB2, HspB3, HspB4, HspB7, HspB9, and HspB10) have been found only in certain tissues.3 sHsps are characterized by their complex oligomeric structures, allowing them to interact with each other to form homo- and hetero-oligomeric structures of dynamic size (up to 700 kDa).4 For instance, the heterooligomeric complex formed by HspB4 and HspB5 plays an important role in keeping the lens transparency.5 In heterooligomeric complexes, HspB6 and HspB1 mutually affect the structure of each other and the formation of heterooligomeric complexes might influence diverse processes depending on sHsps.6
HspB5, also known as CRYAB or αB-Crystallin, has an N-terminal domain, a central domain, and a C-terminal domain.7 Its structural and functional characteristics are shown in Figure 1:8 1) low molecular weight of 22 kDa; 2) N-terminal domain of about 60 residues, a conserved α-crystallin structure of about 90 residues involved in the dimerization domain, and the 25-residue C-terminal domain containing the IXI motif; 3) the ability to form large oligomers; 4) dynamic quaternary structure; and 5) induction by stress conditions.
CRYAB was first discovered proteins in the lens of the eye9 and is also expressed in other parts of the body, such as the heart, skeletal muscle, ovaries, etc.10–12 However, CRYAB protein mutations associate with the different diseases. For instance, domain mutations (D109H, R120G, Q151X, G154S, P155Rfs9X, and R157H) are associated with myopathy.13 The dominant D109A mutation of CRYAB is pathogenic and associated with myofibrils myopathy.14,15 In addition to myopathy, mutations D109H, R120G, and X176Wfs19X are only associated with cardiomyopathy and cataract or discrete lens opacity.16 Other dominant mutations (R11H, P20R, P20S, R69C, D140N, K150Nfs34X, A171T) and recessive mutations (eg, R11C, R12C, R56W) are also described as associated with congenital cataracts, uniformly dispersed throughout the coding sequence.17 The autosomal dominant multisystem phenotype in the residual (D109H) mutation is not only associated with myopathy, but also with cardiomyopathy and lens cataract.18 Even point mutations or short deletions in CRYAB can lead to the development of different hereditary diseases.
CRYAB acts primarily as a chaperone, blocking the aggregation of denatured proteins and keeping aggregation‐prone proteins in reservoirs of non-native, foldable intermediates within large, soluble, multimeric structures.19 The ectopic expression of CRYAB in diverse cell types has been demonstrated to confer protection against a broad range of apoptotic stimuli,20 oxidative stress,21 and exposure to anticancer drugs.22 Simultaneously, silencing its expression by RNA interference sensitizes cells to apoptosis.23 Similarly, a growing number of researchers have described the high expression of CRYAB in human cancers and the significant relationship between CRYAB and unfavorable survival of cancer patients.24–26
So, what is the role of CRYAB in participating in the apoptotic and inflammatory pathways and what role does it play in the diseases? Here, we review recent advances implicating the importance of CRYAB in signaling pathways, its role in cancer progression, and as target molecules in anticancer therapy.
The role of CRYAB in the signaling pathway
CRYAB has multiple functions in cells, but how does it work? Based on the report, the apparent pleiotropic activity of CRYAB may be due to its binding to chaperones and regulation of the activity and half-life involved in many protein targets involved in apoptotic cell death tumorigenesis and metastasis.27–29 And CRYAB contains several serine sites that can be phosphorylated by specific stress or mitogen-activated protein kinases.30 Phosphorylation and oligomeric organization of these proteins are dynamic and are deeply modified due to changes in the cellular environment.31 In fact, these structural modifications are reversible and may be sensors in the cellular environment. Changes in the sHsps structure can lead to at least 300 different stoichiometries to allow them to interact with putative proteins.32
Participation in apoptosis
Apoptosis is a programmed cell death that is negatively regulated by sHsps.33–35 In environmental damage such as heat shock, contrary to the increased expression of sHsps, the expression of these proteins is not up-regulated in apoptosis. In some cells, the expressed sHsps can counteract the apoptotic process mediated by the immune system or therapeutic drugs. CRYAB is now thought to interact with specific proteins and regulate their activity during the initiation and execution phases of apoptosis.36,37
CRYAB is a recognized anti-apoptotic protein,38 whose main property is to negative regulation of the proapoptotic members of the Bcl-2 family, Bax, and caspase-3.39 CRYAB interacts directly with caspase-3, Bax, and Bcl-xS.39,40 In addition to interacting with these proteins, CRYAB inhibits their transfer from the cytoplasm to the mitochondria, thereby preventing stress-induced apoptosis.40 Similarly, CRYAB interacts with p53 to sequester its translocation to the mitochondria, thus indirectly inhibiting its pro-apoptotic effect against the apoptotic Bcl-2 molecule.41 CRYAB protects cells from apoptosis by inhibiting caspase-3 and PARP (poly(ADP-ribose) polymerase) activation.42 CRYAB was also found to inhibit p53-dependent apoptosis mediated by the calcium-activated Raf/MEK/ERK signaling pathway by inhibiting Ras activation.43 CRYAB can also block UVA cell apoptosis by participating in the regulation of PKCα1pha and Raf/MEK/ERK signaling pathway proteins.44 In addition, CRYAB binds directly to the most potent endogenous inhibitor of apoptosis, X-linked inhibitor of apoptosis, to inhibit caspase.45 CRYAB is involved in the regulation of intracellular apoptosis signaling, which inhibits apoptosis by activating the Akt signaling pathway and enhancing PI3K activity.46 The above data indicates the anti-apoptotic effect of the CRYAB protein (Figure 2). Recently, results showed CRYAB protects cardiomyocytes against heat stress, likely by reducing Factin aggregation (thus stabilizing the cytoskeleton), regulating the cell cycle, and preventing caspase-mediated apoptosis.47
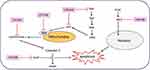 | Figure 2 Schematic diagram of CRYAB protein involved in the regulation of apoptosis (inhibition: ┤, activation: →). |
Involved in inflammation and redox
Intracellular CRYAB has been shown as one of the potent factors in controlling neuroinflammation in several occasions, for instance, multiple sclerosis (MS), an autoimmune demyelinating disease of the central nervous system (CNS). A surprising finding about CRYAB is that patients with MS have at least 70 different proinflammatory mediators (acute phase proteins, complement cascade members, and clotting factors). The interaction with CRYAB reduces the concentration of these peptides, leading to a decrease in inflammatory response.48 In the CNS, microglia and astrocytes are the two main cellular components that participate in the inflammatory process. Recent research reports indicated the molecular and cellular basis of extracellular CRYAB-mediated suppression of neuroinflammation. In EAE mice, the expression of CRYAB was significantly increased in astrocytes. CRYAB was preferentially expressed in astrocytes and can be secreted through exosomes. Expression levels of exosomal CRYAB secreted from astrocytes were markedly increased under stress. Furthermore, incubation of immortalized astrocytes or microglia cell lines with CRYAB remarkably inhibited astrocytes and microgliamediated inflammatory responses in both autocrine and paracrine manners.49
Another anti-inflammatory pathway associated with extracellular CRYAB involves activation of immunoregulatory responses in macrophages via endosomal/phagosome CD14 and Toll-like receptors 1 and 2 (two new CRYAB interacting proteins).50 These reports clearly indicate that CRYAB has a different effect on inflammation.
Inflammation is also associated with oxidative stress, and, in this respect, CRYAB induces a reduced state in cells.51,52 In fact, CRYAB-expressing cells are accompanied by a decrease in mitochondrial membrane potential and reduced glutathione, a decrease in intracellular reactive oxygen species and nitric oxide levels, and iron uptake.53,54 Therefore, it can impair protein oxidation, lipid peroxidation, DNA damage, and cytoskeletal structure damage caused by oxidative stress.55–57
CRYAB in diseases
CRYAB is a structural protein of the lens that helps maintain lens transparency.9 CRYAB is also expressed in non-tissue, including the heart, brain, skeletal muscle, skin, ovaries, and kidneys.10–12 Abnormal expression of CRYAB also occurs in many diseases, such as eye diseases58,59 and heart diseases.60,61 There is increasing evidence that CRYAB is an important regulator of cardiac cytoprotection and is resistant to various forms of cellular stress. Over-expression of CRYAB in cultured cardiomyocytes and transgenic mouse hearts protects against ischemia or reperfusion injury52 and reduces cardiac hypertrophy caused by overload53 CRYAB-20 protein levels are reduced during ischemia, and reperfusion causes clinical damage to cardiac function. Administration of CRYAB-20 peptide reduced the infarct size of the mouse model of myocardial infarction and showed cardioprotective effects of CRYAB.64 Most interesting is the recent discovery that abnormal expression of these chaperones often occurs in tumors.65–67 There is increasing evidence that CRYAB is diversified and significantly associated with cancer.68,69 Emerging strategies to therapeutically target CRYAB and/or interacting proteins to selectively activate apoptosis and/or derail the metastatic cascade in an effort to improve outcomes for patients with metastatic disease.24
CRYAB in breast cancer
Breast cancer (BC) is one of the most common cancers for females and the leading cause of cancer-related death in females globally, with a high incidence rate and mortality.70 BC may have evolved from a process of continuous progression of hyperplasia of mammary gland (HMG).71,72 According to reports, the expression of CRYAB was significantly up-regulated in HMG,73 while it was also highly expressive in BC, such as basal-like, triple-negative breast cancer (TNBC), and mammary metaplastic carcinoma.74–76 Although Her2 has long been reported to be involved in the pathogenesis of brain metastases, it has recently been reported that CRYAB expression is associated with distant recurrence in TNBC patients and as the first distal site compared to early brain recurrence.77–80 Consistent with this pathogenic effect, CRYAB is closely associated with advanced tumor progression, lymphocytic infiltration, and death, and could be a novel oncoprotein biomarker of a poor prognosis in BC, especially in advanced patients.81,82 Paradoxically, the upregulation of CRYAB was not involved in the molecular chaperone in the progression of the disease,83 and did not seem to have an independent impact on patient survival or to interfere with taxane-based therapy in two randomized clinical trials.84
So how is the CRYAB protein regulated in BC? Upregulation of CRYAB may be associated with transcriptional activation. In fact, the CRYAB gene promoter contains the proto-oncogene Ets1, a member of the ETS transcription factor family that bind to DNA at palindromic ETS-binding sites (EBS), which are activated by the oncogenic transcription factor Ets1. Ets1-mediated events appear to be associated with poor survival.85 In addition, Bcl-2 is reported to be a positive prognostic marker for BC, and CRYAB is a marker of poor prognosis. In the correlation analysis, the two proteins demonstrated a weak negative correlation.86 Simultaneously, CRYAB enhances tumorigenesis by regulating the vascular endothelial growth factor (VEGF) and confers anti-VEGF resistance to BC.87,88 It induces EGF and anchorage-independent growth of human mammary gland-like tumors through constitutive activation of the MEK/ERK pathway.89 CRYAB can also be used as an oncoprotein because it transforms immortalized human mammary epithelial cells in invasive BC in nude mice, which have the same characteristics as mammary gland-like tumors.75
CRYAB in lung cancer
Lung cancer (LC) is the most common malignancy worldwide, and also the leading cause of cancer-related mortality in the majority of developed countries.90 The two major types of LC are small cell (SC) and non-small cell lung cancer (NSCLC), and the latter accounts for approximately 85% of all cases.91 Many studies are devoted to NSCLC, but there is still a lack of specific and valuable molecular markers to accurately indicate the prognostic status of NSCLC patients. Early studies have shown that CRYAB is up-regulated in NSCLC by analyzing gene expression profiles of Anip973R and its parental line.92 Is CRYAB an independent marker for prognosis in NSCLC? A study reported that CRYAB did not predict outcomes in patients treated for NSCLC. The reason is that larger studies are required to validate this finding.66 Another study was the first to report on the differential expression of CRYAB with NSCLC, in both mRNA and protein level simultaneously. In addition, high CRYAB protein expression was correlated with certain clinic pathological attributes, including TNM stage and overall survival.93 CRYAB, whose nuclear staining is an independent factor of poor survival, plays an essential role in NSCLC biology.94 What’s more, ERK-regulated CRYAB induction by matrix detachment inhibits anoikis and promotes lung metastasis in vivo.95 Some studies have proved that CRYAB over-expression in idiopathic pulmonary fibrosis (IPF) disrupts Smad4 mono-ubiquitination by interacting with its E3-ubiquitin ligase, TIF1γ, limiting its nuclear export, thus activing TGF-β1-Smad4 pro-fibrotic activity, demonstrating that CRYAB may also be a key target for the development of specific drugs in the treatment of IPF or other fibrotic diseases.96 CRYAB may be distinguished as a novel prognostic biomarker in NSCLC patients, and targeting CRYAB may provide a promising strategy for NSCLC treatment.93
CRYAB in hepatocellular carcinoma
Primary liver cancer is a cancer which occurs in liver and is the third most common cause of cancer-related deaths worldwide. Hepatocellular carcinoma (HCC) is the most prevalent subtype of primary liver cancer.97 Much hope is focused on obtaining a better understanding of the mechanism relevant to this disease in order to develop new preventive, diagnostic, and therapeutic options. First, CRYAB promoted HCC progression in vivo and in vitro. Second, functional and genetic screens demonstrated that CRYAB overexpression fostered HCC progression by inducing EMT. Remarkably, CRYAB complexes with and elevates 14–3-3ζ protein, leading to up-regulation of ERK1/2 activity. Clinically, CRYAB expression correlated with BCLC staging, patients‘ overall survival, and disease recurrence. Simultaneously, CRYAB overexpression activated the ERK1/2/Fra‐1/slug signal to induce HCC cell EMT. The above results support the notion that CRYAB is a positive regulator of HCC growth and aggressiveness.65 Moreover, upregulation of CRYAB is regulated by its upstream heat shock factor 1 (HSF1), the predominant regulator of heat shock response and whose phosphorylation is induced by glucose in HCC cell lines.98 Therefore, expression of the CRYAB gene, which is related with the transferability and invasive capacity of hepatocellular carcinoma cells, can be used as a prognostic indicator in human hepatocellular carcinomas. It may also be involved in the malignant transformation of hepatocytes.99
CRYAB in OS
Osteosarcoma (OS) is the most common primary malignant tumor in children and adolescents.100 Early studies via detection of two-dimensional difference gel electrophoresis, the genomic analysis, and further studies in OS have indicated the amount of CRYAB was significantly increased, especially in advanced stages of the disease.101 CRYAB expression is high in OS tissues and is positively correlated with cell invasiveness and activity of ERK1/2 secreted by MMP-9 (Matrix Metalloprotein-9). Clinically, the high expression of CRYAB is associated with shortened survival and tumor recurrence in postoperative OS patients, and is a new adverse outcomes marker for OS patients.102 A study showed krüppel-like factor 4 (KLF4), a zinc-finger transcription factor, and an essential regulator in many cellular processes, specifically bound the promoter of CRYAB and upregulated CRYAB expression in human osteosarcoma cells.103 Another study revealed microRNAs-491 plays a role in osteosarcoma by directly targeting CRYAB.104 Whether CRYAB protein is actively regulated or passively regulated, it is closely related to OS disease, and may be a new therapeutic target of OS.
CRYAB in colorectal cancer
Colorectal cancer (CRC) is the third most common cancer in both males, and the fourth leading cause of cancer-related deaths worldwide.105 Research on early diagnosis of colorectal carcinogenesis biomarkers is still ongoing, and the selection of “personalized” treatment strategies provides a prognostic marker for colorectal cancer to improve the prognosis of the disease.106 The study was the first to report the expression of CRYAB and splicing changes may mark the risk of cancer by CRC biopsy analysis.79,107 Its high expression, lymph node metastasis, distant metastasis, and tumor TNM stage were all significantly associated with the overall survival CRC patients. CRC patients with high CRYAB expression and positive distant metastasis encountered a significantly poorer overall survival.79 Clinical data indicated that CRYAB expression upregulation had a positive association with TNM stage CRC patients.108
CRYAB high expression could prompt tumor cell proliferation, invasion, and metastasis of CRC through EMT.108,109 Its expression level in CRC patients was closely correlated with MMP7 and E-cadherin, two core EMT gene products. In addition, three significant signaling pathways (PI3K, p38, and ERK) were involved in CRYAB-induced EMT.108,110,111 However, in certain cell types, the ERK, but not PI3K and p38 signaling pathways, may be crucially involved in the invasion, proliferation, and EMT induced by CRYAB over-expression. In summary, CRYAB may trigger the EMT in CRC by activating the ERK signaling pathway and is a potential tumor biomarker for CRC diagnosis and prognosis.108 Also, CG/GG at CRYAB C-802G is correlated with CRC susceptibility, and this polymorphism may be a useful marker for the clinical outcome of CRC.112
CRYAB in head and neck cancer
Head and neck cancers comprise a heterogeneous group of tumors that arise in the paranasal sinus, oral and nasal cavities, pharynx and larynx, and salivary glands. 90% of these tumors are squamous cell carcinomas and are newly diagnosed in 600,000 patients annually.105 Only 40–50% will survive 5 years, making this the fifth-most frequent malignant cancer worldwide.113 Regarding brain cancer, high expression levels of CRYAB are evident in invasive gliomas.114,115 The higher expression of CRYAB may lead to prolonged survival of head and neck squamous cell carcinoma cells under hypoxic conditions, more likely by ROS formation.116 In laryngeal squamous cell carcinoma (LSCC), CRYAB is significantly overexpressed and correlated with malignant phenotypes. CRYAB had a poor prognosis in cancer patients25 and may serve as a novel prognostic factor for LSCC.67,117 In oral squamous cell carcinoma, CRYAB is highly expressed and has a poor prognosis for cancer patients.118 It was first found that the single nucleotide polymorphisms at the promoter region of CRYAB, C-802G, is associated with patient oral cancer susceptibility, recurrence, and 5-year disease-free survival, but not metastasis.119
However, CRYAB staining with cutaneous squamous cell carcinoma of the head and neck (CSCCHN) with clinical Perineural invasion (PNI), a clinical indicator of poor prognosis, showed a decrease compared to non-PNI CSCCHN. Surprisingly, CRYAB is a key component in the machinery leading to degradation of cyclin D1, which is key to understanding how loss of CRYAB can lead to deregulated cellular growth in CSCCHN with PNI. It is possible that under-expression of CRYAB in tumors that exhibit neurotropism contributes to their more aggressive nature.120
Moreover, in nasopharyngeal carcinoma (NPC), CRYAB is down-regulated.121 Activation of CRYAB suppressed NPC tumor formation in nude mice. Overexpression of CRYAB affected NPC progression-associated phenotypes such as loss of cell adhesion, invasion, interaction with the tumor microenvironment, invasive protrusion formation in three dimensional Matrigel culture, as well as expression of epithelial-mesenchymal transition-associated markers. CRYAB functions to suppress NPC progression by associating with the cadherin/catenin adherens junction and modulating the β-catenin function.122
CRYAB expression is down-regulated in highly dedifferentiated malignant anaplastic thyroid carcinoma because of a tumor-specific transcription factor pattern.121 CRYAB gene silencing is present in rapidly growing dedifferentiated anaplastic thyroid carcinomas. The main underlying mechanism seems to be a tumor-specific transcription factor expression pattern, which is most prominently characterized by downregulation of the transcription factor, TFCP2L1.123
CRYAB in other cancers
CRYAB was highly expressed in gastric cancer tissues, contributed to gastric cancer cells migration and invasion via EMT, mediated via the NF-κB signaling pathway, predicting a poor prognosis in patients with gastric cancer and, thus, possibly providing a novel therapeutic target for gastric cancer.124
CRYAB was reported to be expressed in retinoblastomas, but it may not prevent apoptosis of neoplastic cells;125 while in the same laboratory in the same year CRYAB was reported to be highly expressed in retinoblastoma after chemotherapy and may protect tumor cells from apoptotic signals produced by anticancer drugs.126
Integrative analysis of transcriptomics and clinical data uncovered in the prostate a novel direct transcriptional regulation of CRYAB by Microphthalmia-associated transcription factor (MITF), a basic helix-loop-helix leucine zipper transcription factor that regulates the expression of lineage commitment programs that are essential for propagation of the melanocyte lineage.127 Although there is no direct or mechanistic evidence of the MITF-CRYAB transcriptional axis in other cancer types, in melanoma both MITF and CRYAB expression are upregulated by BRAF/MEK-inhibitor treatments.128,129 In addition, the correlation between MITF and CRYAB is also present in colorectal cancer, but not in breast nor lung cancer.130 These suggested that the MITF-CRYAB axis controls beyond prostate biology and CRYAB as a novel direct target of the transcription factor that is, at least in part, responsible for its tumor-suppressive activity in the prostate and could be considered as potentially protective against prostate cancer.127,131
CRYAB, a proposed negative regulator of tumor necrosis factor-related apoptosis inducing ligand (TRAIL)- and cisplatin-induced mediated apoptosis, displayed low expression level significantly associated with adverse patient survival and acts as a molecular marker for the outcome of patients with ovarian cancer. The data showed molecular mechanisms underlying ovarian cancer cell apoptosis and resistance to TRAIL as an obstacle for its therapeutic efficacy.132,133
Conclusions and prospects
As noted above, the expression and functional roles of CRYAB in cancers (Table 1), and further research needs to be done to reveal its underlying role in the progression and metastasis of diseases. Although the CRYAB protein has multiple functions, the most important function is to act as an anti-apoptotic polypeptide in various stress-induced apoptosis69,134 and as a molecular chaperone to block aggregation of denatured proteins by exposure to heat stress.134,136 Moreover, although the pre-clinical results of CRYAB-based anti-cancer drugs in vivo and in vitro are promising, there is still a long way to go from the laboratory to clinical trials. Another important issue is the off-target effect. Is the drug targeting CRYAB sufficient to control the ultimate anti-cancer effects when other HSPs are present? If the answer is yes, what is the underlying mechanism? Only if these events are fully explored can the treatment of CRYAB be used for clinical applications.
 | Table 1 Expression and functional characterization of CRYAB in cancer |
Therefore, according these data, we speculate that the plausible reasons for the ambiguous expression and function of CRYAB in cancers are listed as follows: i) CRYAB expression is developmentally related in some tissues; ii) CRYAB may be specific in certain tissues or populations; iii) cytoprotective and anti-apoptotic activities play a leading role in determining the role of CRYAB in promoting or inhibiting the development of certain types of cancer; and iv) the final functional role of CRYAB is affected by the synergistic or inhibitory effects of other HSPs regulatory molecules.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Haslbeck M, Vierling E. A first line of stress defense: small heat shock proteins and their function in protein homeostasis. Cell Stress Chaperones. 2015;427(7):1537–1548.
2. Kappe G, Franck E, Verschuure P, Boelens WC, Leunissen JA, de Jong WW. The human genome encodes 10 alpha-crystalline-related small heat shock proteins: hspB1-10. Cell Stress Chaperones. 2003;8(1):53–61.
3. Vos MJ, Kanon B, Kampinga HH. HSPB7 is a SC35 speckle resident small heat shock protein. Biochim Biophys Acta. 2009;1793(8):1343–1353. doi:10.1016/j.bbamcr.2009.05.005
4. Simon S, Dimitrova V, Gibert B, et al. Analysis of the dominant effects mediated by wild type or R120G mutant of alphaB-crystallin (HspB5) towards Hsp27 (HspB1). PLoS One. 2013;8(8):e70545. doi:10.1371/journal.pone.0070545
5. Srinivas PN, Reddy PY, Reddy GB. Significance of alpha-crystallin heteropolymer with a 3:1 alphaA/alphaB ratio: chaperone- like activity, structure and hydrophobicity. Biochem J. 2008;414(3):453–460. doi:10.1042/BJ20080544
6. Bukach OV, Glukhova AE, Seit-Nebi AS, Gusev NB. Heterooligomeric complexes formed by human small heat shock proteins HspB1 (Hsp27) and HspB6 (Hsp20). Biochim Biophys Acta. 2009;1794(3):486–495. doi:10.1016/j.bbapap.2008.11.010
7. Rajagopal P, Tse E, Borst AJ, et al. A conserved histidine modulates HSPB5 structure to trigger chaperone activity in response to stress-related acidosis. Elife. 2015;4:e07304. doi:10.7554/eLife.06416
8. Acunzo J, Katsogiannou M, Rocchi P. Small heat shock proteins HSP27 (HspB1), αB-crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. Int J Biochem Cell Biol. 2012;44(10):1622–1631. doi:10.1016/j.biocel.2012.04.002
9. Carver JA, Ecroyd H, Truscott RJW, Thorn DC, Holt C. Proteostasis and the regulation of intra- and extracellular protein aggregation by ATP-independent molecular chaperones: lens α-Crystallins and milk caseins. Acc Chem Res. 2018;51(3):745–752. doi:10.1021/acs.accounts.7b00250
10. Harakalova M, Kummeling G, Sammani A, et al. A systematic analysis of genetic dilated cardiomyopathy reveals numerous ubiquitously expressed and muscle-specific genes. Eur J Heart Fail. 2015;17(5):484–493. doi:10.1002/ejhf.255
11. Hao D, Li J, Jia S, et al. Integrated analysis reveals tubal-and ovarian-originated serous ovarian cancer and predicts differential therapeutic responses. Clin Cancer Res. 2017;23(23):7400–7411. doi:10.1158/1078-0432.CCR-17-0638
12. Mullins CR, Zerby HN, Fitzpatrick LA, Parker AJ. Bosindicus cattle possess greater basal concentrations of HSP27, alpha B-crystallin, and HSP70 in skeletal muscle in vivo compared with cattle. J Anim Sci. 2016;94(1):424–429. doi:10.2527/jas.2016-0811
13. Jiao X, Khan SY, Irum B, et al. Missense mutations in CRYAB are liable for recessive congenital cataracts. PLoS One. 2015;10(9):e0137973. doi:10.1371/journal.pone.0137973
14. Fichna JP, Potulska-Chromik A, Miszta P, et al. A novel dominant D109A CRYAB mutation in a family with myofibrillar myopathy affects αB-crystallin structure. BBA Clin. 2016;7:1–7. doi:10.1016/j.bbacli.2016.11.004
15. Kley RA, Olivé M, Schröder R. New aspects of myofibrillar myopathies. Curr Opin Neurol. 2016;29(5):628–634. doi:10.1097/WCO.0000000000000357
16. Lim EF, Musa A, Frederick A, Ousman SS. AlphaB-crystallin expression correlates with aging deficits in the peripheral nervous system. Neurobiol Aging. 2017;53:138–149. doi:10.1016/j.neurobiolaging.2017.01.006
17. Patel R, Zenith RK, Chandra A, Novel AA. Mutations in the crystallin gene in age-related cataract patients from a North Indian population. Mol Syndromol. 2017;8(4):179–186. doi:10.1159/000471992
18. Sacconi S, Féasson L, Antoine JC, et al. A novel CRYAB mutation resulting in multisystemic disease. Neuromuscul Disord. 2012;22(1):66–72. doi:10.1016/j.nmd.2011.07.004
19. Parcellier A, Schmitt E, Brunet M, Hammann A, Solary E, Garrido C. Small heat shock proteins HSP27 and alphaB-crystallin: cytoprotective and oncogenic functions. Antioxid Redox Signal. 2005;7(3–4):404–413. doi:10.1089/ars.2005.7.404
20. Mehlen P, Kretz-Remy C, Preville X, Arrigo AP. Human hsp27, Drosophila hsp27 and human alphaB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNF alpha-induced cell death. Embo J. 1996;15(11):2695–2706. doi:10.1002/j.1460-2075.1996.tb00630.x
21. Launay N, Goudeau B, Kato K, Vicart P, Lilienbaum A. Cell signaling pathways to alphaB-crystallin following stresses of the cytoskeleton. Exp Cell Res. 2006;312(18):3570–3584. doi:10.1016/j.yexcr.2006.07.025
22. Muchowski PJ, Valdez MM, Clark JI. AlphaB-crystallin selectively targets intermediate filament proteins during thermal stress. Invest Ophthalmol Vis Sci. 1999;40(5):951–958.
23. Gangalum RK, Schibler MJ, Bhat SP. Small heat shock protein alphaB-crystallin is part of cell cycle‐dependent golgi reorganization. J Biol Chem. 2004;279(42):43374–43377. doi:10.1074/jbc.C400371200
24. Malin D, Petrovic V, Strekalova E, Sharma B, Cryns VL. αB-crystallin: portrait of a malignant chaperone as a cancer therapeutic target. Pharmacol Ther. 2016;160:1–10. doi:10.1016/j.pharmthera.2016.01.012
25. Mao Y, Zhang DW, Lin H, et al. Alpha B-crystallin is a new prognostic marker for laryngeal squamous cell carcinoma. J Exp Clin Cancer Res. 2012;31(1):101. doi:10.1186/1756-9966-31-95
26. Gruvberger-Saal SK, Parsons R. Is the small heat shock protein alphaB-crystallin an oncogene? J Clin Invest. 2006;116(1):30–32. doi:10.1172/JCI27462
27. Raju I, Abraham EC. Mutants of human αB-crystallin cause enhanced protein aggregation and apoptosis in mammalian cells: influence of co-expression of HspB1. Biochem Biophys Res Commun. 2013;430(1):107–112. doi:10.1016/j.bbrc.2012.10.142
28. Arrigo AP, Gibert B. Protein interactomes of three stress inducible small heat shock proteins: HSPB1, HspB5 and HspB8. Int J Hyperthermia. 2013;29(5):409–422. doi:10.3109/02656736.2013.796529
29. Arrigo AP. Human small heat shock proteins: protein interactomes of homo- and hetero-oligomeric complexes: an update. FEBS Lett. 2013;587(13):1959–1969. doi:10.1016/j.febslet.2013.05.011
30. Zhu Z, Li R, Stricker R, Reiser G. Extracellular α-crystallin protects astrocytes from cell death through activation of MAPK, PI3K/Akt signaling pathway and blockade of ROS release from mitochondria. Brain Res. 2015;1620:17–28. doi:10.1016/j.brainres.2015.05.011
31. Arrigo AP. Analysis of HspB1 (Hsp27) oligomerization and phosphorylation patterns and its interaction with specific client polypeptides. Methods Mol Biol. 2018;1709:163–178. doi:10.1007/978-1-4939-7477-1_11
32. Hochberg GK, Benesch JL. Dynamical structure of αB-crystallin. Prog Biophys Mol Biol. 2014;115(1):11–20. doi:10.1016/j.pbiomolbio.2014.03.003
33. Lee SW, Rho JH, Lee SY, et al. Alpha B-crystallin protects rat articular chondrocytes against casein kinase II inhibition-induced apoptosis. PLoS One. 2016;11(1):e0166450. doi:10.1371/journal.pone.0166450
34. Cesa LC, Shao H, Srinivasan SR, et al. X-linked inhibitor of apoptosis protein (XIAP) is a client of heat shock protein 70 (Hsp70) and a biomarker of its inhibition. J Bio Chem. 2018;293(7):2370–2380. doi:10.1074/jbc.RA117.000634
35. Ganguly S, Mitra A, Sarkar S. Role of α-crystallin B in regulation of stress induced cardiomyocyte apoptosis. Cardiovasc Hematol Agents Med Chem. 2014;12(2):60–65. doi:10.2174/1871525713666150123151731
36. Ciocca DR, Arrigo AP, Calderwood SK. Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: an update. Arch Toxicol. 2013;87(1):19–48.
37. Arrigo AP. Editorial: heat shock proteins in cancer. Curr Mol Med. 2012;12:1099–1101. doi:10.2174/156652412803306738
38. Xu F, Yu H, Liu J, Cheng L. αB-crystallin regulates oxidative stress-induced apoptosis in cardiac H9c2 cells via the PI3K/AKT pathway. Mol Biol Rep. 2013;40(9):2517–2526. doi:10.1007/s11033-012-2332-2
39. Hu WF, Gong L, Cao Z, et al. αA- and αB-crystallins interact with caspase-3 and Bax to guard mouse lens development. Curr Mol Med. 2012;12(2):177–187. doi:10.2174/156652412798889036
40. Mao YW, Liu JP, Xiang H, Li DW. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11(5):512–526. doi:10.1038/sj.cdd.4401384
41. Liu S, Yan B, Lai W, et al. As a novel p53 direct target, bidirectional gene HspB2/αB-crystallin regulates the ROS level and Warburg effect. Biochim Biophys Acta. 2014;1839:592–603. doi:10.1016/j.bbagrm.2014.05.017
42. Sreekumar PG, Kannan R, Kitamura M, et al. αB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS One. 2010;5(10):e12578. doi:10.1371/journal.pone.0012578
43. E S L, Raimann A, B T C, et al. c-Raf promotes angiogenesis during normal growth plate maturation. Development. 2016;143(2):348–355. doi:10.1242/dev.127142
44. Abdelwahid E, Stulpinas A, Kalvelyte A. Effective agents targeting the mitochondria and apoptosis to protect the heart. Curr Pharm Des. 2017;23(8):1153–1166. doi:10.2174/1381612822666161229150120
45. Lee JS, Kim HY, Jeong NY, et al. Expression of alphaB-crystallin overrides the anti-apoptotic activity of XIAP. Neuro Oncol. 2012;14(11):1332–1345. doi:10.1093/neuonc/nos069
46. Pasupuleti N, Matsuyama S, Voss O, et al. The anti-apoptotic function of human αA-crystallin is directly related to its chaperone activity. Cell Death Dis. 2011;1(3):e31. doi:10.1038/cddis.2010.3
47. Yin B, Tang S, Xu J, et al. CRYAB protects cardiomyocytes against heat stress by preventing caspase-mediated apoptosis and reducing F-actin aggregation. Cell Stress Chaperones. 2018;24(1)1–10.
48. Rothbard JB, Kurnellas MP, Brownell S, et al. Therapeutic effects of systemic administration of chaperone alphaB-crystallin associated with binding proinflammatory plasma proteins. J Biol Chem. 2012;287:9708–9721. doi:10.1074/jbc.M111.337691
49. Guo YS, Liang PZ, Lu SZ, Chen R, Yin YQ, Zhou JW. Extracellular αB-crystallin modulates the inflammatory responses. Biochem Biophys Res Commun. 2019;508(1):282–288. doi:10.1016/j.bbrc.2018.10.159
50. van Noort JM, Bsibsi M, Nacken PJ, et al. Activation of an immune-regulatory macrophage response and inhibition of lung inflammation in a mouse model of COPD using heat-shock protein alpha B-crystallin-loaded PLGA microparticles. Biomaterials. 2013;34(3):831–840. doi:10.1016/j.biomaterials.2012.10.028
51. Park H, Park H, Hwang HJ, et al. Alpha B-crystallin prevents ventricular arrhythmia by attenuating inflammation and oxidative stress in rat with autoimmune myocarditis. Int J Cardiol. 2015;182:399–402. doi:10.1016/j.ijcard.2014.12.152
52. Grazioli E, Dimauro I, Mercatelli N, et al. SFRR-E young investigator Awardeeαb-crystallin modulation after acute exercise in skeletal muscle: the role of oxidative stress and fiber composition. Free Radic Biol Med. 2014;75:S13–14. doi:10.1016/j.freeradbiomed.2014.10.819
53. Prabhu S, Srinivas V, Ramakrishna T, Raman B, Rao C. Inhibition of Cu2+-mediated generation of reactive oxygen species by the small heat shock protein αB-crystallin: the relative contributions of the N- and C-terminal domains. Free Radic Biol Med. 2011;51(3):755–762. doi:10.1016/j.freeradbiomed.2011.04.011
54. Christopher KL, Pedler MG, Shieh B, et al. Alpha-crystallin-mediated protection of lens cells against heat and oxidative stress-induced cell death. Biochim Biophys Acta. 2014;1843(2):309–315. doi:10.1016/j.bbamcr.2013.11.025
55. Adhikari AS, Singh BN, Rao KS, Rao C. αB-crystallin, a small heat shock protein, modulates NF-κB activity in a phosphorylation-dependent manner and protects muscle myoblasts from TNF-α induced cytotoxicity. Biochim Biophys Acta. 2011;1813(8):1532–1542. doi:10.1016/j.bbamcr.2011.04.009
56. Dieterich LC, Huang H, Massena S, Golenhofen N, Phillipson M, Dimberg A. αB-crystallin/HspB5 regulates endothelial-leukocyte interactions by enhancing NF-κB-induced up-regulation of adhesion molecules ICAM-1, VCAM-1 and E-selectin. Angiogenesis. 2013;16(4):975–983. doi:10.1007/s10456-013-9367-4
57. Christians ES, Ishiwata T, Benjamin IJ. Small heat shock proteins in redox metabolism: implications for cardiovascular diseases. Int J Biochem Cell Biol. 2012;44(10):1632–1645. doi:10.1016/j.biocel.2012.06.006
58. Ruebsam A, Dulle JE, Myers AM, et al. A specific phosphorylation regulates the protective role of αA-crystallin in diabetes. JCI Insight. 2018;3(4):97919. doi:10.1172/jci.insight.97941
59. Moreno-Gonzalo O, Fernandez-Delgado I, Sanchez-Madrid F. Post-translational add-ons mark the path in exosomal protein sorting. Cell Mol Life Sci. 2018;75(1):1–19. doi:10.1007/s00018-017-2690-y
60. Jabłońska J, Dubińska-Magiera M, Jagla T, et al. Drosophila Hsp67Bc hot-spot variants alter muscle structure and function. Cell Mol Life Sci. 2018;75(23):4341–4356. doi:10.1007/s00018-018-2875-z
61. Dimauro I, Antonioni A, Mercatelli N, Caporossi D. The role of αB-crystallin in skeletal and cardiac muscle tissues. Cell Stress Chaperones. 2018;23(4):1–15. doi:10.1007/s12192-017-0866-x
62. Li J, Horak KM, Su H, et al. Enhancement of proteasomal function protects against cardiac proteinopathy and ischemia/reperfusion injury in mice. J Clin Invest. 2011;121(9):3689–3700. doi:10.1172/JCI57873
63. Kumarapeli AR, Su H, Huang W, et al. Alpha B-crystallin suppresses pressure overload cardiac hypertrophy. Circ Res. 2008;103(12):1473–1482. doi:10.1161/CIRCRESAHA.108.180117
64. Cubedo J, Vilahur G, Casaní L, et al. Targeting the molecular mechanisms of ischemic damage: protective effects of alpha-crystallin-B. Int J Cardiol. 2016;215:406–416. doi:10.1016/j.ijcard.2016.04.072
65. Huang XY, Ke AW, Shi GM, et al. αB-crystallin complexes with 14-3-3ζ to induce epithelial-mesenchymal transition and resistance to sorafenib in hepatocellular carcinoma. Hepatology. 2013;57(6):2235–2247. doi:10.1002/hep.26255
66. Campbell-Lloyd AJ, Mundy J, Deva R, et al. Is alpha-B crystallin an independent marker for prognosis in lung cancer? Heart Lung Circ. 2013;22(9):759–766. doi:10.1016/j.hlc.2013.01.014
67. Yilmaz M, Karatas OF, Yuceturk B, Dag H, Yener M, Ozen M. Alpha-B-crystallin expression in human laryngeal squamous cell carcinoma tissues. Head Neck. 2015;37(9):1344–1348. doi:10.1002/hed.v37.9
68. Dimberg A, Rylova S, Dieterich LC, et al. alphaB-crystallin promotes tumor angiogenesis by increasing vascular survival during tube morphogenesis. Blood. 2008;111(4):2015–2023. doi:10.1182/blood-2007-04-087841
69. Arrigo AP, Gibert B. HspB1, HspB5 and HspB4 in human cancers: potent oncogenic role of some of their client proteins. Cancers (Basel). 2014;6(1):333–365. doi:10.3390/cancers6010333
70. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi:10.3322/caac.21208
71. Coopey SB, Mazzola E, Buckley JM, et al. The role of chemoprevention in modifying the risk of breast cancer in women with atypical breast lesions. Breast Cancer Res Treat. 2012;136(3):627–633. doi:10.1007/s10549-012-2318-8
72. Hayes MM, Konstantinova AM, Kacerovska D, et al. Bilateral gigantomastia, multiple synchronous nodular pseudoangiomatous stromal hyperplasia involving breast and bilateral axillary accessory breast tissue, and perianal mammary-type hamartoma of anogenital mammary-like glands: a case report. Am J Dermatopathol. 2016;38(5):374–383. doi:10.1097/DAD.0000000000000498
73. Wang ZC, E D, Batu DL, Saixi YL, Zhang B, Ren LQ. 2D-DIGE proteomic analysis of changes in estrogen/progesterone-induced rat breast hyperplasia upon treatment with the Mongolian remedy RuXian-Ⅰ. Molecules. 2011;16(4):3048–3065. doi:10.3390/molecules16043048
74. Guo J, Gong G, Zhang B. Screening and identification of potential biomarkers in triple-negative breast cancer by integrated analysis. Oncol Rep. 2017;38(4):2219–2228. doi:10.3892/or.2017.5851
75. Chen Z, Ruan Q, Han S, et al. Discovery of structure-based small molecular inhibitor of αB-crystallin against basal-like/triple-negative breast cancer development in vitro and in vivo. Breast Cancer Res Treat. 2014;145(1):45–59. doi:10.1007/s10549-014-2940-8
76. Chan SK, Lui PC, Tan PH, et al. Increased alpha-B-crystallin expression in mammary metaplastic carcinomas. Histopathology. 2011;59(2):247–255. doi:10.1111/j.1365-2559.2011.03882.x
77. Malin D, Strekalova E, Petrovic V, et al. αB-crystallin: a novel regulator of breast cancer metastasis to the brain. Clin Cancer Res. 2014;20(1):56–67. doi:10.1158/1078-0432.CCR-13-3045
78. van de Schootbrugge C, van Asten F, Nagtegaal ID, et al. αB-crystallin expression is correlated with phospho-ERK1/2 expression in human breast cancer. Int J Biol Markers. 2013;28(4):e365–370. doi:10.5301/JBM.5000032
79. Tsang JY, Lai MW, Wong KH, et al. αB-crystallin is a useful marker for triple negative and basal breast cancers. Histopathology. 2012;61(3):378–386. doi:10.1111/j.1365-2559.2012.04254.x
80. Pedrosa RM, Mustafa DA, Soffietti R, et al. Breast cancer brain metastasis: molecular mechanisms and directions for treatment. Neuro oncol. 2018;20(11):1439–1449.
81. Kim HS, Lee Y, Lim YA, Kang HJ, Kim LS. αB-Crystallin is a novel oncoprotein associated with poor prognosis in breast cancer. J Breast Cancer. 2011;14(1):14–19. doi:10.4048/jbc.2011.14.1.14
82. Kim MS, Lee HW, Jun SY, Lee EH. Expression of alpha B crystallin and BCL2 in patients with infiltrating ductal carcinoma. Int J Clin Exp Pathol. 2015;8(8):8842–8856.
83. Kabbage M, Trimeche M, Ben Nasr H, et al. Expression of the molecular chaperone αB-crystallin in infiltrating ductal breast carcinomas and the significance thereof: an immunohistochemical and proteomics-based strategy. Tumour Biol. 2012;33(6):2279–2288. doi:10.1007/s13277-012-0490-4
84. Koletsa T, Stavridi F, Bobos M, et al. alphaB-crystallin is a marker of aggressive breast cancer behavior but does not independently predict for patient outcome: a combined analysis of two randomized studies. BMC Clin Pathol. 2014;14(1):28. doi:10.1186/1472-6890-14-28
85. Bosman JD, Yehiely F, Evans JR, Cryns VL. Regulation of alphaB-crystallin gene expression by the transcription factor Ets1 in breast cancer. Breast Cancer Res Treat. 2010;119(1):63–70. doi:10.1007/s10549-009-0330-4
86. Voduc KD, Nielsen TO, Perou CM, et al. αB-crystallin expression in breast cancer is associated with brain metastasis. NPJ Breast Cancer. 2015;1:15014. doi:10.1038/npjbcancer.2015.14
87. Chen W, Lu Q, Lu L, Guan H. Increased levels of alphaB-crystallin in vitreous fluid of patients with proliferative diabetic retinopathy and correlation with vascular endothelial growth factor. Clin Exp Ophthalmol. 2017;45(4):379–384. doi:10.1111/ceo.12891
88. Ruan Q, Han S, Jiang WG, et al. alphaB-crystallin, an effector of unfolded protein response, confers anti-VEGF resistance to breast cancer via maintenance of intracrine VEGF in endothelial cells. Mol Cancer Res. 2011;9(12):1632–1643. doi:10.1158/1541-7786.MCR-11-0168
89. Shi C, He Z, Hou N, Ni Y, Xiong L, Chen P. Alpha B-crystallin correlates with poor survival in colorectal cancer. Int J Clin Exp Pathol. 2014;7(9):6056–6063.
90. Fu M, Fan W, Pu X, et al. Elevated expression of SHIP2 correlates with poor prognosis in non-small cell lung cancer. Int J Clin Exp Pathol. 2013;6(10):2185–2191.
91. Esposito L, Conti D, Ailavajhala R, et al. Lung cancer: are we up to the challenge? Curr Genomics. 2010;11(7):513–518. doi:10.2174/138920210793175903
92. Xu QY, Gao Y, Liu Y, Yang WZ, Xu XY. Identification of differential gene expression profiles of radioresistant lung cancer cell line established by fractionated ionizing radiation in vitro. Chin Med J (Engl). 2008;121(18):1830–1837. doi:10.1097/00029330-200809020-00014
93. Qin H, Ni Y, Tong J, et al. Elevated expression of CRYAB predicts unfavorable prognosis in non-small cell lung cancer. Med Oncol. 2014;31(8):142. doi:10.1007/s12032-014-0374-0
94. Cherneva R, Petrov D, Georgiev O, et al. Expression profile of the small heat-shock protein alpha-B-crystallin in operated-on non-small-cell lung cancer patients: clinical implication. Eur J Cardiothorac Surg. 2010;37(1):44–50. doi:10.1016/j.ejcts.2009.06.038
95. Malin D, Strekalova E, Petrovic V, et al. ERK-regulated αB-crystallin induction by matrix detachment inhibits anoikis and promotes lung metastasis in vivo. Oncogene. 2015;34(45):5626–5634. doi:10.1038/onc.2014.462
96. Bellaye PS, Wettstein G, Burgy O, et al. The small heat-shock protein αB-crystallin is essential for the nuclear localization of Smad4: impact on pulmonary fibrosis. J Pathol. 2014;232(4):458–472. doi:10.1002/path.4314
97. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132.
98. Ma W, Zhang Y, Mu H, et al. Glucose regulates heat shock factor 1 transcription activity via mTOR pathway in HCC cell lines. Cell Biol Int. 2015;39(11):1217–1224. doi:10.1002/cbin.10493
99. Tang Q, Liu YF, Zhu XJ, et al. Expression and prognostic significance of the αB-crystallin gene in human hepatocellular carcinoma. Hum Pathol. 2009;40(3):300–305. doi:10.1016/j.humpath.2009.01.003
100. Messerschmitt PJ, Garcia RM, Abdul-Karim FW, Greenfield EM, Getty PJ. Osteosarcoma. J Am Acad Orthop Surg. 2009;17(8):515–527. doi:10.5435/00124635-200908000-00005
101. Folio C, Mora MI, Zalacain M, et al. Proteomic analysis of chemonaive pediatric osteosarcomas and corresponding normal bone reveals multiple altered molecular targets. J Proteome Res. 2009;8(8):3882–3888. doi:10.1021/pr900113w
102. Shi QM, Luo J, Wu K, Yin M, Gu YR, Cheng XG. High level of αB-crystallin contributes to the progression of osteosarcoma. Oncotarget. 2016;7(8):9007–9016.
103. Zhang L, Zhang L, Xia X, He S, He H, Zhao W. Krüppel-like factor 4 promotes human osteosarcoma growth and metastasis via regulating CRYAB expression. Oncotarget. 2016;7(21):30990–31000.
104. Wang SN, Luo S, Liu C, et al. miR-491 inhibits osteosarcoma lung metastasis and chemoresistance by targeting αB-crystallin. Mol Ther. 2017;25(9):2140–2149. doi:10.1016/j.ymthe.2016.10.004
105. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386. doi:10.1002/ijc.29210
106. Yiu AJ, Yiu CY. Biomarkers in colorectal cancer. Anticancer Res. 2016;36(3):1093–1102.
107. Pesson M, Volant A, Uguen A, et al. A gene expression and pre-mRNA splicing signature that marks the adenoma-adenocarcinoma progression in colorectal cancer. PLoS One. 2014;9:e87761. doi:10.1371/journal.pone.0087761
108. Li Q, Wang Y, Lai Y, Xu P, Yang Z. HspB5 correlates with poor prognosis in colorectal cancer and prompts epithelial-mesenchymal transition through ERK signaling. PLoS One. 2017;12(8):e0182588. doi:10.1371/journal.pone.0182588
109. Shi C, Yang X, Bu X, Hou N, Chen P. Alpha B-crystallin promotes the invasion and metastasis of colorectal cancer via epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2017;489(4):369–374. doi:10.1016/j.bbrc.2017.05.070
110. Giordano G, Febbraro A, Tomaselli E, et al. Cancer-related CD15/FUT4 overexpression decreases benefit to agents targeting EGFR or VEGF acting as a novel RAF-MEK-ERK kinase downstream regulator in metastatic colorectal cancer. J Exp Clin Cancer Res. 2015;34:108. doi:10.1186/s13046-015-0225-7
111. Worthley DL, Leggett BA. Colorectal cancer: molecular features and clinical opportunities. Clin Biochem Rev. 2010;31(2):31–38.
112. Wu X, Zheng YZ, Han B, Wang K. Alpha B-crystallin C-802G polymorphism and colorectal cancer susceptibility and clinical outcome in Chinese population. Sci Rep. 2018;8(1):11731. doi:10.1038/s41598-018-29589-y
113. Morris LGT, Chandramohan R, West L, et al. The molecular landscape of recurrent and metastatic head and neck cancers: insights from a precision oncology sequencing platform. JAMA Oncol. 2017;3:244–255. doi:10.1001/jamaoncol.2016.1790
114. Avliyakulov NK, Rajavel KS, Le KMT, et al. C-terminally truncated form of αB-crystallin is associated with IDH1 R132H mutation in anaplastic astrocytoma. J neuro oncol. 2014;117(1):53–65. doi:10.1007/s11060-014-1371-z
115. Kore RA, Abraham EC. Phosphorylation negatively regulates exosome mediated secretion of cryAB in glioma cells. Biochim Biophys Acta. 2016;1863(2):368–377. doi:10.1016/j.bbamcr.2015.11.027
116. van de Schootbrugge C, Schults EM, Bussink J, et al. Effect of hypoxia on the expression of αB-crystallin in head and neck squamous cell carcinoma. BMC Cancer. 2014;14:252.
117. Peyvandi H, Peyvandi AA, Safaei A, et al. Introducing potential key proteins and pathways in human laryngeal cancer: a system biology approach. Iran J Pharm Res. 2018;17(1):415–425.
118. Annertz K, Enoksson J, Williams R, Jacobsson H, Coman WB, Wennerberg J. Alpha B-crystallin-a validated prognostic factor for poor prognosis in squamous cell carcinoma of the oral cavity. Acta Otolaryngol. 2014;134(5):543–550. doi:10.3109/00016489.2013.872293
119. Bau DT, Tsai CW, Lin CC, et al. Association of alpha B-crystallin genotypes with oral cancer susceptibility, survival, and recurrence in Taiwan. PLoS One. 2011;6(9):e16374. doi:10.1371/journal.pone.0016374
120. Solares CA, Boyle GM, Brown I, Parsons PG, Panizza B. Reduced alphaB-crystallin staining in perineural invasion of head and neck cutaneous squamous cell carcinoma. Otolaryngol Head Neck Surg. 2010;142(3 suppl 1):S15–19.
121. Dai W, Zheng H, Cheung AKL, et al. Genetic and epigenetic landscape of nasopharyngeal carcinoma. Chin Clin Oncol. 2016;5(2):16. doi:10.21037/cco.2016.03.06
122. Huang Z, Cheng Y, Chiu PM, et al. Tumor suppressor Alpha B-crystallin (CRYAB) associates with the cadherin/catenin adherens junction and impairs NPC progression-associated properties. Oncogene. 2012;31(32):3709–3720. doi:10.1038/onc.2011.627
123. Mineva I, Gartner W, Hauser P, et al. Differential expression of alphaB-crystallin and Hsp27-1 in anaplastic thyroid carcinomas because of tumor-specific alphaB-crystallin gene (CRYAB) silencing. Cell Stress Chaperones. 2005;10(3):171–184. doi:10.1379/CSC-107R.1
124. Chen D, Cao G, Qiao C, et al. Alpha B-crystallin promotes the invasion and metastasis of gastric cancer via NF-κB-induced epithelial-mesenchymal transition. J Cell Mol Med. 2018;22(6):3215–3222. doi:10.1111/jcmm.13602
125. Kase S, Parikh JG, Rao NA. Expression of alpha-crystallin in retinoblastoma. Arch Ophthalmol. 2009;127(2):187–192. doi:10.1001/archophthalmol.2008.580
126. Kase S, Parikh JG, Rao NA. Expression of heat shock protein 27 and alpha-crystallins in human retinoblastoma after chemoreduction. Br J Ophthalmol. 2009;93(4):541–544. doi:10.1136/bjo.2008.153007
127. Valcarcel-Jimenez L, Macchia A, Martín-Martín N, et al. Integrative analysis of transcriptomics and clinical data uncovers the tumor-suppressive activity of MITF in prostate cancer. Cell Death Dis. 2018;9(10):1041. doi:10.1038/s41419-018-1111-y
128. Hu R, Aplin AE. alphaB-crystallin is mutant B-RAF regulated and contributes to cyclin D1 turnover in melanocytic cells. Pigment Cell Melanoma Res. 2010;23(2):201–209. doi:10.1111/j.1755-148X.2010.00668.x
129. Smith MP, Brunton H, Rowling EJ, et al. Inhibiting drivers of non-mutational drug tolerance is a salvage strategy for targeted melanoma therapy. Cancer Cell. 2016;29(3):270–284. doi:10.1016/j.ccell.2016.02.003
130. Cortazar AR, Torrano V, Martín-Martín N, et al. CANCERTOOL: a visualization and representation interface to exploit cancer datasets. Cancer Res. 2018;78(21):6320–6328. doi:10.1158/0008-5472.CAN-18-1669
131. Altintas DM, Allioli N, Decaussin M, et al. Differentially expressed androgen-regulated genes in androgen-sensitive tissues reveal potential biomarkers of early prostate cancer. PLoS One. 2013;8(6):e66278. doi:10.1371/journal.pone.0066278
132. Volkmann J, Reuning U, Rudelius M, et al. High expression of crystallin αB represents an independent molecular marker for unfavourable ovarian cancer patient outcome and impairs TRAIL- and cisplatin-induced apoptosis in human ovarian cancer cells. Int J Cancer. 2013;132(12):2820–2832. doi:10.1002/ijc.27975
133. Stronach EA, Sellar GC, Blenkiron C, et al. Identification of clinically relevant genes on chromosome 11 in a functional model of ovarian cancer tumor suppression. Cancer Res. 2003;63(24):8648–8655.
134. Andley UP, Song Z, Wawrousek EF, Fleming TP, Bassnett S. Differential protective activity of alpha A- and alphaB-crystallin in lens epithelial cells. J Biol Chem. 2000;275(47):36823–26831. doi:10.1074/jbc.M004233200
135. Bang HS, Choi MH, Kim CS, Choi SJ. Gene expression profiling in undifferentiated thyroid carcinoma induced by high-dose radiation. J Radiat Res. 2016;57(3):238–249. doi:10.1093/jrr/rrw002
136. Den Engelsman J, van de Schootbrugge C, Yong J, Pruijn GJ, Boelens WC. Pseudoph-osphorylated alphaB-crystallin is a nuclear chaperone imported into the nucleus with help of the SMN complex. PLoS One. 2013;8(9):e73489. doi:10.1371/journal.pone.0073489
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

