Back to Journals » OncoTargets and Therapy » Volume 10
Progression-free survival of up to 8 months of an advanced intrahepatic cholangiocarcinoma patient treated with apatinib: a case report
Authors Ma FC, Yu Q, Zeng ZM, He RQ, Mo CH, Zhong JC, Ma J, Feng ZB, Chen G , Hu XH
Received 13 July 2017
Accepted for publication 30 September 2017
Published 1 November 2017 Volume 2017:10 Pages 5237—5242
DOI https://doi.org/10.2147/OTT.S146051
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
Fu-Chao Ma,1 Qian Yu,1 Zhi-Ming Zeng,1 Rong-Quan He,1 Chao-Hua Mo,2 Jin-Cai Zhong,1 Jie Ma,1 Zhen-Bo Feng,2 Gang Chen,2 Xiao-Hua Hu1
1Department of Medical Oncology, First Affiliated Hospital of Guangxi Medical University, Nanning, 2Department of Pathology, First Affiliated Hospital of Guangxi Medical University, Nanning, People’s Republic of China
Abstract: Intrahepatic cholangiocarcinoma (ICC) arises from the biliary epithelium and is a relatively rare and highly fatal neoplasm. The prognosis is poor, and survival is limited to a few months. Here, we report a case of advanced ICC that was successfully treated with apatinib, a new oral tyrosine kinase inhibitor that targets the intracellular domain of vascular endothelial growth factor receptor-2. To the best of our knowledge, this is the first case report of the successful use of apatinib for advanced ICC; this treatment has demonstrated fewer toxic effects than traditional cytotoxic chemotherapy. The progression-free survival time was 8 months. The only toxicity observed was mild hand–foot syndrome. Therefore, apatinib may be an additional option for the treatment of advanced ICC, but further prospective studies are needed to optimize the treatment.
Keywords: advanced intrahepatic cholangiocarcinoma, apatinib, PFS, cholangiocarcinoma, VEGFR-2, targeted therapy
Introduction
Intrahepatic cholangiocarcinoma (ICC) arises from the biliary epithelium and is a relatively rare and highly fatal neoplasm. Patients who experience ICC usually present at a late stage of the disease, and the outcomes are poor; as few as 5%–10% of ICC patients with unresectable disease are still alive after 5 years.1 Surgery may be the only potentially curative treatment, but for advanced and metastatic ICC, systemic chemotherapy and radiotherapy remain poorly defined and have only a modest therapeutic effect.2,3 Therefore, patients with ICC require newer, safer, and more effective treatments.
Apatinib (Hengrui Pharmaceutical Co. Ltd, Shanghai, People’s Republic of China), which is a small-molecule tyrosine kinase inhibitor that is taken orally, selectively inhibits vascular endothelial growth factor receptor-2 (VEGFR-2). This is currently a treatment for patients with advanced gastric cancer. Apatinib plays antiangiogenic and antitumor roles through the inhibition of the VEGFR-2 signaling pathway, which prevents tumor growth. The effects of apatinib on advanced gastric cancer4,5 and hepatocellular carcinoma (HCC)6 have been demonstrated by several clinical trials. This drug also shows a wide potential efficacy in a variety of solid tumors including metastatic lung, colon, and breast cancers.7,8 Moreover, apatinib is currently used to treat breast and lung cancer patients.9 Here, we report a unique case of ICC that was treated with apatinib at our hospital. To the best of our knowledge, this is the first case report of a patient with ICC who responded to apatinib.
Case report
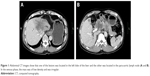
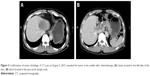
The patient was a 59-year-old male with a 2-month history of upper abdominal pain. He had a history of chronic hepatitis B for >30 years, but no family history of cancer. Physical examination showed no positive signs. The Eastern Cooperative Oncology Group (ECOG) performance status was 0. The concentration of CA19–9 (carbohydrate antigen 19–9) was 60.2 U/mL (0–37 U/mL). He did not appear jaundiced, and his initial liver function was scored as Child-Pugh A. The patient underwent abdominal computed tomography (CT) on January 27, 2015, which demonstrated several irregular and low-density masses in the liver as well as abdominal para-aortic lymph node metastasis, with slight delay enhancement. These masses were located in the left and right lobes of the liver and had a maximum volume of 8.1×7.8 cm, while the volume of the abdominal para-aortic lymph node was 5.4×4.1 cm (Figure 1). He underwent a liver puncture, and the mass was histologically confirmed as ICC (Figure 2). The immunohistochemical analysis showed that the neoplastic cells were positive for CK 18, CK 19, CK 7, and Cox-2 and negative for CDX 2, CK 20, Glypican 3, and hepatocyte markers (Figure 2).
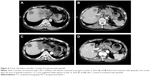
This patient did not undergo surgery or radiotherapy because of extensive lesions and metastasis to the para-aortic lymph node. There was no remote organ metastasis. The patient was initially treated with a traditional chemotherapeutic regimen that consisted of six cycles of gemcitabine/albumin–bound paclitaxel (gemcitabine, 1,000 mg/m2, days 1 and 8, albumin-bound paclitaxel 125 mg/m2, days 1, 8, and 15). A CT scan of the upper abdomen was done after every two cycles of treatment and showed that the regimen was effective. After six cycles of treatment, the mass measured 4.8×6.2 cm (left lobe of the liver, decreased 23.5%), while the abdominal para-aortic lymph node measured 2.7×2.8 cm (decreased 48.1%) on CT (August 5, 2015), which indicated a 33.33% decrease compared with the baseline measurement. This also met the RECIST (Response Evaluation Criteria in Solid Tumors) criteria for a partial response (PR) (Figure 3). During and after the treatment, thrombocytopenia was observed, and no improvement was seen after repeated treatment of the symptoms. Cytotoxic chemotherapy was not given at that time due to the severe thrombocytopenia and was thereafter discontinued. However, the upper abdominal CT scan (January 9, 2016) indicated clear evidence of disease progression, with more intrahepatic metastases and larger metastasis in the abdominal para-aortic lymph node. Since progressive disease (PD) was apparent, the therapy regimen was changed to Keytruda® (Merck Sharp & Dohme Corp, Kenilworth, NJ, USA), a programmed death-1 (PD-1) inhibitor. During 4 months of the treatment, an upper abdominal CT scan (June 3, 2016) revealed that the disease continued to progress (Figure 4A and B).
After written informed consent was provided by the patient to have the case details and any accompanying images published, and the study was approved by The Ethics Committee of the First Affiliated Hospital of Guangxi Medical University, the regimen was then changed to apatinib, at a dose of 500 mg/day (June 4, 2016). The liver function had been scored as Child-Pugh A before the treatment with apatinib. Following 1 month of therapy, a CT scan suggested stable disease (SD) on July 16, 2016 (Figure 4C and D). After that, the patient stopped using apatinib, and a CT scan after 2 months suggested PD (Figure 5). The patient then began treatment with apatinib once again. At that time, an upper abdominal CT scan (May 11, 2017) obtained after 10 months of apatinib treatment suggested SD (Figure 6), and the progression-free survival was 8 months. The ECOG performance status was 1 and the CA19–9 level was 181 U/mL (0–37 U/mL). The only toxicity observed was mild hand–foot syndrome. The entire timeline of treatment is presented in Figure 7.
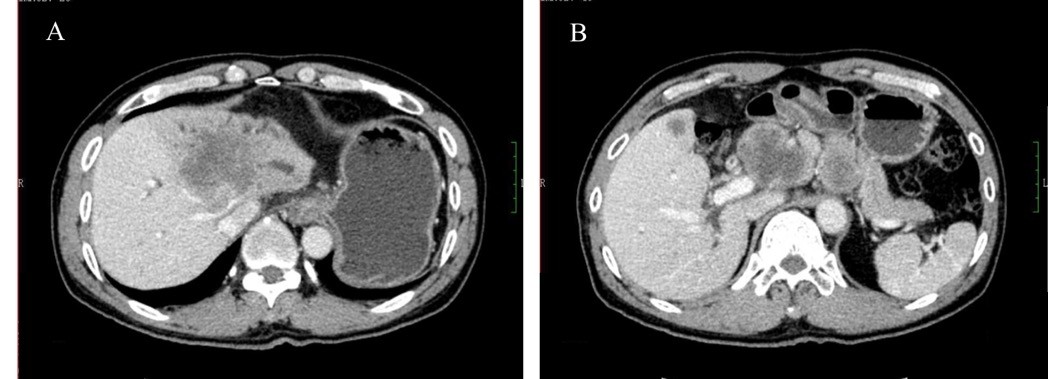
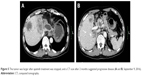  | Figure 5 The tumor was larger after apatinib treatment was stopped, and a CT scan after 2 months suggested progressive disease (A and B, September 9, 2016). |
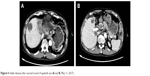  | Figure 6 Stable disease after second round of apatinib use (A and B, May 11, 2017). |
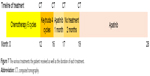  | Figure 7 The various treatments the patient received as well as the duration of each treatment. |
Discussion
ICC is a relatively rare disease, but its incidence continues to increase worldwide.1,10 For patients with local and early ICC, surgery is a potentially curative therapy. It is often asymptomatic until later stages of the disease, and patients are frequently diagnosed at an advanced and unresectable stage. However, in patients with advanced ICC, treatment options are complicated and limited due to recurrence, metastasis, and poor patient outcome, as the median reported survival is 3–6 months.11 No standard treatment guidelines exist for advanced ICC, and the role of adjuvant chemotherapy is limited and controversial. Regimens are primarily based on 5-fluorouracil or gemcitabine as first-line treatments, which have slightly prolonged survival times.12,13 However, the use of 5-fluorouracil or gemcitabine has not improved the 1-year median survival times.
Considering that the prevalence of ICC is usually in late adulthood and that the toxicities of combined chemotherapeutic regimens often lead to treatment cessation, ICC requires novel, effective, and safer treatment options, especially for older patients.1 An understanding of the molecular mechanisms of malignancies will help in the development of novel targeted therapies for this disease. Targeted VEGFR-2 therapies represent a potential treatment of choice. Apatinib promotes apoptosis in ICC via the inhibition of vascular endothelial growth factor (VEGF) signaling in vitro and delayed xenograft tumor growth in vivo.14
VEGF mediates angiogenesis, which plays an essential role in the process of malignant tumor growth.15 The proliferation of the vascular endothelium is activated by the downstream signals that are generated when VEGF binds to vascular epidermal growth factor receptor (VEGFR). Proteins of the VEGFR family are membrane receptor tyrosine kinases that include VEGFR-1, VEGFR-2, and VEGFR-3.16 VEGFR-2 is the major mediator of the mitogenic, angiogenic, and permeability-enhancing effects of VEGF.17 The inhibition of VEGFR-2 might be a promising strategy to inhibit tumor-induced angiogenesis.
Apatinib is a novel oral small-molecule tyrosine kinase inhibitor that targets VEGFR-2 that can suppress tumor angiogenesis. Several studies have confirmed the efficacy of the inhibition of VEGFR-2, which is a promising therapeutic option for a variety of tumor types since this treatment inhibits angiogenesis.14,18,19 In several Phase II clinical trials, it has been demonstrated to be effective in diverse tumor types.20–22 In a Phase II clinical trial, apatinib, which showed a survival benefit in patients with gastric cancer, also demonstrated a manageable toxicity profile.20 However, the successful use of apatinib in ICC has not yet been reported.
In the Phase I study of apatinib, the maximum-tolerated dose was 850 mg once daily, and the recommended dose was 750 mg. The most frequent adverse events of apatinib were hypertension (70%), proteinuria (50%), and hand–foot syndrome (46%).19
In this case report, 6 months after the end of chemotherapy, CT indicated disease progression. Due to older age and thrombocytopenia, the patient could not tolerate toxic chemotherapy, and apatinib was then used to control the disease. Considering his older age and thrombocytopenia, a dose of 500 mg was given. The disease was controlled successfully with an SD and a progression-free survival time of 8 months. In addition, the administration of apatinib resulted in no obvious side effects.
Gene expression analysis revealed that VEGFR-2 is overexpressed in the tissues of patients with ICC.23 Apatinib inhibits VEGF signaling and promotes apoptosis in ICC,17 which suggests that apatinib leads to a clinical response via the inhibition of VEGFR-2 tyrosine kinase activity.
Conclusion
Apatinib may serve as an additional option for the treatment of ICC, especially for older patients and those with poor performance status. In this case report, apatinib exhibited good efficacy and safety in the treatment of the patient. Further large-scale prospective studies are required to optimize the use of apatinib in the treatment of ICC and to identify which patients will benefit from the agent.
Acknowledgments
We are grateful to the patient for participating in this study. We also thank Wen-guang Bao, Zhi-gang Peng, and all of the clinicians for their contributions to the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24(2):115–125. | ||
Farges O, Fuks D, Boleslawski E, et al. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg. 2011;254(5):824–830. | ||
Mechteld C, de Jong, Hari Nathan, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29(23):3140–3145. | ||
Jin Li, Shukui Qin, Jianming Xu, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31(26):3219–3225. | ||
Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34(13):1448–1454. | ||
Qin S. Apatinib in Chinese patients with advanced hepatocellular carcinoma: a phase II randomized, open- label trial. J Clin Oncol. 2014;32(Suppl 5):abstract 4019. | ||
Hu X, Zhang J, Xu B, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer. 2014;135(8):1961–1969. | ||
Scott AJ, Messersmith WA, Jimeno A. Apatinib: a promising oral antiangiogenic agent in the treatment of multiple solid tumors. Drugs Today (Barc). 2015;51(4):223–229. | ||
Fontanella C, Ongaro E, Bolzonello S, et al. Clinical advances in the development of novel VEGFR2 inhibitors. Ann Transl Med. 2014;2(12):123. | ||
Doherty B, Nambudiri VE, Palmer WC. Update on the diagnosis and treatment of cholangiocarcinoma. Curr Gastroenterol Rep. 2017;19(1):2. | ||
Cunningham SC, Choti MA, Bellavance EC, Pawlik TM. Palliation of hepatic tumors. Surg Oncol. 2007;16(4):277–291. | ||
Lim KH, Han SW, Oh DY, Im SA, Kim TY, Bang YJ. Outcome of infusional 5-fluorouracil, doxorubicin, and mitomycin-C (iFAM) chemotherapy and analysis of prognostic factors in patients with refractory advanced biliary tract cancer. Oncology. 2012;83(2):57–66. | ||
Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–1281. | ||
Peng H, Zhang Q, Li J, et al. Apatinib inhibits VEGF signaling and promotes apoptosis in intrahepatic cholangiocarcinoma. Oncotarget. 2016;7(13):17220–17229. | ||
Ferrara N. Vascular endothelial growth factor as a target for anticancer therapy. Oncologist. 2004;9(Suppl 1):2–10. | ||
Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. | ||
Glade-Bender J, Kandel JJ, Yamashiro DJ. VEGF blocking therapy in the treatment of cancer. Expert Opin Biol Ther. 2003;3(2):263–276. | ||
Scott AJ, Messersmith WA, Jimeno A. Apatinib: a promising oral antiangiogenic agent in the treatment of multiple solid tumors. Drugs Today (Barc). 2015;51(4):223–229. | ||
Li J, Zhao X, Chen L, et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer. 2010;10:529. | ||
Li J, Qin S, Xu J, et al. Apatinib for chemotherapy- refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31(26):3219–3225. | ||
Hu X, Cao J, Hu W, et al. Multicenter phase II study of apatinib in non-triple-negative metastatic breast cancer. BMC Cancer. 2014;14:820. | ||
Langer CJ, Mok T, Postmus PE. Targeted agents in the third/fourth-line treatment of patients with advanced (stage III/IV) non-small cell lung cancer (NSCLC). Cancer Treat Rev. 2013;39(3):252–260. | ||
Sang H, Li T, Li H, Liu J. Gab1 regulates proliferation and migration through the PI3K/Akt signaling pathway in intrahepatic cholangiocarcinoma. Tumor Biol. 2015;36(11):8367–8377. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.