Back to Journals » Vascular Health and Risk Management » Volume 11
Progression of carotid-artery disease in type 2 diabetic patients: a cohort prospective study
Authors Bosevski M, Stojanovska L
Received 11 December 2014
Accepted for publication 7 July 2015
Published 16 October 2015 Volume 2015:11 Pages 549—553
DOI https://doi.org/10.2147/VHRM.S79079
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Daniel Duprez
Marijan Bosevski,1 Lily Stojanovska2
1Faculty of Medicine, University Cardiology Clinic, Skopje, Macedonia; 2Centre for Chronic Disease, College of Health and Biomedicine, Victoria University, Melbourne, VIC, Australia
Abstract: In order to assess the progression of carotid-artery disease in type 2 diabetic cohort (n=207 patients), the dynamic change in carotid intima-media thickness (CIMT) and the occurrence of plaques were followed for a period of 31.35±10.59 months. The mean CIMT at the beginning of the study was 0.9178±0.1447 mm, with a maximal value of 1.1210±0.2366 mm. The maximal value of CIMT changed by 0.07 mm/year. Progression of CIMT was noted in 86.8% and its regression in 7.8% of patients. The occurrence of carotid plaques was detected in 41.8% of patients. Multiple regression analysis revealed the maximal value of CIMT to be associated with diastolic blood pressure, despite mean CIMT being predicted by body mass index. The presence of peripheral arterial disease and hypo-high-density lipoproteinemia were found to be predictors for the occurrence of carotid plaques. Our data have clinical implications in predicting risk factors for the progression of carotid-artery disease in type 2 diabetic patients for their appropriate management.
Keywords: carotid IMT, type 2 diabetes, progression of atherosclerosis, risk factors
Introduction
Diabetes has been defined as an independent factor for the presence of high-grade carotid-artery stenosis in the general population.1 Type 2 diabetes (T2D) poses a substantial risk factor for the progression of atherosclerosis, measured by rise in carotid intima-media thickness (CIMT), expressed in length unit per patient in 1 year, and compared with the occurrence of plaques on the internal carotid artery in 1–2 years.2,3
There is a clear correlation among arterial hypertension, hypercholesterolemia, and inflammatory markers (c-reactive protein and serum amyloid concentration) as predictors of carotid atherosclerosis in patients with diabetes.4,5 We determined the dynamics of progression of carotid-artery disease (CAD) in patients with T2D and determined factors that influence it.
Patients and methods
In this prospective study, a cohort of T2D patients (n=207) were followed for a period of 31.35±10.59 months for dynamic changes in CIMT and the occurrence of plaques. T2D was defined based on the criteria of the International Diabetes Federation.
CIMT was measured by B-mode ultrasound using a linear transducer (7.5–10 MHz) and was performed by a single sonographer. CIMT is presented as a mean value of two measurements from both sides of the common carotid arteries. CIMT is defined as the distance from the leading edge of the first echogenic line to the leading edge of the second echogenic line on the scans, with the first line representing the lumen–intima interface and the second line representing the collagen-containing upper layer of the adventitia. Plaque was defined as a localized thickening lesion (≥1.1 mm). Carotid stenosis greater than 60% was considered significant. In each longitudinal projection, the site with the greatest thickness (including plaques) was detected along the vessel from the common carotid artery to the internal carotid artery. Peripheral arterial disease (PAD) was defined as ankle-brachial index (ABI) <0.9 or >1.3. The observer was blinded to patient risk factors.
Standard laboratory analyses were performed in all patients. Multivariate analysis was performed in order to assess the predictors for CIMT progression. The model was adjusted for age. The study was conducted in accordance with the Helsinki declaration. This study which was a part of project: Diabetic polyvascular disease, was approved by the scientific committee of Medical Faculty Skopje. At this timepoint there was no need for approval from an ethics committee. Informed consent was obtained from all patients.
Results
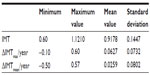
In the study population, a mean value of IMT =0.9178 mm and maximal value of CIMT =1.1210 mm, respectively, were obtained. The maximal value of CIMT changed by 0.07 mm annually (Table 1).
Progression of carotid atherosclerosis was noted in 86.8%, followed by a change in CIMT. In 17.8% of patients, the occurrence of carotid stenosis was evident, while in 41.8% of patients, the development of new carotid plaques was noted.
Multiple linear regression analysis was performed when continuous variables from Table 2 were put into this model. The change in CIMT showed that its maximal value was predicted by the diastolic pressure value and the change in mean value of CIMT by body mass index (BMI). Predictors for the change in CIMT values annually are shown in Table 3.
  | Table 2 Continuous variables in the study population |
Multiple regression analysis demonstrated low high-density lipoprotein (HDL) and the presence of PAD to be independent factors for the progression of atherosclerotic plaques (Table 4). In addition, ABI values, presence of carotid plaques at the beginning of the study, and the presence of intermittent claudication were also found to be independent factors.
  | Table 4 Predictors for plaque progression |
Since postprandial glycemia was included in the multiple linear regression analysis in the progression of CAD, the same value was shown as a substantive value compared to degree of carotid stenosis. Raised amounts of glycated hemoglobin (HbA1c) >7% were in linear correlation with maximal change in CIMT (Figure 1).
  | Figure 1 Relationship of glycemia to CIMT. |
In order to analyze the operator’s influence on carotid measurements, a homogeneity test of regression was used when the mean and maximal values of IMT were included. It was clear that there was minimal influence of the operator on measurements (F=17.061, P=0.018).
Discussion
The progression rate of CAD in high-risk populations has been previously reported to be 15%–20% annually.6–8 Our results are comparable with previous studies – ARIC (Atherosclerosis Risk in Communities Cohort) and IRAS (Insulin Resistance Atherosclerosis Study) – with regard to progression of CIMT of 0.1 mm annually in the T2D population.6–8
Randomized controlled studies have shown an inverse correlation between HDL cholesterol and CIMT value and its progression in the population with high cardiovascular risk and those with T2D.9 IMT, which comprises the intima and media, is susceptible to the influence of risk factors for artery disease. The enlargement of the intima is a result of the atherosclerotic process, and media enlargement is a response to vascular remodeling in high-artery-pressure conditions.10 The SANDS study11 demonstrated that the arterial pressure and low-density lipoprotein (LDL) cholesterol are defined as independent factors with the greatest risk for progression of CAD. Our results show logistic regression dependency in plaque progression; respectively, they showed linear regression dependency on change in IMT mean (ΔIMTmean) from hypo-HDL-emia. Total cholesterol is an independent predictor for the occurrence of carotid plaques according to our results. Lipid fractions, LDL cholesterol, and triglycerides are important factors in the progression of carotid atherosclerosis in the general population and in those with diabetes.11 However, lipid fractions were not shown as independent factors in the progression of carotid atherosclerosis in the Rotterdam study.12 This is most likely due to the high atherogenicity of LDL cholesterol and non-HDL cholesterol, even when they are within their normal range. Accomplishing lower LDL and non-HDL values is important in decreasing the progression of CAD in T2D.
Arterial hypertension is a predictor for CIMT value. In a meta-analysis, it was clear that for every decrease of 5 mmHg in arterial pressure, regression of IMT of 0.01 mm/year resulted.13
Age, male sex, smoking, and BMI are the best independent factors in carotid-atherosclerosis progression.14,15 Accordingly, our data demonstrate that BMI is a predictor for progression of IMT values (ΔIMTmean and ΔIMTmax) annually. The long prediabetic phase and late diagnosis of diabetes are independent factors in the progression of CIMT;16 however, in our study this was not evident.
PAD occurrence and ABI value have been shown as predictors in CAD progression, which is demonstrated in our results. The relationship of ABI with CAD defines not only PAD but the presence of diabetic polyvascular disease.17
We also examined the influence of glycemic control on the progression of carotid stenosis. The obtained glycemia values and IMT were in log-linear correlation. The degree of carotid stenosis was correlated with the glycemic value.
The correlation of vascular disease in diabetic patients with glycemic control measured by HbA1c was shown in Selvin et al’s meta-analysis.18 Postprandial glycemia has been shown to be a powerful predictor against HbA1c compared to the correlation with diabetic vascular disease.19 In the DECODE study, postprandial glycemia is an independent predictor for CAD and mortality in patients with T2D.20 Our results demonstrate the correlation of carotid atherosclerosis with fasting glycemia.
In our study, bias might have been present because of the selected values of CIMT through which the progression of atherosclerosis is defined. For this reason, a multiple linear regression analysis for continuous variables of CIMT was used.
Progression of the carotid plaque due to multivariate regression analysis is predicted with the former presence of plaques, low HDL cholesterol and presence of PAD, ABI value, age, systolic blood pressure, and total cholesterol. Change in CIMT value is predicted with diastolic blood pressure and BMI according to linear regression analysis.
These results are also meaningful when making comparative analysis of certain risk factors in the progression of vascular disease in certain localizations in T2D. Hyperglycemia is a crucial factor in the development of PAD in diabetes, and arterial hypertension and low HDL cholesterol for the development of CAD in diabetes. This spectrum of significance on certain risk factors was supported with our results.
Conclusion
Our results indicate that the dynamics of CIMTmax were determined with diastolic blood pressure, despite CIMTmean being predicted by BMI. Presence of PAD and hypo-HDL-emia were found to be predictors for the occurrence of carotid plaques. These results have clinical implications in finding the risk factors for the progression of CAD in T2D patients for appropriate clinical management.
Acknowledgment
The authors thank Filip Janushevski for participation in preparation of the manuscript and Golubinka Bosevska for lab analyses.
Disclosure
The authors report no conflicts of interest in this work.
References
Göksan B, Erkol G, Bozluolcay M, Ince B. Diabetes as a determinant of high-grade carotid artery stenosis: evaluation of 1,058 cases by Doppler sonography. J Stroke Cerebrovasc Dis. 2001;10(6):252–256. | |
Kiechl S, Willeit J. The natural course of atherosclerosis. Part 1: incidence and progression. Arterioscler Thromb Vasc Biol. 1999;19(6):1484–1490. | |
Yang B, Li TD, Wang JS, Zhi G, Jin WS, Xu Y. Insulin resistance and carotid atherosclerosis in 221 patients with potential hyperglycemia. Chin Med Sci J. 2005;20(2):108–111. | |
Baldassarre D, De Jong A, Amato M, et al. Carotid intima-media thickness and markers of inflammation endothelial damage and hemostasis. Ann Med. 2007;40(1):21–44. | |
Wang JG, Staessen JA, Li Y, et al. Carotid intima-media thickness and antihypertensive treatment: a meta-analysis of randomized controlled trials. Stroke. 2006;37(7):1933–1940. | |
Kallio M, Forsblom C, Groop PH, Groop L, Lepäntalo M. Development of new peripheral arterial occlusive disease in patients with type 2 diabetes during a mean follow-up of 11 years. Diabetes Care. 2003;26(4):1241–1245. | |
Salonen R, Nyyssönen K, Porkkala E, et al. Kuopio Atherosclerosis Prevention Study (KAPS). A population-based primary preventive trial of the effect of LDL lowering in atherosclerotic progression in carotid and femoral arteries. Circulation. 1995;92(7):1758–1764. | |
Wagenknecht LE, Zaccaro D, Espeland MA, Karter AJ, O’Leary DH, Haffner SM. Diabetes and progression of carotid atherosclerosis: the Insulin Resistance Atherosclerosis Study. Arterioscler Thromb Vasc Biol. 2003;23(6):1035–1041. | |
Davidson M, Meyer PM, Haffner S, et al. Increased high-density lipoprotein cholesterol predicts the pioglitazone-mediated reduction of carotid intima-media thickness progression in patients with type 2 diabetes mellitus. Circulation. 2008;117(16):2123–2130. | |
Furberg CD, Adams HP, Aplegate WB. Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Asymptomatic Carotid Artery Progression Study (ACAPS) research group. Circulation. 1994;90(4):1679–1687. | |
Howard BV, Roman MJ, Devereux RB, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes: the SANDS randomized trial. JAMA. 2008;299(14):1678–1689. | |
van der Meer IM, Iglesias del Sol A, Hak AE, Bots ML, Hofman A, Witteman JC. Risk factors for progression of atherosclerosis measured at multiple sites in the arterial tree: the Rotterdam study. Stroke. 2003;34(10):2374–2379. | |
Wang JG, Staessen JA, Li Y. Carotid intima-media thickness and antihypertensive treatment: a meta-analysis of randomized controlled trials. Stroke. 2006;37(7):1933–1946. | |
Zuriek M, Touboul PJ, Bonithon-Kopp C, et al. Cross sectional and 4-year longitudinal associations between brachial pulse pressure and common carotid intima-media thickness in a general population. The EVA study. Stroke. 2002;30(3):550–555. | |
Mackinnon AD, Jerrard-Dunne P, Sitzer M, Biehler A, von Kegler S, Markus HS. Rates and determinants of site-specific progression of carotid intima-media thickness: the Carotid Atherosclerosis Progression study. Stroke. 2004;35(9):2150–2154. | |
Bosevski M, Peovska I. Clinical usefulness of assessment of ankle-brachial index and carotid stenosis in type 2 diabetic population – three-year cohort follow-up of mortality. Angiology. 2013;64(1):64–68. | |
Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141(6):421–431. | |
Temelkova-Kurktschiev TS, Koehler C, Henkel E, Leonhardt W, Fuecker K, Hanefeld M. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care. 2000;23(12):1830–1834. | |
Hu G. Gender difference in all-cause and cardiovascular mortality related to hyperglycemia and newly-diagnosed diabetes. Diabetologia. 2003;46(5):608–617. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.