Back to Journals » Cancer Management and Research » Volume 12
Progress of Artificial Intelligence in Gynecological Malignant Tumors
Authors Zhou J , Zeng ZY, Li L
Received 2 September 2020
Accepted for publication 22 October 2020
Published 14 December 2020 Volume 2020:12 Pages 12823—12840
DOI https://doi.org/10.2147/CMAR.S279990
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bilikere Dwarakanath
Jie Zhou,1,2,* Zhi Ying Zeng,3,* Li Li1
1Department of Gynecologic Oncology, Guangxi Medical University Cancer Hospital, Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, Ministry of Education, Nanning 530021, Guangxi, People’s Republic of China; 2Department of Gynecology, The Second Affiliated Hospital, University of South China, Hengyang 421001, Hunan, People’s Republic of China; 3Department of Anesthesiology, The Second Affiliated Hospital, University of South China, Hengyang 421001, Hunan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Li Li
Department of Gynecologic Oncology, Guangxi Medical University Cancer Hospital, Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, Ministry of Education, Nanning 530021, Guangxi, People’s Republic of China
Email [email protected]
Abstract: Artificial intelligence (AI) is a sort of new technical science which can simulate, extend and expand human intelligence by developing theories, methods and application systems. In the last five years, the application of AI in medical research has become a hot topic in modern science and technology. Gynecological malignant tumors involves a wide range of knowledge, and AI can play an important part in these aspects, such as medical image recognition, auxiliary diagnosis, drug research and development, treatment scheme formulation and other fields. The purpose of this paper is to describe the progress of AI in gynecological malignant tumors and discuss some problems in its application. It is believed that AI improves the efficiency of diagnosis, reduces the burden of clinicians, and improves the effect of treatment and prognosis. AI will play an irreplaceable role in the field of gynecological malignant oncology and will promote the development of medicine and further promote the transformation from traditional medicine to precision medicine and preventive medicine. However, there are also some problems in the application of AI in gynecologic malignant tumors. For example, AI, inseparable from human participation, still needs to be more “humanized”, and needs to further protect patients’ privacy and health, improve legal and insurance protection, and further improve according to local ethnic conditions and national conditions. However, it is believed that with the continuous development of AI, especially ensemble classifier, and deep learning will have a profound influence on the future of medical technology, which is a powerful driving force for future medical innovation and reform.
Keywords: artificial intelligence, gynecological malignant tumor, diagnosis, treatment, prognosis
With the rapid development of human society, cancer-related knowledge is also growing exponentially, which has caused a knowledge gap for practicing oncologists.1 With the increasing understanding of each patient, more and more information needs to be absorbed from literature in providing evidence-based cancer treatment. Researchers have shown that due to the heavy clinical tasks and limited time for clinicians to acquire professional knowledge, it is difficult to invest a lot of energy in timely access to the latest literature, which is more obvious in relatively backward countries and regions. This contributes to the relative delay of information absorption, leading to a gradually widening gap between what results the academic research centers really achieved and what is practiced in fact.2 However, compared with doctors in other clinical disciplines, clinical oncologists urgently need to acquire evidence-based medicine knowledge in time to support patient's personalized treatment plans. Consequently, clinicians need some new types of tools to bridge this knowledge gap, analyze historical data, predict future results and determine the best treatment plan in the current situation.3 In this way, clinicians can support and adopt new prediction, diagnosis and treatment methods in an evidence-based manner, and provide actionable insights for improving the delivery of health care4 so that more people can benefit from social investment in research and development.5 At this moment, with the academic circles announcing new breakthroughs and technologies at a breakneck pace, artificial intelligence (AI) naturally appears in the consciousness of medical personnel and the public. At the same time, AI, which is currently believed to fully revolutionize many aspects of current clinical practice in the foreseeable future, will play an important role in the fields of oncology such as medical image recognition, auxiliary diagnosis, drug research and development, treatment plan formulation, etc.
The Background of AI in the Application of Medical and Gynecological Malignant Tumors
AI first appeared in the early 1950s. In October 2016, the National Academy of Science, Engineering and Medicine held a meeting to discuss the impact of the fourth industrial revolution on manufacturing, society and economy.6 Experts unanimously recognized that an area of great influence on manufacturing industry is AI, which refers to the creation of intelligent machines with functions and reactions similar to human beings.7 The goal of AI is to replicate the human mind, that is to say, it can perform tasks such as identification, interpretation, reasoning, and transformation, with the acuteness and influence typically attributed to human beings. It seems that the human mind tends to reach its limits. Aimed at enhancing human’s abilities, Machine intelligence (MI, also called augmented intelligence) is a more concerned area of AI. It is good at the areas that human beings are not good at, such as absorbing a large amount of qualitative information that can recognize the patterns of relevant information.8 The most famous example of AI is that it has defeated human experts in knowledge and strategy games, such as Alpha Go, which it has learned from its own competition and defeated the world’s strongest Go player.9,10 Although it is only a primary AI, the success of Alpha Go has made human beings aware of the possibility that computer technology can rival or even surpass human beings.
Medical personnel also realized the importance of AI and gradually applied it to the medical field. Therefore, AI has been closely related to medicine from the moment of its birth. In the field of medicine, the goal of AI is to make the behavior of machines look like the intelligent behavior of medical personnel. However, with the rapid development of AI, early AI such as the automatic interpretation of electrocardiogram, which appeared in the 1970s, now most people will be surprised to hear that it was once described as AI. Recent examples of the application of AI to medicine are also numerous. In 2015, Atomwise’s progress in finding a cure for Ebola was remarkable. They used AI to find two predictive drugs that could be used to fight Ebola. The whole process took only a week and cost less than $1000. In the application of intelligent diagnosis and treatment, IBM Watson is currently the most mature case. Da Vinci Surgical System, a well-known example of robotic-assisted surgical system, has also developed to the fourth generation.
Just because medicine has always been regarded as one of the most promising application fields of AI, both shallow learning (SL) and recent deep learning (DL) are widely integrated into medicine. It is worth noting that, unlike SL based on shallow structure algorithm, DL is an algorithm that attempts to abstract data at a high level by using multiple processing layers including complex structures or multiple nonlinear transformations. In the past decades, more and more scholars have used advanced classification technology to predict various diseases. At the same time, implementation of models incorporating AI, such as classification regression trees (CARTs),11 have been applied to cancer prediction in many fields. At present, the most successful domain of medical AI applications is automatic medical image diagnosis. Many medical specialties, including radiology, pathology and so on, which are closely related to tumor patients, rely on image-based diagnosis, so they are very suitable for DL technology and have made great progress in clinical application, especially in tumor patients. DL and Radiomics are creating a paradigm shift in radiology and precision medicine by developing a new area of research to be used for precision medicine.12 For example, AI can be useful in the detection of lung nodules from CT images,13–15 prediction of complete response after neoadjuvant chemoradiation for locally advanced rectal cancer,16 prediction of the response to individual induction chemotherapy in advanced nasopharyngeal carcinoma,17 as well as the detection of cell mitosis,18 and lymph node metastasis19 in breast cancer from pathological images, etc. In recent years, with the further penetration of AI into the field of medical treatment, gynecologic oncologists are unwilling to lag behind. So the ongoing research of AI methods has strengthened and supplemented many aspects of gynecological tumors, such as diagnosis, treatment planning, and prognosis of patients (see Figure 1). This paper will briefly introduce the latest progress and problems of AI in the field of gynecological malignant tumors, and discuss whether AI can optimize the diagnosis and treatment of gynecological malignant tumors.
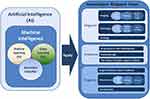 |
Figure 1 Schematic diagram of application of artificial intelligence in gynecological malignant tumors. |
The Value of AI in the Diagnosis of Gynecological Malignant Tumors and Precancerous Lesions
Medical Imaging Diagnosis
Ultrasound Imaging
Nowadays, one of the most challenging problems that medical staffs often meet in gynecologic practice is the differential diagnosis of benign and malignant adnexal masses in ultrasound images. Transvaginal ultrasound is a very useful way to identify differential diagnosis and it is thought to be the first-line imaging technology. However, it is highly dependent on the examiner’s experience; that is to say, a major defect with this technology is its diagnostic performance which mainly based on the subjective impression of diagnostic medical sonographers. In order to improve the diagnostic accuracy of ultrasound examination, it is necessary to develop a computer-aided design (CAD) technology for ultrasound images. In the study of Aramendía-Vidaurreta et al20 a new technology for automatic identification of adnexal masses based on a neural network (NN) method was tested. They firstly calculated seven different types of features (local binary pattern, entropy, law texture energy, invariant motion, gray level co-occurrence matrix, Gabor wavelet and fractal dimension) from ultrasound images of the ovary, extracted several parameters from these features and collected them together with the age of gynecological patients. Images of 39 malignant and 106 benign, obtained from patients with ovarian tumor, were used to verify the proposed technology, which corresponded to the probability of its occurrence in the general population. By using this CAD technology, which based on a NN, they found the accuracy of automatic identification of malignant adnexal masses was 98.78%, the sensitivity was 98.50%, the specificity was 98.90%, and the area under the receiver operating characteristic curve (AUC) reached 0.997. Therefore, it is believed that the performance of DL is obviously better than that of less experienced examiners and SL such as support vector machine (SVM), which can overcome the limitations of available approaches. The highlight of this study lies in considering a wide range of texture features and making use of advantages that NNs can generalize. In primary health care, due to the lack of input from specialist, algorithms are crucial for proper patient guidance, as is the early screening of endometrial cancer. In the study by Pergialiotis et al,21 three different methods including logistic regression, artificial neural networks (ANNs) and CARTs were used to compare diagnostic accuracy of endometrial carcinoma in postmenopausal women presenting with endometrial thickness of 5 mm or abnormal vaginal bleeding. The diagnostic accuracy of three methods was determined by ultrasonography combined with the final pathological results. The study found that the sensitivity of the ANN model (86.79%) was much higher than that of CARTs (78.3%) and logistic regression model (76.4%) (p<0.05 in both comparisons). The specificity of ANN model (83.33%) was higher than CARTs (76.4%) and logistic regression model (66.7%) (in the logistic regression model, p<0.05) (see Table 1). In terms of total accuracy, the total accuracy of ANN (85.39%) was higher (in the logistic regression model, p<0.05). As a result, the performance of DL (ANN) is obviously better than SL (CART) and logistic regression. Therefore, AI, especially DL with particularly high sensitivity and specificity, is a powerful and useful mathematical tool, which can be used in primary health care and considerably promote public health.
 |
Table 1 Performance Indices of the Three Applied Classification Models for Diagnosis of Endometrial Carcinoma |
Magnetic Resonance Imaging (MRI)
In the treatment of endometrial cancer, histological grade, International Federation of Gynecology and Obstetrics (FIGO) staging, lymphovascular space invasion (LVSI) and deep myometrial invasion (DMI) are important prognostic factors, which can be used for risk stratification. However, most of these prognostic factors only can be evaluated in operative specimens obtained during comprehensive staging. As a result, a comprehensive noninvasive diagnostic method for preoperative risk stratification is needed in clinical practice, which can predict tumor staging and invasion. A study by Ueno et al22 included 137 women with endometrial cancer with a maximum diameter of more than 1 cm who underwent 1.5-T MRI before hysterectomy. Texture analysis was performed using commercial research software and the surrounding region of interest was manually delineated. The results show that texture analysis and radio-frequency modeling based on MRI can accurately diagnose the presence of DMI, LVSI, and high-level tumors, and the diagnostic accuracy is equivalent to that of the most experienced radiologists. It shows that texture features based on MRI can be used for computer-aided diagnosis, and MRI combined with AI can distinguish clinical-pathologic prognosticators before treatment thus providing enough clinical benefit for patients with endometrial cancer. Dong et al23 recently verified the accuracy of using DL with a convolutional neural network (CNN) approach to detect depth of myometrial invasion of endometrial cancer in MRIs. No significant difference in diagnostic accuracy was found between radiologists (accuracy rate: 77.8%) and AI for both T1w (79.2%) and T2w (70.8%). It is believed that AI has the potential to assist radiologists or serve as a reasonable alternative for preoperative evaluation of myometrial invasion depth of stage I endometrial carcinoma. However, this study also pointed out that AI is more likely to provide the wrong interpretations in patients with coexisting benign leiomyomas or polypoid tumors. Malek et al24 submitted detailed opinions on a semi-automatic computer-aided analysis framework based on perfusion weighted magnetic resonance imaging (PWI) in a study to distinguish uterine sarcoma from leiomyomas. In the framework, radiologists drew the regions of interest (ROI) on myometrium, psoas muscle and tumor regions, and then extracted seven parameters from each ROI to characterize contrast agent dynamics. Subsequently, the information was input into a decision tree (DT) ensemble classifier, which classified lesions into benign uterine leiomyoma or malignant uterine sarcoma. The classifier achieved 100% sensitivity and 90% specificity at its optimal operating point. These preliminary results indicate that the proposed way to obtain a promising discriminative power can be used together with conventional MRI sequences to distinguish sarcomas from myomas.
Spectroscopy Imaging
As a preinvasive lesion, cervical intraepithelial neoplasia (CIN) can be detected by a screening program to obtain an appropriate treatment. The automatic detection and segmentation of abnormal areas in cervical image play a vital role in the diagnosis of cervical lesions. Xue et al25 developed a diffuse reflectance spectrum detection and analysis system based on LabWindows development software and MariaDB database, which can collect spectrum data and save it to the database. The ANN model based on a spectral database was established to distinguish cervical tissue from normal tissue. The nude mouse tumor model test and the human volunteer test have been carried out. The results showed that the hemoglobin content in unit tissue increased during cell proliferation, so the absorption coefficient of light increased. Therefore, with the aggravation of preinvasive lesion, the spectral peak value changes obviously in the wavelength range of 500~600 nm. It is proved that the system can distinguish CIN from normal cervical and can be applied to screening preinvasive cervical lesions. So far, cervical carcinoma is still the main reason of cancer-related deaths in women around the world. Elayaraja and Suganathi26 proposed a theory for screening the cervical carcinoma using cervigram image. Oriented local histogram technique (OLHT) was put into use in the image of cervix to enhance the edge, and then dual tree-complex wavelet transform (DT-CWT) was put into use in the enhanced image to obtain the multi-resolution image. The preprocessed image is then used to extract features such as wavelet features, gray level co-occurrence matrix (GLCM) features, moment invariant features and local binary pattern (LBP) features. In order to classify a given cervical image as benign or malignant, these extracted features are repeatedly trained and then tested by a feed-forward back propagation neural network (FFBPNN) so that abnormal cervical images could be detected and tumor regions could be segmented by morphological operation. The study also analyzed the performance of this cervical carcinoma detection system. The results showed that the performance index of the proposed detection system can achieve the sensitivity up to 97.42%, specificity up to 99.36%, accuracy up to 98.29%, positive predictive value (PPV) of 97.28%, negative predictive value (NPV) of 92.17%, likelihood ratio positive (LRP) of 141.71, likelihood ratio negative (LRN) of 0.0936, precision of 97.38%, false positive rate (FPR) of 96.72% and false negative rate (FNR) of 91.36%. The study also pointed out that SL has its limitations, such as being only applicable to high-resolution cervical images, detecting only the external boundary regions of cancer regions, and the sensitivity and accuracy rate for further cervical cancer diagnosis are not optimal. Therefore, the results of DL (NN) for cervical cancer detection and classification are better than SL (SVM) (see Table 2).
 |
Table 2 Performance Indices of the Three Methodologies for Diagnosis of Cervical Cancer |
Jaya and Kumar27 proposed another cervical cancer detection and segmentation methodology based on an adaptive neuro fuzzy inference system (ANFIS) classifier. They made use of the advantages of ANFIS, which combines the learning mechanism of NNs and the cognitive linguistic ability of fuzzy systems, to make up for their respective deficiencies. For noncancerous and cancerous cervical images, the proposed system achieved classification accuracy of 97.14% and 100%, respectively. This proposed methodology for cervical image classification achieved 98.57% of the total classification accuracy. The performance analysis of the proposed cervical cancer detection and segmentation system showed that its sensitivity was 97.42%, specificity was 99.36%, and segmentation accuracy was 99.36%. Therefore, the simulation on these cervical image data sets shows that the new method is superior to the traditional cervical cancer detection and segmentation methods and has higher performance in clinical practice.
Pathological Diagnosis
Pap Smear
At present, the methods of screening cervical cancer (ie, Pap smear and liquid based cytology) are time-consuming and depend on the skill of the cell pathologist, which is quite subjective. Therefore, many researchers focus on the development of intelligent computer vision system to help clinicians overcome these problems, so as to produce more accurate results. Based on the morphological characteristics of cervical cancer cells, Bora et al28 classified Pap smear images in their research by using the integrated classifier which was designed with three popular individual classifiers: SVM, neural network multilayer perceptron (MLP) and random forest classifier (RF). Then the generated database was used for three-level classification, and the three characteristics of shape, texture and color were correctly analyzed, thus the accuracy of smear level reached 98.11%, the accuracy of cell level reached 98.38%, respectively. However, the accuracy rate was 96.51% (Grade 2) and 91.71% (Grade 3) using the Herlev Pap smear database. Therefore, all features are proved that it is very important for the classification of Pap smear samples, and a single feature cannot provide high accuracy in the classification process. The study also found that the performance of the ensemble classifier is the best, and the performance of MLP and SVM are similar, both of which are better than RF (see Figure 2).
 |
Figure 2 Output of different classifiers for Pap smear level classification. Abbreviations: MLP, multilayer perceptron; RF, random forest classifier; LSSVM, least square support vector machine. Note: Reprinted from Comput Methods Programs Biomed, 138, Bora K, Chowdhury M, Mahanta LB, et al, Automated classification of Pap smear images to detect cervical dysplasia, 31–47, Copyright (2017), with permission from Elsevier.28 |
Liquid Based Cytology
Zhang et al29 proposed a cervical cell classification method based on CNN, which is different from previous methods, which depend on cytoplasm/nucleus segmentation and manual features. This technology can automatically extract image patches coarsely centered on the nucleus as network input, which means that it can extract deep features embedded in cell image blocks for classification. It was found that this method yielded the highest performance not only on the Herlev Pap smear, but also on the H&E staining manual liquid-based cytology (HEMLBC) liquid-based cytology datasets. CNN’s performance was better than that of LBP, SVM and even ANN, etc. Therefore, it is expected that this type of cervical cell classification system with segmentation-free and high accuracy will be developed into an automatic assisted reading system for primary cervical screening. In order to facilitate the diagnosis of cervical intraepithelial neoplasia of different grades, Sheikhzadeh et al30 developed a DL network called Whole Image (WI)-Net to judge molecular biomarkers of immunohistochemistry images. The testing results showed that the automatic labeling method of WI-Net was comparable to that of manual labeling by professional cell technicians (positive p16, positive Ki-67, positive p16 and Ki-67, negative p16 and Ki-67) (the average F value was 0.96). The advantage of this method is that it does not need to find and quantify the complex characteristics of cells expressing specific biomarkers. Therefore, this method may be far more reproducible than those that rely on the extraction of predefined or handmade nuclear or cell appearance features. It also offers an accurate evaluation of epithelial cell proliferation characteristics, which helps to describe the classification of diseases (distinguish CIN 1, CIN 2, CIN 3 form normal cervical epithelial cells). More importantly, this method based on fully convolutional network (FCN) can be easily applied to diverse ways of tissue types and different staining combinations, as well as other research questions in modern pathology.
Kyrgiou et al31 developed a clinical decision support scoring system (DSSS) based on an ANN of MLP for personalized management of women with cervical abnormalities, and the date was used from 2267 women’s HPV biomarkers (E6, E7, p16INK4a) and cytology results. Detailed patient characteristics and colposcopy results were also collected, and a series of biomarkers were performed using liquid-based cytology samples. Compared to cytology with or without HPV, the sensitivity and specificity of this novel ANN for predicting CIN 2 or worse were 93% and 99.2%, respectively. The results show that the clinical DSSS based on MLP can accurately predict the histological status of women who participate in cytological screening, which also means that it can optimize the personalized management of women who have abnormalities at cervical screening. The study also pointed out that the total accuracy rate of MLP is higher than that of conventional cytological examination, which can correctly classify this cervical disease.
Other Cytology
In recent experiments, cell light scattering analysis was used for unlabeled cell analysis, while a directional gradient histogram (HOG) algorithm was used to extract the anisotropic features of two-dimensional light scattering patterns. In order to collect a certain amount of light scattering patterns of epithelial ovarian cancer cells and normal epithelial ovarian cells for HOG processing, Chen and Zhang32 designed a single cell light scattering pattern collection platform for ovarian cancer. They performed five independent 10-fold cross-validation processes by SVM algorithm to verify whether the classification results were more reliable. The verification results showed that the accuracy was over 90.20%, the maximum accuracy reached 91.55%, while the average value was 90.81%; the highest sensitivity was 95.95%, the average accuracy was 94.05%, and the average specificity was 87.57%. This research shows that the characteristics of HOG could specifically identify ovarian cancer cells and the normal ovarian cells. This light scattering pattern can be applied as a marker-free way to distinguish two kinds of cells, and the two-dimensional light scattering technology is expected to obtain high accuracy in the clinical identification of ovarian cancer cells.
Serological Diagnosis
Serological diagnosis has always been a common method for malignant tumor patients, especially for ovarian cancer (OC) patients. Kawakami et al34 have established a specific predictive framework for pretreatment estimation of histotypes, clinical stage, residual tumor burden and prognosis of epithelial ovarian cancer (EOC) patients using machine learning algorithms based on multiple biomarkers and clinical variables. They randomly assigned 334 patients with EOC and 101 patients with benign ovarian tumor into training group and testing group. Seven supervised machine learning classifiers, including RF, SVM, conditional random forest (CRF), gradient boosting machine (GBM), naïve Bayes algorithm (NB), elastic network (EN) and NN, were used to obtain diagnosis and prognostic information from 32 parameters commonly used in pretreatment peripheral blood test. The results showed that machine learning technology had an advantage over traditional regression-based analysis in predicting multiple clinical parameters of EOC. When they combine the integration method of weak decision trees (such as GBM, RF, and CRF), this new system showed the best performance in receiver operating characteristic (ROC) curve prediction for segregating EOC from benign ovarian tumors, the accuracy and the AUC with RF were 92.4% and 0.968, respectively. The highest accuracy and AUC with RF in predicting clinical staging were 69.0% and 0.760 respectively. Moreover, RF could predict the histotypes of high-grade serous and mucinous EOC before operation. More importantly, unsupervised cluster analysis identified the subgroups with significantly worse survival in patients with early-stage EOC. It suggests that the machine learning system, especially integrated classifiers, can provide critical diagnosis and prognosis prediction for patients with EOC before initial intervention, and the use of predictive algorithm can promote personalized treatment selection and avoid “one-size fits all” treatment approach through pretreatment stratification of patients. The study also pointed out that in order to further improve the accuracy of prediction, AI should be used in future studies to determine the prediction characteristics of preoperative blood value time series.
Application of AI in Gynecologic Malignant Tumor Treatment
Drug Research and Development
With the individual differences of malignant tumor patients and the emergence of multidrug resistance, many patients with gynecological cancer have poor drug sensitivity resulting in unsatisfactory clinical treatment results. In the process of drug research and development, AI is also playing an increasingly important role to meet this challenge. Sherin et al.35 used modeling and AI to map the anticancer effect of synthetic gallic acid analogs in a wide scope of concentrations and exposure times, explore the basic mechanism of drug action, and carefully infer the heterogeneity of drug response. They first synthesized esters and amides, which were two series of gallic acid derivatives. Then they characterized the new compounds by spectral data, such as mass spectrometry (MS), Fourier transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR). The anticancer activity of these compounds was tested in vitro by A2780 cell line (a wild-type human ovarian cancer cell). In order to characterize completely optimal anticancer activities, a new method, a comprehensive model containing Hill function, was put into use to quantitatively evaluate the in vitro anticancer effects of the test compound. They also used SVM combined with pharmacodynamic modeling paradigm not only to identify the test compounds that trail forecasting algorithm by analyzing the data collected at different temporal values, but also to verify the anticancer efficiency of the compounds. The results showed that all the tested compounds were biocompatible, and had strong anticancer activity against ovarian cancer cells A2780. According to the concentration for 50% of maximal effect (EC50) value, compounds 7b, 7c, 7g, 7h, 7m, 9c, and 9b were identified as the most effective anticancer agent against A2780 cell line. At the same time, a pharmacodynamic model of the anticancer potential of synthetic compounds was established, and it was found that the therapeutic effect could be optimized by adjusting the drug efficacy and response heterogeneity through changing the exposure time. Combining the experimental results of anticancer activity with the prediction of drug action based on AI, compounds 7b, 7g, and 9b can be used as a promising nature mediated anticancer medicine precursors and can be recommended for in vivo research, and the results of these compounds may provide theoretical basis for preclinical studies of their anticancer effects. Therefore, it is believed that this method may effectively infer the in vitro results of lead compounds produced both in vivo and preclinical research.
Chemotherapy
Nowadays, chemotherapy is still the most effective maintenance therapy for all kinds of cancers, although it has many shortcomings such as drug resistance and serious side effects. Recently, many clinical studies have shown that short-term application of antitumor angiogenesis drugs and chemotherapy drugs can achieve ideal therapeutic effects. After long-term use, it will lead to necrosis of blood vessels in the central area of the tumor, resulting in hypoxia and acidic microenvironment in the tumor, so as to reduce the sensitivity of solid tumor cells to chemotherapy drugs and lead to drug resistance, and patients will relapse. In order to solve such problems, Li et al36 have constructed an autonomous DNA robot by using DNA origami technology and named it nanorobot-Th, which was programmed to transport payloads (chemotherapeutics, peptide drugs or siRNA) for studying the antitumor activity of nanorobots in tumors with fewer blood vessels. In the experiment, SK-OV-3 (a human ovarian cancer cell line) was selected because it has been reported to be lowly vascularized and has a relatively poor permeability and retention for Evans blue. They transplanted SK-OV-3 cells subcutaneously in mice, and found that compared with the control group, nanorobot-Th exhibited significantly restrained tumor growth after treatment, and also conspicuously prolonged the survival time of animals with xenografts. It was found that limiting tumor permeability does not stop nanorobots from playing an antitumor role, and it was confirmed that the autonomous DNA robot is absolutely safe in normal tissues of large mammals. Therefore, it can be considered that the DNA nanorobot is applicable to tumors of various levels of vascularization, and stands for a promising tactics for precise drug delivery in cancer treatment. In the past few years, intraperitoneal (IP) chemotherapy has rekindled the hope of inhibiting intraperitoneal transmission of gynecologic cancer. However, poor drug permeability is one of the major shortcomings of IP chemotherapy. It is necessary to develop new strategies to improve the efficacy of IP chemotherapy. Shamsi et al37 proposed a novel magnetically assisted tactic to improve drug penetration into peritoneal surface malignancy and reduce disease recurrence in their research. In order to evaluate the ability of magnetic drug targeting (MDT) technology in surpassing interstitial barriers and increasing the performance of anticancer drugs, they established a computer model consisting of three main parts: the transport of magnetic nanoparticles (MNPs) in cancer tissues, magnetic force, and interstitial fluid. The model was also used to evaluate the predictability and controllability of their established active drug delivery technology. Then, the experiment simulated the penetration of intraperitoneally injected MNPs inside cancerous tissues and compared with the penetration of paclitaxel and cisplatin, two widely used free cytotoxic agents. The results of magnetically assisted delivery presented that the final concentration of drug-coated MNPs in cancerous tissues increased by an order of magnitude compared with free cytotoxic agent, which was expected to improve the therapeutic effect of IP chemotherapy in the future.
The Clinical Decision Support System—Watson for Oncology
Watson for Oncology (WFO), an AI computer program, was developed by IBM Corporation (USA) with the help of top oncologists from Memorial Sloan Kettering Cancer Center (MSK). Their goal was to create a cognitive computing system to meet today’s big data information challenges. The scientists who developed WFO integrated natural language processing, information retrieval, knowledge expression, machine learning, and general reasoning modes, acquired and evaluated a large amount of structured and unstructured data from previous medical records through machine learning and natural language processing to make recommendations for cancer treatment. As for supported cases, the treatment recommendations provided by WFO are divided into three groups: recommended, ie green “buckets”, which represents a treatment supported by obvious evidence; for consideration, ie yellow “buckets”, which represents a potentially suitable alternative; and not recommended, ie red “buckets”, which stands for a treatment with contraindications or obvious evidence against its use. Although WFO helps to reduce the time required for clinical treatment planning,38 due to the recent development of cognitive computing technology, there is still a lack of large-scale data applied outside the US. Several studies have analyzed the consistency of treatment schemes determined by multidisciplinary team (MDT) and WFO, including gynecologic tumors. Liang et al39 have re-identified and analyzed concordance studies published by WFO in 2017–2018 in nine unique institutions located in seven provinces in China. By comparing cancer types and institutions respectively, he announced the concordance rate. The results showed that concordance rate of all included cases was 59% (2012/3388), and the concordance rate varied according to cancer types and institutions. Concordance of ovarian cancer was 96% and 43% respectively in two different hospitals. The concordance of gastric cancer was the lowest, only 12%. Therefore, it is believed that the concordance rate between therapeutic options from WFO and clinical treatment schemes is obviously different in different cancer types and institutions in China, indicating that there are noticeable practical variations. Zhou et al40 expanded the sample size and cancer spectrum using the updated version of WFO to explore the concordance of the suggested therapeutic regimen between WFO and MDT, which could reflect the similarities and differences between East and West in the treatment of cancer. In the study, it was found that the treatment recommendation concordance of cervical cancer was only 50%, and the remaining half was physician’s choice. By contrast, the treatment recommendation concordance of ovarian cancer was up to 95.83%, and only 4.17% of cases was physician’s choice. Besides showing a high degree of consistency with most MDT programs, WFO, as an AI clinical decision support system, has the following advantages: First, it improves doctors’ work efficiency and reduces workload. Researchers found that it takes an average of 20 min for oncologists to capture and analyze data manually and make recommendations. When oncologists became familiar with these cases, the average time could be reduced to approximately 12 min. However, compared with oncologists, WFO only took 40 seconds to complete these tasks.38 Second, it can prevent man-made calculation errors. Chemotherapy scheme and drug selection involve multiple clinical formulas, which need to be calculated one by one in sequence. The process is complicated and time consuming. At the same time, due to the fact that doctors often estimate through experience in actual work, there may be errors in selection;41 WFO can calculate all the input data of the patient and generate a recommendation scheme through a computer program step by step, thereby achieving accurate medication and preventing such errors.42 Finally, it can improve the quality of doctor–patient communication and prevent doctor–patient disputes. Nowadays, due to a variety of reasons, patients’ distrust of doctors is increasing in China.43,44 Therefore, the more patients participate in the decision-making of their own therapeutic regimen, the more they understand about their own diseases. Once they have more confidence in the therapeutic regimen, they will cooperate with doctors more actively.45 But at the same time, WFO still has certain limitations, which lead to differences in the coincidence rate when other countries apply the system. These are mainly related to different drug choices,46 different treatment options,47 coexisting diseases of patients,48 and economic factors49 in different countries. Only by solving these problems can the clinical decision support system benefit more people in the world.
Application of AI in Predicting Prognosis of Gynecological Malignant Tumor
Surgical Method Value Prediction
Ovarian cancer is the deadliest gynecologic cancer in the developed world.50 Even though more and more medical and operative attempts are raising curative effect of first-line treatment for ovarian cancer, most patients still develop recurrent disease. It is well known that chemotherapy is still the gold standard for the treatment of recurrent ovarian cancer,51 but more and more data show that secondary cytoreductive surgery (SCS) may be a valuable treatment in selected patients. Despite the lack of mature evidence, some studies emphasize that SCS may improve the prognosis of patients with platinum-sensitive relapse.52 AI is thus used to measure the importance of individual patients and disease variables to determine who among the many recurrent ovarian cancer patients is worth SCS. Bogani et al53 conducted a retrospective study to evaluate 194 patients with recurrent ovarian cancer who have been treated by SCS. ANN analysis was used to estimate the importance of different variables and predict complete cytoreduction (CC) and survival. In general, 82.9% of patients could reach CC during SCS. Using ANN, they found several main factors could affect the abilities of achieving CC. These factors included disease-free interval (DFI), retroperitoneal recurrence only, residual diseases at primary surgical treatment and FIGO staging, and importance of factors predicting for CC were 0.231, 0.178, 0.138, and 0.088, respectively (see Figure 3A). Interestingly, the experimental data confirm that the existence of retroperitoneal diseases alone, rather than peritoneal diseases, is related to the ability to achieve CC. Single or multiple peritoneal mass or nodule have little impact on achieving CC, suggesting that the presence of peritoneal carcinomatosis should not be a surgical contraindication of SCS. By observing the relationship between different covariates and overall survival (OS), they found that DFI was the most significant variable which influenced OS (importance: 0.306), and other significant factors including CC and FIGO stages (importance: 0.217 and 0.100, respectively), while retroperitoneal or peritoneal recurrence was not (see Figure 3B). In this study, the main novelty is to use ANN, and it is also proved that the performance of ANN is better than the two most reliable preexisting models (AGO OVAR and MSK criteria). Using AI, the variables used to predict CC and OS can be more deeply understood.
 |
Figure 3 The results of ANN and the importance of various predicting factors for recurrent ovarian cancer. Importance of factors predicting for complete cytoreduction (A). Importance of factors predicting for survival (B). Abbreviations: DFI, disease-free interval; ECOG, Eastern Cooperative Oncology Group; RD, residual disease; CC, complete cytoreduction. Note: Reproduced from Bogani G, Rossetti D, Ditto A, et al. Artificial intelligence weights the importance of factors predicting complete cytoreduction at secondary cytoreductive surgery for recurrent ovarian cancer. J Gynecol Oncol. 2018;29(5):e66. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/.53 |
Recurrence Prediction
Medical Imaging Prediction
Modern medical science has transformed from “traditional medicine” (follows a one-size-fits-all approach) to preventive medicine and precision medicine (also called personalized medicine). This requires a great deal of information to extend existing knowledge of the disease process. AI is considered as the principal tool to realize personalized medicine by synthesizing data on complex oncology. As we know, recurrence is one of the major risk factors for high-grade serous ovarian cancer (HGSOC). In order to predict ovarian cancer recurrence better, Wang et al54 established a novel DL model, which provides a noninvasive method for the recurrence prediction of HGSOC patients. A total of 8917 CT images of 245 HGSOC patients were included in the study. In order to extract DL features (prognostic biomarkers) of HGSOC, they gradually trained a new DL network from the CT images. Subsequently, in order to predict the individual recurrence probability of patients with HGSOC, they established a DL-CPH model that combines DL characteristics and Cox proportional hazards (Cox-PH) regression. The results showed that DL features showed a greater value in prognosis than clinical characteristics. In the primary cohort and two independent validation cohorts, the performance of the new model in predicting recurrence-free survival (RFS) and three-year recurrence was better than that of the existing clinical model (see Table 3). Therefore, the DL-method is considered an effective prognostic biomarker for HGSOC, which extracts data from CT images, and this novel model provides a new prognostic analysis method. This method can provide noninvasive and preoperative models for individualized recurrence prediction of HGSOC. The intrinsic characteristics of HGSOC can be mined through unsupervised learning by this new prognostic analysis method, and it can utilize the large data volume such as CT without the need for follow-on prognostic biomarker extraction.
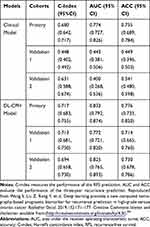 |
Table 3 Model Performance on Predicting Recurrence-free Survival and Three-year Recurrence of High-grade Serous Ovarian Cancer (HGSOC) |
Serological Prediction
In order to evaluate the clinical potential of plasma metabolic profiling before and after surgery in prognosticating EOC recurrence and to obtain a generated model from the combination that accurately predicts EOC recurrence, Zhang et al55 collected 35 pairs of pre- and postoperative plasma samples and recurrence information from 35 EOC patients who were followed-up, Rapid separation liquid chromatography-mass spectrometry was used for metabonomic studies to extract and analyze metabolic characteristics related to EOC recurrence. They also used the SVM model to predict EOC recurrence by using identified predictive biomarkers. The results showed that metabonomic significant changes of plasma before and after operation can be used to distinguish recurrent from nonrecurrent EOC. Ten common significant metabolites, such as uric acid, carnitine, and hydroxyphenyllactic acid, were selected as recurrent predictive biomarkers. The AUC values of biomarkers involved for distinguishing recurrence from the nonrecurrence were as follows: postoperative biomarkers performed better than preoperative biomarker (0.909 vs 0.815), combining the two sets showed the best performance (reached 0.964). By contrasting, the AUC value of the most known tumor marker cancer antigen-125 (CA-125) was only 0.6126 (see Figure 4). As a result, it was believed that the combination of the selected candidate biomarkers performed best and had great potential for predicting recurrent EOC. More importantly, this promising metabonomics approach may provide a new treatment strategy for selecting appropriate cure or even realizing “personalized treatment”.
 |
Figure 4 Receiver operator characteristic (ROC) curves for predicting epithelial ovarian cancer (EOC) recurrence with candidate biomarkers: combining biomarkers (AUC=0.964), preoperative biomarkers (AUC=0.815), postoperative biomarkers (AUC=0.909), CA125 (AUC=0.6126). Abbreviation: AUC, the area under the receiver operating characteristic curve. Note: Reprinted by permission from Springer Nature, Metabolomics, Zhang F, Zhang Y, Ke C, et al. Predicting ovarian cancer recurrence by plasma metabolic profiles before and after surgery. 2018;14(5):65.55 |
Genomic Prediction
In a recent study, Zhou et al56 predicted the recurrence of OC through SVM classifier established by retrieving and identifying target genes. They retrieved and downloaded the expression dataset from the Gene Expression Omnibus by searching keywords and further analyzing whether the set criteria were fulfilled. At the same time, in order to further verify the results of SVM classifier, another set of independent data from ovarian cancer patients were downloaded from the Cancer Genome Atlas (TCGA). The results show that the prediction accuracy of SVM classifier for three downloaded datasets (GSE17260, GSE44104, and GSE51088 datasets) and independent data downloaded from TCGA was 92.7%, 93.3%, 96.6%, and 90.4%, respectively. In addition, the survival time of patients with predicted recurrent OC was significantly shorter than that of patients with predicted nonrecurrent OC (p=6.598×10−6). In this study, an SVM classifier consisting of 39 specific genes, which was more economical and effective than conditional sequencing technology, was constructed and verified to predict OC recurrence. More importantly, the 39 included feature genes may represent therapeutic biomarkers and new therapeutic targets of OC and play a significant role in the development of OC. However, the study also pointed out that in order to further verify the results of this study, further studies on independent cohorts of nonrecurrent and recurrent OC patients are needed in the future.
Survival Rate Prediction
As for cervical carcinoma, the FIGO staging system has long served as the primary tool for evaluating general prognosis and is still the established platform for formulating treatment plans. But the established prognostic factors including histological subtypes, deep interstitial infiltration, etc are not included in this staging system. In order to find a useful way to predict the survival rate in cervical cancer and to facilitate patient consultation on palliative treatment, AI is involved. Obrzut et al.57 collected 102 early stage cervical cancer patients (FIGO Ia2-IIb) who underwent radical operation to evaluate the effectiveness of the AI model in predicting the five-year overall survival rate of these patients, which was the first time that AI predicted the survival rate of such patients. They also collected tumor-related parameters, demographic characteristics, and selected perioperative variables of each patient. Then they simulated computational intelligence methods which included six classifiers: SVM, MLP, k-means method, probabilistic NN (PNN), radial basis function NN (RBFN), and gene expression programming classifier (GEP). And they measured the prediction ability of the model by the sensitivity, accuracy, specificity and the area under the receiver operating characteristics curve (AUC). As a reference model, the results of linear regression analysis were compared with the results of the AI. Finally, it was found that the PNN model obtains the best results, which provided very high prediction ability with high sensitivity (97.5%) and accuracy (89.2%) (see Table 4). The AUC of the PNN receiver was also high, reached 0.818, while SVM algorithm and K-means method could not be used as predictive classifiers, because the AUC value was low (see Figure 5). Therefore, the PNN model is considered a kind of effective tool to predict the overall five-year survival rate in patients with cervical carcinoma treated by radical hysterectomy.
 |
Table 4 The Accuracy, Sensitivity, Specificity, and the Area Under the Receiver Operating Characteristic Curve Obtained for the Set of 23 Variables |
 |
Figure 5 Receiver operator characteristic (ROC) curves for predicting five-year overall survival in cervical cancer patients treated with radical hysterectomy with different models. Abbreviations: PNN, probabilistic neural network; MLP, multilayer perceptron network; GEP, gene expression programming classifier; RBFN, radial basis function neural network; LRA, linear regression analysis. Notes: Reproduced from Obrzut B, Kusy M, Semczuk A, et al. Prediction of 5-year overall survival in cervical cancer patients treated with radical hysterectomyusing computational intelligence methods. BMC Cancer. 2017;17(1):840. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/.57 Plots are shown for the models with AUC >0.5. |
Matsuo et al.58 have proposed a DL model by evaluating certain clinico-laboratory parameters used generally in daily practice. Compared with the historical approach (linear regression model), the experimental model had an obviously better prediction for three-month (AUC 0.747 vs 0.652, p<0.0001) and six-month (AUC 0.724 vs 0.685, p<0.0001) survival rate in patients with cervical cancer. In this model, it was found that the decreased three-month survival rate was associated with eight unique variables (clinical: older age, increasing pain score, decreasing body mass index, and decreasing systolic blood pressure, and laboratory: increasing platelet counts, decreasing albumin level, decreasing white blood cell count, and decreasing hemoglobin levels) (all p<0.05). Similar results were showed in the six-month survival predictors, too. Therefore, it is considered that DL is better than traditional methods in predicting survival rate.
In order to find a noninvasive, reliable, economical, and effective prognostic marker approach to guide precision treatment of ovarian cancer patients, Enshaei et al59 set up a database which contained 668 cases of EOC during a 10-year period. At the same time, they collected the data which was routinely available in a clinical environment for all cases. This database also contained survival data of each patient. Subsequently, they established an ANN algorithm which was able to predict the overall survival rate, because they found that the performance of ANN was better than the Bayesian network and DT in the process of verification. The result showed that the AI model can predict five-year survival with a high accuracy of 93% and AUC of 0.74, and this is better than logical regression. The AI model was also used to predict the result of operation (complete, optimal, or suboptimal cytoreduction), and again proved that AI could predict outcome (complete and optimal group vs suboptimal group) with an accuracy of 77.7% and an AUC of 0.73.
Recently, Lu et al60 found and validated a new mathematical description of tumor phenotype and prognosis. The researchers developed a new software program (Texlab 2.0), which extracted 657 descriptors from the preoperative CT images of 364 EOC patients by radiomic analysis. The biological basis of the new descriptor was further investigated through the research of relevant comprehensive molecular profile which included gene expression, protein expression and copy-number alterations (CNAs). The study found HGSOC had distinctive characteristics, which frequently featured CNAs and usually had worse outcomes. Then, an AI system called “Radiomic Prognostic Vector” (RPV) was designed which could identify patients whose median overall survival time was less than two years and had been verified in two independent multicenter cohorts. It had significantly better prognosis ability than the existing prognostic markers including CA-125 and was considered a potential predictive marker in HGSOC. At the same time, it was also found that high RPV was significantly correlated with primary chemotherapy resistance and poor surgical prognosis. RPV could be utilized to guide personalized therapy of EOC and other cancer types.
AI in Gynecologic Malignant Tumor Diagnosis and Treatment Problems, Possible Solutions and Choices
AI was initially defined as the study of algorithms. These massive and repeated studies give machines the ability to reason and perform cognitive functions, so machines can solve problems, recognize objects, and make decisions.60 AI has increasingly become the topic of many different industries and has unimaginable potential. Meanwhile, AI has achieved comparable performance with that of medical experts in specific medical fields, of course, the field of gynecologic malignant tumors is also among them. The predictive performance and streamlined efficiency pertaining to AI in disease diagnosis—especially in medical imaging tasks—are on a par with or even transcend that of human doctors, and they are endowed with the advantages of being tireless and having stable characteristics.62 Such AI-assisted imaging-related clinical tasks can increase the efficiency of health-care delivery by reducing the cognitive burden of human experts.63 In the latest research mentioned in this review, it is found that the application performance of AI in gynecologic oncology at present mostly exceeds the existing methods and models in prognosis and diagnosis21,27,29,31,53,54,56–60 and it is also superior to the less experienced clinicians,20 or equivalent to the most experienced clinicians.22,23,30 In the comparison of AI itself, it is also found that the performance of the ensemble classifier combining DL and SL is often the best,28,34 DL (ANN, CNN, FFBPNN, and PNN, etc) is often better than SL (CARTs, SVM, and RF, etc),20,21,26,29,57,59 but a small part of them are the same28 or even the opposite.34 This just reflects the advantages of ensemble classifier and the development trend that DL may even replace SL. The reason why DL has the best performance may be that it can automatically learn the features that can reflect the differences of data through a large number of data, which is more representative; while the traditional methods are all based on manual feature extraction, which requires experts in the field to manually design through years of accumulation and experience. But at the same time, the development of DL also faces its own obstacles, such as the small sample size and the curse of dimensionality in machine learning are obstacles for correct evaluation of DL features for radiomic analysis, which needs to be solved in the future development of AI itself. Of course, most of the latest studies mentioned in this review are related to experimental data and need further clinical verification.
Due to the extensive application of AI in medicine, the number of AI papers has exponentially increased over the past five years.64 Despite being a propitious moment for AI, there are still some problems to be solved in the future. So far, AI is still immature, in its start-up step, and is still not a standalone procedure. Clinicians are still required to use AI sufficiently to generate their hypotheses and optimize the applications of AI in clinical practice. When AI and clinicians contradict each other, clinicians still need to interpret the data in a clinically meaningful way. Human physicians must critically evaluate the results produced by AI. At present, it is not clear whether AI can completely transform the current clinician-dominant assessment in clinical procedures. The hybrid system jointly contributed by AI and doctors will lead to more effective diagnostic practice63 and bring about improved health care. However, data interpretation still seems to be a major challenge for AI, and future research may pay more attention to this topic. In view of this relationship between AI and human users, the applicability and clinical significance of advanced AI cannot be completely isolated. The development of AI technology itself may provide an encouraging prospect for medical application, but the evaluation conducted by medical experts plays a vital part in the sustainable development of AI. The feasibility of AI is inevitably determined by human experts through cumulative clinical experience.65 In other words, clinical experts seek the desired predicted patient-related results from AI, which still cannot interpret what it has obtained from the data and its related clinical significance. Therefore, the ultimate success of AI is conditionally limited by medical professionals, who are the real evaluators of diagnostic and therapeutic performance. In medical applications, AI cannot exist without human engagement because it has artificial nature in a human-dominated medical environment, the final results obtained by AI need human beings to give real-world implications. The patient-oriented medical principle stipulates the nature of the need to be patient-centered in the establishment and learning of AI. Every successful AI, no matter what type, needs to ultimately improve the patient’s health. At present, AI cannot reach the same sympathetic characteristics as human doctors, and the use of AI in the medical process also needs to be more “humanized”. Like the application of AI in other medical specialties, the application of AI in gynecologic tumor patients also has many risks and challenges. For example, the new model based on machine learning may be better on average, but it may perform worse in some gynecologic tumor patients. This requires a clear guideline for the certification of the teams that develop and revise the AI systems to protect these patients.
In the process of using AI, some patient information needs to be collected, which inevitably involves the health privacy of gynecologic tumor patients. This requires us to pay enough attention to the use of this advanced technology, that is, to do a good job of screening and pre-judging the data usage rights in the process of data aggregation, and to seek the opinions of the parties in advance, so as to avoid many contradictions. Similarly, AI in medical will inevitably face legal challenges in medical negligence, such as missed diagnosis and misdiagnosis of gynecological tumor detection system, medical negligence caused by complex decision support system, etc. Therefore, when medical malpractice cases occur, the legal system will need to provide clear guidance on which entity is responsible, and insurance companies need to be clear about insurance coverage. In addition, when WFO is used in different countries outside the US, it is found that the system has certain limitations, and the consistency rate is different when the system is used in other countries. This shows that different regions and different ethnic groups have different characteristics. The AI system used in the US is not necessarily suitable for eastern countries. The system needs to be improved according to the local ethnic conditions and actual conditions of other countries.
Similar to the use of WFO, medical personnel should regard AI as “a tool, not a crutch”.66 If AI is properly used and its applications in clinical practice are optimized,67 it will be regarded as a valuable tool. Proper use requires AI to be only in the position of a complement to the doctor’s work, not a replacement.38 AI cannot only be used as a promising tool in gynecologic malignant tumors, but also as a method to resolve several long-term challenges. AI may also be a means to increase knowledge and help clinicians to make decisions in various fields of gynecology.68 It is necessary to recognize the practical value of AI to current clinical practice, but at the same time the foundation of human clinical experience and patient-centered principle should be retained in future AI applications. People often say that AI will change medicine. In fact, we can look forward to how AI can enable people all over the world to obtain the best quality medical services fairly, no matter where or who the patients are.
Funding
This study was funded by the Scientific Research and Technology Development Program of Guangxi (NO. Guike 14124004) and the Natural Science Foundation of Guangxi (NO. GXNSFAA118147).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Denu RA, Hampton JM, Currey A, et al. Influence of patient, physician, and hospital characteristics on the receipt of guideline-concordant care for inflammatory breast cancer. Cancer Epidemiol. 2016;40:7–14. doi:10.1016/j.canep.2015.11.003
2. American Society of Clinical Oncology. The state of cancer care in America, 2016: a report by the American society of clinical oncology. J Oncol Pract. 2016;12(4):339–383. doi:10.1200/JOP.2015.010462.
3. Wang Y, Hajli N. Exploring the path to big data analytics success in healthcare. J Bus Res. 2017;70:287–299. doi:10.1016/j.jbusres.2016.08.002
4. Mehta N, Pandit A. Transforming healthcare with big data analytics and artificial intelligence: a systematic mapping study. J Biomed Inform. 2019;100:103311. doi:10.1016/j.jbi.2019.103311
5. Castaneda C, Nalley K, Mannion C, et al. Clinical decision support systems for improving diagnostic accuracy and achieving precision medicine. J Clin Bioinforma. 2015;5:4. doi:10.1186/s13336-015-0019-3
6. National Academies of Sciences, Engineering, and Medicine. The Fourth Industrial Revolution: Proceedings of a Workshop-In Brief. The National Academies Collection: Reports Funded by National Institutes of Health. Washington (DC); 2017.
7. Musib M, Wang F, Tarselli MA, et al. Artificial intelligence in research. Science. 2017;357(6346):28–30. doi:10.1126/science.357.6346.28
8. Dayarian A, Romero R, Wang Z, et al. Predicting protein phosphorylation from gene expression: top methods from the IMPROVER species translation challenge. Bioinformatics. 2015;31(4):462–470. doi:10.1093/bioinformatics/btu490
9. Hassabis D. Artificial intelligence: chess match of the century. Nature. 2017;544:413–414. doi:10.1038/544413a
10. Silver D, Huang A, Maddison CJ, et al. Mastering the game of go with deep neural networks and tree search. Nature. 2016;529:484–489. doi:10.1038/nature16961
11. Pouliakis A, Karakitsou E, Margari N, et al. Artificial neural networks as decision support tools in cytopathology: past, present, and future. Med Biol Eng Comput. 2016;7:1–18. doi:10.4137/BECB.S31601
12. Parekh VS, Jacobs MA. Deep learning and radiomics in precision medicine. Expert Rev Precis Med. 2019;4(2):59–72. doi:10.1080/23808993.2019.1585805
13. Li X, Guo F, Zhou Z, et al. Performance of deep-learning-based artificial intelligence on detection of pulmonary nodules in chest CT. Zhongguo Fei Ai Za Zhi. 2019;22(6):336–340. doi:10.3779/j.issn.1009-3419.2019.06.02
14. Prayer F, Röhrich S, Pan J, et al. Artificial intelligence in lung imaging. Radiologe. 2020;60(1):42–47. doi:10.1007/s00117-019-00611-2
15. Gong J, Liu J, Hao W, et al. A deep residual learning network for predicting lung adenocarcinoma manifesting as ground-glass nodule on CT images. Eur Radiol. 2020;30(4):1847–1855. doi:10.1007/s00330-019-06533-w
16. Bibault JE, Giraud P, Housset M, et al. Deep learning and radiomics predict complete response after neo-adjuvant chemoradiation for locally advanced rectal cancer. Sci Rep. 2018;8(1):12611. doi:10.1038/s41598-018-30657-6
17. Peng H, Dong D, Fang MJ, et al. Prognostic value of deep learning PET/CT-based radiomics: potential role for future individual induction chemotherapy in advanced nasopharyngeal carcinoma. Clin Cancer Res. 2019;25(14):4271–4279. doi:10.1158/1078-0432.CCR-18-3065
18. Mahmood T, Arsalan M, Owais M, et al. Artificial intelligence-based mitosis detection in breast cancer histopathology images using faster R-CNN and deep CNNs. J Clin Med. 2020;9(3):749. doi:10.3390/jcm9030749
19. Steiner DF, MacDonald R, Liu Y, et al. Impact of deep learning assistance on the histopathologic review of lymph nodes for metastatic breast cancer. Am J Surg Pathol. 2018;42(12):1636–1646. doi:10.1097/PAS.0000000000001151
20. Aramendía-Vidaurreta V, Cabeza R, Villanueva A, et al. Ultrasound image discrimination between benign and malignant adnexal masses based on a neural network approach. Ultrasound Med Biol. 2016;42(3):742–752. doi:10.1016/j.ultrasmedbio.2015.11.014
21. Pergialiotis V, Pouliakis A, Parthenis C, et al. The utility of artificial neural networks and classification and regression trees for the prediction of endometrial cancer in postmenopausal women. Public Health. 2018;164:1–6. doi:10.1016/j.puhe.2018.07.012
22. Ueno Y, Forghani B, Forghani R, et al. Endometrial carcinoma: MR imaging-based texture model for preoperative risk stratification-a preliminary analysis. Radiology. 2017;284(3):748–757. doi:10.1148/radiol.2017161950
23. Dong HC, Dong HK, Yu MH, et al. Using deep learning with convolutional neural network approach to identify the invasion depth of endometrial cancer in myometrium using mr images: a pilot study. Int J Environ Res Public Health. 2020;17(16):5993. doi:10.3390/ijerph17165993
24. Malek M, Gity M, Alidoosti A, et al. A machine learning approach for distinguishing uterine sarcoma from leiomyomas based on perfusion weighted MRI parameters. Eur J Radiol. 2019;110:203–211. doi:10.1016/j.ejrad.2018.11.009
25. Xue Y, Zhao Y, Yao L, et al. Development of diffuse reflectance spectroscopy detection and analysis system for cervical cancer. Zhongguo Yi Liao Qi Xie Za Zhi. 2019;43(3):157–161. doi:10.3969/j.issn.1671-7104.2019.03.001
26. Elayaraja P, Suganthi M. Automatic approach for cervical cancer detection and segmentation using neural network classifier. Asian Pac J Cancer Prev. 2018;19(12):3571–3580. doi:10.31557/APJCP.2018.19.12.3571
27. Jaya BK, Kumar SS. Image registration based cervical cancer detection and segmentation using ANFIS classifier. Asian Pac J Cancer Prev. 2018;19(11):3203–3209. doi:10.31557/APJCP.2018.19.11.3203
28. Bora K, Chowdhury M, Mahanta LB, et al. Automated classification of Pap smear images to detect cervical dysplasia. Comput Methods Programs Biomed. 2017;138:31–47. doi:10.1016/j.cmpb.2016.10.001
29. Zhang L, Lu L, Nogues I, et al. DeepPap: deep convolutional networks for cervical cell classification. IEEE J Biomed Health Inform. 2017;21(6):1633–1643. doi:10.1109/JBHI.2017.2705583
30. Sheikhzadeh F, Ward RK, van Niekerk D, et al. Automatic labeling of molecular biomarkers of immunohistochemistry images using fully convolutional networks. PLoS One. 2018;13(1):e0190783. doi:10.1371/journal.pone.0190783
31. Kyrgiou M, Pouliakis A, Panayiotides JG, et al. Personalised management of women with cervical abnormalities using a clinical decision support scoring system. Gynecol Oncol. 2016;141(1):29–35. doi:10.1016/j.ygyno.2015.12.032
32. Chen Q, Zhang J. Classification and recognition of ovarian cells based on two-dimensional light scattering technology. J Med Syst. 2019;43:127. doi:10.1007/s10916-019-1211-y
33. Bergmeir C, García Silvente M, Benitez JM. Segmentation ofcervical cell nuclei in high-resolution microscopic images:a new algorithm and a web-based software framework. Comput Methods Programs Biomed. 2012;107(3):497–512. doi:10.1016/j.cmpb.2011.09.017
34. Kawakami E, Tabata J, Yanaihara N, et al. Application of artificial intelligence for preoperative diagnostic and prognostic prediction in epithelial ovarian cancer based on blood biomarkers. Clin Cancer Res. 2019;25(10):3006–3015. doi:10.1158/1078-0432.CCR-18-3378
35. Sherin L, Sohail A, Shujaat S. Time-dependent AI-modeling of the anticancer efficacy of synthesized gallic acid analogues. Comput Biol Chem. 2019;79:137–146. doi:10.1016/j.compbiolchem.2019.02.004
36. Li S, Jiang Q, Liu S, et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat Biotechnol. 2018;36(3):258–264. doi:10.1038/nbt.4071
37. Shamsi M, Sedaghatkish A, Dejam M, et al. Magnetically assisted intraperitoneal drug delivery for cancer chemotherapy. Drug Deliv. 2018;25(1):846–861. doi:10.1080/10717544.2018.1455764
38. Printz C. Artificial intelligence platform for oncology could assist in treatment decisions. Cancer. 2017;123(6):905. doi:10.1002/cncr.30655
39. Liang J, Dankwa-Mullan I, Ren Y-P, et al. Employing an oncology decision-support system to quantify treatment variation. J Clin Oncol. 2019;37(15_suppl):e18067. doi:10.1200/JCO.2019.37.15_suppl.e18067
40. Zhou N, Zhang CT, Zhang XC, et al. Concordance study between IBM watson for oncology and clinical practice for patients with cancer in China. Oncologist. 2019;24(6):812–819. doi:10.1634/theoncologist.2018-0255
41. Keiffer MR. Utilization of clinical practice guidelines: barriers and facilitators. Nurs Clin North Am. 2015;50(2):327–345. doi:10.1016/j.cnur.2015.03.007
42. Svenstrup D, Jørgensen HL, Winther O. Rare disease diagnosis: a review of web search, social media and large-scale data-mining approaches. Rare Dis. 2015;3(1):e1083145. doi:10.1080/21675511.2015.1083145
43. Zhou M, Zhao L, Campy KS, et al. Changing of China׳s health policy and doctor-patient relationship: 1949–2016. Health Policy Technol. 2017;6(3):358–367. doi:10.1016/j.hlpt.2017.05.002
44. Chan CS. Mistrust of physicians in China: society, institution, and interaction as root causes. Dev World Bioeth. 2018;18(1):16–25. doi:10.1111/dewb.12162
45. Fang JM, Zhu ZZ, Wang H, et al. The establishment of a new medical model for tumor treatment combined with Watson for oncology, MDT and patient involvement. J Clin Oncol. 2018;36(15_suppl):e18504. doi:10.1200/JCO.2018.36.15_suppl.e18504
46. Lu S, Li L, Luo Y, et al. A multicenter, open-label, randomized Phase II controlled study of rh-endostatin (Endostar) in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer. J Thorac Oncol. 2015;10(1):206–211. doi:10.1097/JTO.0000000000000343
47. Strong VE, Russo A, Yoon SS, et al. Comparison of young patients with gastric cancer in the United States and China. Ann Surg Oncol. 2017;24(13):3964–3971. doi:10.1245/s10434-017-6073-2
48. Liu C, Liu X, Hu C, et al. Using artificial intelligence (Watson for oncology) for treatment recommendations amongst Chinese patients with lung cancer: feasibility study. J Med Internet Res. 2018;20(9):e11087. doi:10.2196/11087
49. Kim EJ, Woo HS, Cho JH, et al. Early experience with Watson for oncology in Korean patients with colorectal cancer. PLoS One. 2019;14(3):e0213640. doi:10.1371/journal.pone.0213640
50. Kumar A, Torres ML, Cliby WA, et al. Inflammatory and nutritional serum markers as predictors of peri-operative morbidity and survival in ovarian cancer. Anticancer Res. 2017;37(7):3673–3677. doi:10.21873/anticanres.11738
51. Colombo N, Lorusso D, Scollo P. Impact of recurrence of ovarian cancer on quality of life and outlook for the future. Int J Gynecol Cancer. 2017;27(6):1134–1140. doi:10.1097/IGC.0000000000001023
52. Cowan RA, Eriksson AG, Jaber SM, et al. A comparative analysis of prediction models for complete gross resection in secondary cytoreductive surgery for ovarian cancer. Gynecol Oncol. 2017;145(2):230–235. doi:10.1016/j.ygyno.2017.02.010
53. Bogani G, Rossetti D, Ditto A, et al. Artificial intelligence weights the importance of factors predicting complete cytoreduction at secondary cytoreductive surgery for recurrent ovarian cancer. J Gynecol Oncol. 2018;29(5):e66. doi:10.3802/jgo.2018.29.e66
54. Wang S, Liu Z, Rong Y, et al. Deep learning provides a new computed tomography-based prognostic biomarker for recurrence prediction in high-grade serous ovarian cancer. Radiother Oncol. 2019;132:171–177. doi:10.1016/j.radonc.2018.10.019
55. Zhang F, Zhang Y, Ke C, et al. Predicting ovarian cancer recurrence by plasma metabolic profiles before and after surgery. Metabolomics. 2018;14(5):65. doi:10.1007/s11306-018-1354-8
56. Zhou J, Li L, Wang L, et al. Establishment of a SVM classifier to predict recurrence of ovarian cancer. Mol Med Rep. 2018;18(4):3589–3598. doi:10.3892/mmr.2018.9362
57. Obrzut B, Kusy M, Semczuk A, et al. Prediction of 5-year overall survival in cervical cancer patients treated with radical hysterectomyusing computational intelligence methods. BMC Cancer. 2017;17(1):840. doi:10.1186/s12885-017-3806-3
58. Matsuo K, Purushotham S, Moeini A, et al. A pilot study in using deep-learning to predict limited life-expectancy in women with recurrent cervical cancer. Am J Obstet Gynecol. 2017;217(6):703–705. doi:10.1016/j.ajog.2017.08.012
59. Enshaei A, Robson CN, Edmondson RJ, et al. Artificial intelligence systems as prognostic and predictive tools in ovarian cancer. Ann Surg Oncol. 2015;22(12):3970–3975. doi:10.1245/s10434-015-4475-6
60. Lu H, Arshad M, Thornton A, et al. A mathematical-descriptor of tumor-mesoscopic- structure from computed-tomography images annotates prognostic- and molecular- phenotypes of epithelial ovarian cancer. Nat Commun. 2019;10(1):764. doi:10.1038/s41467-019-08718-9
61. Hashimoto DA, Rosman G, Rus D, et al. Artificial intelligence in surgery: promises and perils. Ann Surg. 2018;268(1):70–76. doi:10.1097/SLA.0000000000002693
62. Shen J, Zhang CJP, Jiang B, et al. Artificial intelligence versus clinicians in disease diagnosis: systematic review. JMIR Med Inform. 2019;7(3):e10010. doi:10.2196/10010
63. Rajpurkar P, Irvin J, Ball RL, et al. Deep learning for chest radiograph diagnosis: a retrospective comparison of the CheXNeXt algorithm to practicing radiologists. PLoS Med. 2018;15(11):e1002686. doi:10.1371/journal.pmed.1002686
64. Cook TS. The importance of imaging informatics and informaticists in the implementation of AI. Acad Radiol. 2020;27(1):113–116. doi:10.1016/j.acra.2019.10.002
65. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. J Am Med Assoc. 2016;316(22):2402–2410. doi:10.1001/jama.2016.17216
66. Hamilton JG, Genoff Garzon M, Westerman JS. “A tool, not a crutch”: patient perspectives about IBM Watson for oncology trained by memorial sloan kettering. J Oncol Pract. 2019;15(4):e277–e288. doi:10.1200/JOP.18.00417
67. Krittanawong C, Zhang H, Wang Z, et al. Artificial intelligence in precision cardiovascular medicine. J Am Coll Cardiol. 2017;69(21):2657–2664. doi:10.1016/j.jacc.2017.03.571
68. Emin EI, Emin E, Papalois A. Artificial intelligence in obstetrics and gynaecology: is this the way forward? In Vivo (Brooklyn). 2019;33(5):1547–1551. doi:10.21873/invivo.11635
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
