Back to Journals » Drug Design, Development and Therapy » Volume 16
Programmed Intermittent Epidural Bolus in Comparison with Continuous Epidural Infusion for Uterine Contraction Pain Relief After Cesarean Section: A Randomized, Double-Blind Clinical Trial
Authors Mo X, Zhao T, Chen J, Li X, Liu J, Xu C, Song X
Received 26 November 2021
Accepted for publication 23 March 2022
Published 2 April 2022 Volume 2022:16 Pages 999—1009
DOI https://doi.org/10.2147/DDDT.S350418
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Xiaofei Mo,1 Tianyun Zhao,1 Jinghui Chen,1 Xiang Li,1 Jun Liu,2 Cuiyi Xu,1 Xingrong Song1
1Department of Anesthesiology, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, People’s Republic of China; 2Department of Medical Records, The Sixth Affiliated Hospital, Sun Yat-Sen University, Guangzhou, People’s Republic of China
Correspondence: Xingrong Song; Tianyun Zhao, Department of Anesthesiology, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, 9 Jinsui Road, Tianhe District, Guangzhou, 510623, People’s Republic of China, Tel +86 13922416303 ; +86 18198907639, Fax +86 20 38076243, Email [email protected]; [email protected]
Purpose: Programmed intermittent epidural bolus (PIEB) was reported to provide superior maintenance of labour analgesia with better pain relief and less motor block than continuous epidural infusion (CEI). Whether this is also evident for uterine contraction pain relief after cesarean section remains unknown.
Patients and Methods: Parturients scheduled for cesarean section were recruited for the study. At the end of the surgery, after a similar epidural loading dose given, patients received either PIEB (6 mL·h− 1) or CEI (6 mL·h− 1) of 0.1% ropivacaine. The primary outcome was the uterine contraction pain assessed with visual analog scale (VAS-U) at the postoperative 36 h. Secondary outcomes included incision pain at the rest (VAS-R) and in the movement-evoked (VAS-P), and lower extremity motor block (defined as Bromage score > 0). The whole profile of VAS scores between groups was analyzed using linear mixed model. When significant differences were found, the pairwise comparison was done with the Mann Whitney U-test followed by Bonferroni correction.
Results: One hundred and twenty parturients were studied (PIEB, 60; CEI, 60). VAS-U at the postoperative 36 h in the PIEB group was lower than in the CEI group (Bonferroni-adjusted P < 0.01). The linear mixed model indicated that VAS-U, VAS-R and VAS-P were lower in the PIEB group compared with the CEI group (all P < 0.01). Motor block was higher in the CEI group than in the PIEB group during the study period except 2 h (all P < 0.05). No differences of adverse events such as hypotension and urinary retention were observed between the two groups.
Conclusion: Programmed intermittent epidural bolus provides more effective uterine contraction and incision pain relief and less motor block after cesarean section than continuous epidural infusion without an increased risk of urinary retention and blood pressure instability.
Keywords: cesarean section, epidural, pain, postoperative analgesia, uterine contraction
Introduction
Women often experience uterine contraction pain after cesarean section. This type of pain results in extensive neuroendocrine stress responses1,2 and inadequate analgesia increased the risk of breastfeeding failure,3 impaired maternal-infant interaction,3 chronic pain4 and postpartum depression,5 and postoperative morbidities and negated quality of life.6
Multimodal analgesia approach with intrathecal opioids, acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs) greatly improved pain control for the majority of patients after cesarean section.7 However, there remains a proportion of women for whose postoperative pain relief and patient satisfaction are still inadequate,8 and some women are concerned about the potential negative effects of these drugs during breastfeeding.9,10 Compared with systemic administration (such as intravenous, intramuscular and oral) of analgesics, epidural administration of local anesthetics transferred into breast milk was minimal and thus epidural analgesia was considered to be safely used for postpartum analgesia.10,11
Previous meta-analysis12,13 showed that epidural analgesia provided significantly superior analgesia compared with patient-controlled intravenous analgesia and parenteral opioids. Several studies14–16 demonstrated that epidural analgesia was safely and effectively used for post-cesarean section analgesia. Painful impulses caused by uterine contractions are transmitted via visceral afferent nerve fibers which accompany sympathetic nerve fibers and enter into the spinal cord at the T10-L1 spinal segments.17 If most of these pathways can be successfully blocked by epidural analgesia, uterine contractions pain would be alleviated.
Programmed intermittent epidural boluses (PIEB), a newly established technology, provides better analgesia, less motor block and lower local anesthetic drug consumption compared with traditional continuous epidural infusion (CEI), which has being used in labour analgesia.18–21 Therefore, we hypothesized that PIEB may provide superior uterine contraction pain and incision pain relief and less motor block than CEI. Our primary aim was to evaluate the effectiveness of uterine contraction pain relief assessed with visual analog scale (VAS) score at the postoperative 36 h.
Materials and Methods
Study Design and Participants
Patient inclusion criteria were ASA Physical Status 2 or 3, aged 18 to 45 years, term gestation (≥ 37 weeks), singleton pregnancy, parity 0 or 1, scheduled for elective cesarean section under combined spinal-epidural anesthesia, planned on breastfeeding. Exclusion criteria were contraindications to neuraxial anaesthesia, BMI ≥ 40 kg·m−2, severe pregnancy complications, use of sedative or analgesic drugs 2 h before surgery, allergy to drugs used in the study, or known fetal abnormalities.
Ethics
The trial was registered prior to patient enrolment at chictr.org.cn (ChiCTR2000032645). The study protocol was approved by the local ethics committee, the Guangzhou Women and Children’s Medical Centre Ethics Committee, Guangzhou, China on 27 May, 2020 (The reference number 2020-27601). This trial adheres to the Consolidated Standards of Reporting Trials guidelines and complies with Declaration of Helsinki. All participants gave written informed consent prior to study commencement. The trial was carried out from 31 May 2020 to 11 October 2020.
Randomization and Blinding
Consenting parturients were randomly assigned to the PIEB group or the CEI group using a computer-generated randomization list (in a 1:1 ratio). The intensity of uterine contraction pain increased with parity, so to avoid imbalances due to parity, randomization was stratified by parity 0 or 1 in a 1:2 ratio (nulliparous parturients, 40; multiparous parturients, 80). Two sets of random numbers (40 and 80, respectively) were generated by computer and allocation was concealed by sequentially numbered sealed opaque black and white envelopes, respectively. Patients of nulliparous parturients were assigned into the PEIB or CEI group according to random numbers in black envelopes while patients of multiparous parturients were assigned into the PEIB or CEI group according to random numbers in white envelopes. A staff who did not participate in the study organized and kept randomization code until study completion. Patients, anaesthesia providers, obstetricians and researchers were blind to the group assignments.
Anaesthesia, Surgery, and Postoperative Analgesia
After an intravenous catheter was placed, and the patient was transported to the operating room, where standard ASA monitors were placed and a fluid co-load with Lactated Ringer’s was started. With the patient in a left lateral decubitus position, a 25-gauge pencil-point needle was introduced into the subarachnoid space at the L3-L4 or L2-L3 interspace in a standard sterile fashion. After return of clear cerebrospinal fluid, 12–15 mg of 0.5% ropivacaine which was diluted by cerebrospinal fluid was administered. Then a single-orifice epidural catheter was placed 4 cm into the epidural space and a 3 mL epidural test dose of 1% lidocaine was administered. After immediately positioned supine with a 15° left lateral tilt, pinprick test was done to ensure that sensory block was above T6. If sensory block was not achieved to the T6 level, or the patient experienced intraoperative pain, 2% lidocaine was administered into the epidural space to achieve adequate anaesthesia. All subjects received a prophylactic intravenous phenylephrine infusion initiated at 0.5 μg·kg−1·min−1 at the time of spinal injection.
After delivery of the baby, oxytocin was administered as per hospital protocol and each patient received 12.5 mg dolasetron intraoperatively for vomiting prophylaxis. At the end of the surgery, the same solution of loading dose contained 0.125% ropivacaine 8 mL with hydromorphone 0.6 mg and naloxone 0.04 mg was administered in two groups via the epidural catheter and group assignments were opened by an anesthesia research nurse. Epidural analgesia was maintained with a solution of 0.1% ropivacaine alone. In the PIEB group, the epidural pump (Apon MC ZZB—IV; Jiangsu Apon Medical Technology, Jiangsu, China) was programmed to deliver a 6-mL bolus at a rate of 360 mL·h−1 every hour beginning 30 minutes after administration of the loading dose. In the CEI group, the pump (Apon ZZB—I; Jiangsu Apon Medical Technology, Jiangsu, China) was programmed to deliver at a constant rate of 6 mL·h−1 immediately after the loading dose. Both pump settings were set with a patient-controlled epidural analgesia (PCEA) of 6 mL with a lockout interval of 15 min and a maximum hourly volume of 24 mL. The pumps were programmed by an anesthesiologist not involved in data collection and inserted into an opaque, portable bag to maintain blinding. Before leaving the operating room, patients were instructed to use PCEA with a standardized instruction whenever she felt uncomfortable. If the parturient still felt pain after activating the PCEA bolus twice in a 30-min period, rescue analgesia with 100 mg rectal diclofenac potassium suppository was allowed as a rescue option.
Peri-operative monitoring consisted of hourly respiratory rate, pulse rate and non-invasive blood pressure measurements for 6 h and thereafter at intervals of 8 h by nursing staff. In the obstetrical ward, 10 units oxytocin was intravenously administered for three times at 12-hour intervals after surgery. All other treatments were followed a routine clinical practice including in case of hypotension (defined as systolic blood pressure 20% less than the baseline value or less than 90 mmHg), it was treated with intravenous phenylephrine (20–40 µg) as necessary. Baseline blood pressure was defined as the usual blood pressure at home. The definition of urinary retention was, lack of spontaneous micturition 6 h after the removal of catheter after cesarean section, requiring catheterization.22
Pain Evaluation
All data were collected by research team members blinded to the groups at the postoperative 2, 6, 12, 24 and 36 h. The VAS score was used to assess uterine contractile pain and incision pain after cesarean section as reported previously.2 It is presented as a 100-mm horizontal line (0, no pain; 100 mm, worst imaginable pain). Breastfeeding and oxytocin administration stimulates the uterine contraction and increases the severity of the pains.23,24 Therefore, the highest VAS score of uterine contractions pain evoked with breastfeeding or oxytocin administration experienced over the designated time interval was defined as the VAS-U. The incision pain at the rest (VAS-R) and in the movement-evoked mainly by changing position in bed (VAS-P) was also evaluated. Uterine contraction pain was defined as an intermittent, indefinite location, short-lasting, while the incision pain was defined as a constant, definite location and evoked abdominal pain over the wound and adjacent region. At the postoperative 36 h, the parturient was also asked to report an overall postpartum experience satisfaction using VAS score (0, completely dissatisfied; 100 mm, completely satisfied).
Outcomes
Primary Outcome
The primary outcome was the VAS-U at the postoperative 36 h.
Secondary outcomes
The key secondary outcomes included VAS-R and VAS-P during the study period, and VAS-U at 2, 6, 12, 24 h, and lower extremity motor block (defined as Bromage score > 0). The degree of motor block was assessed in both lower extremity using a modified Bromage score [0 = no motor paralysis; 1 = unable to raise the extended leg, but able to move knee and foot; 2 = unable to raise the extended leg as well as flex knees, able to move foot; 3 = not able to flex ankle, foot or knee (complete block)].25 If the Bromage score of both lower limbs was inconsistent, the higher score was taken. Other secondary outcomes included the PCEA boluses, time to first PCEA bolus, proportion of patients requiring diclofenac potassium suppository rescue. The presence of vomiting, pruritus, hypotension, or urinary retention was recorded throughout the study period as yes or no.
Sample Size Calculation
The sample size estimation was based on the primary outcome. Data from a pilot study including 20 patients suggested that the mean ± standard deviation (SD) VAS-U score at the postoperative 36 h was 30.3 ± 12.8 mm in the PIEB group and 40.2 ± 16.3 mm in the CEI group. A sample size of 48 subjects per group had 90% power at α = 0.05 in a 2-sided 2-sample t-test to identify this difference calculated using PASS software (version 11. NCSS, LLC. Kaysville, Utah, USA). To take dropouts into account, 120 subjects was planned to be recruited.
Statistical Analysis
Statistical analysis were performed using SPSS (version 25.0, IBM Corp., Armonk, NY, USA). Data were expressed as the mean ± SD, the median (interquartile range, IQR), or the number (proportion) as appropriate. For the normality test, the Kolmogorov–Smirnov test was performed. The differences between groups were compared using the Student’s t-test (normally distributed data) or Mann Whitney U-test (skewed data). Categorical and proportions were analyzed using the Pearson Chi-square test or Fisher exact test as appropriate. The whole profile of VAS scores were assessed between groups with linear mixed model. The models consisted of main effects for treatment group and time. The test was followed the Mann Whitney U-test with Bonferroni adjustment for multiple pairwise comparisons (5 comparisons) if a significant intergroup difference was found. A Bonferroni-adjusted P value <0.01 (0.05/5) was considered statistically significant. Time to first PCEA bolus was analyzed by using the Kaplan Meier curves with a Log rank test. An intention-to-treat analysis was performed with a P value < 0.05 considered to be of statistically significant.
Results
Participants
A total of 133 patients were assessed for eligibility. After exclusion, 120 participants were included and randomized to either the PIEB group or the CEI group (Figure 1). Baseline characteristics are presented in Table 1.
 |
Table 1 Patient Characteristics |
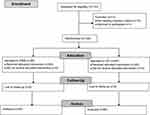 |
Figure 1 Flowchart of the study. Abbreviations: CEI, continuous epidural infusion; PIEB, programmed intermittent epidural bolus. |
Pain Scores
The linear mixed model showed a significant main effect of group and a significant effect of time and a significant group-by-time interaction in VAS-U, VAS-R and VAS-P. Three types of pain VAS scores were all significantly lower in the PIEB group compared with the CEI group (For VAS-U, P = 0.001; For VAS-R, P < 0.001; For VAS-P, P = 0.008). Three types of pain levels were increased over time in both groups (all P < 0.001) (Figure 2). The group-by-time interaction was significant (all P < 0.05), indicating that the PIEB group had significantly lower three types of pain VAS scores over time than the CEI group. The primary outcome, VAS-U at the postoperative 36 h, was significantly lower in the PIEB group [median (IQR), 30 (20 to 40) mm] compared with the CEI group [40 (30 to 50) mm], with an estimated difference of −10 mm (95% CI −15 to −5 mm; Bonferroni-adjusted P < 0.01 after Mann Whitney U-test) (Figure 2). The VAS-R scores at the postoperative 12 and 36 h were significantly lower in the PIEB group than in the CEI group (Bonferroni-adjusted P < 0.01) (Figure 2). The VAS-P scores at the postoperative 36 h were significantly lower in the PIEB group than in the CEI group (Bonferroni-adjusted P < 0.01) (Figure 2).
Motor Block
Motor block occurred more frequent in the CEI group compared with the PIEB group at the postoperative 6 h (40% vs 16.7%; P = 0.005), 12 h (25% vs 3.3%; P = 0.001), 24 h (15% vs 1.7%; P = 0.008) and 36 h (10% vs 0%; P = 0.003) (Figure 3).
PCEA Administrations, Rescue Analgesia Usages and Patient Satisfaction
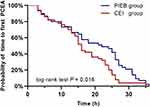
The number of PCEA boluses was less in the PIEB group than that of the CEI group [median (IQR), 1 (0 to 5) vs 3 (1 to 10); P = 0.02]. The PIEB group had a longer median time to first PCEA (22 h; 95% CI, 10–28 h vs 16 h; 95% CI, 11–24 h; P = 0.016; Figure 4) compared with the CEI group. The percentage of patients who received diclofenac potassium suppository rescue was 27.8% in the PIEB group and 30.0% in the CEI group (P = 0.800). Maternal satisfaction with overall postpartum experience was greater in the PIEB group than in the CEI group [median (IQR), 88 (80 to 90) vs 80 (70 to 90); P = 0.004].
Side Effects
Complications are shown in Table 2. The incidence of vomiting, pruritus, hypotension, or urinary retention did not differ between the two groups.
 |
Table 2 Complications: Vomiting, Pruritus, Hypotension and Urinary Retention |
Discussion
This randomized, double-blind clinical trial demonstrated that PIEB provided more effective uterine contraction and incision pain relief in patients after cesarean section compared with CEI. In addition, PIEB was associated with reduced incidence of motor block, less PCEA boluses, longer time to first PCEA bolus, and higher patient satisfaction without an increased risk of hypotension and urinary retention in patients after cesarean section when compared with CEI.
In recent years, PIEB becomes popular with the anesthesiologist and parturients for vaginal birth pain relief because PIEB has demonstrated superiority to CEI for providing better pain relief, reduced motor block, and improved patient satisfaction,21,25–29 which is consistent with our findings that PIEB provided more effective post-cesarean uterine contraction pain relief and less motor block. Recently, PIEB was reported to be used for incision pain management in surgical patients in previous studies.30–33 We also found that PIEB provided more effective post-cesarean incision pain relief. These findings were similar to the findings reported by previously.33 In contrast, Su et al30 found that the average pain severity after major abdominal surgery was not different between PIEB and CEI groups. This was likely due to that all subjects received a postoperative multimodal analgesic regimen, which might mask the advantages of PIEB.
Our study demonstrated that the peak pain level of incision pain evoked by movement and at the rest, and uterine contraction pain were at 36 h after cesarean section in the both groups, which is consistent with the findings reported previously.2 The reasons may be as follows: First, the sensory blockade of ropivacaine for spinal anaesthesia lasted approximately 200 min,34 and a single-dose epidural hydromorphone also extended its effects for up to up to 24 h.35–37 These results may suggest that postoperative analgesia regimen should be applied to patients during the whole hospitalization, especially the second day after cesarean section.
An in vitro study demonstrated that intermittent bolus of the epidural catheter has a wider spread of methylene blue dye solution compared with continuous infusion.38 In a porcine model, the mean longitudinal extent spread of 1 mL dye administration was 8.9 ± 2.6 cm in the continuous infusion group compared with 15.2 ± 2.7 cm in the bolus group.39 In another porcine model study, the spread of epidural radiopaque contrast dye was 5.6 vertebral levels in the continuous infusion group compared with 10.4 in the bolus group.40 Furthermore, a clinical study comparing epidural bolus and CEI administration after abdominal surgery showed that the median number of blocked spinal segments was 19.5 in the epidural bolus group and 11.5 in the CEI group, indicating that epidural bolus administration provided greater longitudinal extension of sensory blockade than CEI.41 Therefore, it is very likely that PIEB providing more effective uterine contraction pain and incision pain relief after cesarean section than CEI was due to that the PIEB blocked the sensory-nerve fibers more extensively.
A randomized study of labour analgesia showed that the incidence of motor block was 2.7% in parturients administered PIEB and 37% in those received CEI,29 which is comparable to these of our study at 12 h (3.3% in the PIEB group vs 25% in the CEI group). Two meta-analysis19,21 of labour analgesia also demonstrated that PIEB produced less motor block compared with CEI. Analgesia and motor block are produced by the movement of local anaesthetic from the extraneural space into the intraneural space along a diffusion gradient. If low concentrations of local anaesthetic are given in CEI, the concentration of local anaesthetic in the extraneural is persistently higher than in the intraneural space, which increases the concentration in the nerve and reaches the threshold for motor fiber block.42 However, in the case of PIEB, blockade of motor fibers is less because the total amount of local anaesthetic in the nerve is insufficient.42 The advantage of PIEB in reducing motor block suggests that it allows early mobilization to enable these women to care for their newborn and facilitates early discharge from hospital. The overall satisfaction of mothers was higher in PIEB group than in the CEI group, which may be due to the lower pain score and less motor block in the PIEB group.
While improving the analgesic effect, one may be concerned about the increased risk of hypotension and urinary retention. Our study found no hypotension requiring treatment in both groups, and urinary retention did not differ between groups. However, Wei et al32 compared continuous infusion with intermittent bolus for postoperative analgesia in patients after thoracic surgery, reported the incidence of hypotension was 18.5% in the intermittent group and 53.6% in the continuous group. Two possible explanations for the discrepancy between our study and Wei’s study are that the epidural catheter was placed in T7-T8 interspace in their study, which may lead to cardiac sympathetic block, and they used a higher local anaesthetic concentration (0.3% ropivacaine) than our study.
Our study also has limitations. First, although the study was designed to be double-blinded, it is possible that patients and investigators might have been aware of grouping by the different sounds of pump injection. Second, although continuous postpartum epidural analgesia is not a common analgesic model clinically, this study may provide a reference for PIEB as the part of a multimodal analgesia regimen. In addition, if PIEB combined with NSAIDS and opioids, it may reduce the dosage and side effects of each other.
Conclusion
In conclusion, we found that the maintenance of epidural analgesia with PIEB provided better uterine contraction and incision pain relief, less motor block, fewer PCEA boluses, longer time to first PCEA bolus, and higher patient satisfaction without an increased risk of hypotension and urinary retention in patients after cesarean section when compared with CEI.
Data Sharing Statement
The data collected for this study can be shared with researchers in de-identified form after the publication date, and in the presence of a data transfer agreement, and if it complies with China legislation. Requests for data and study proposal should be directed to [email protected], including a proposal that must be approved by the trial’s steering committee.
Acknowledgments
Assistance with the study: we would like to thank Chongyang Duan, PhD of Department of Biostatistics, School of Public Health, Southern Medical University, China, for his statistical analysis guidance during this project and Prof Daqing Ma, Imperial College London, UK, for his critical comments during manuscript preparation.
Funding
This work was supported by the National Science Foundation of China (grant number 81870823), Guangzhou Institute of Pediatrics/Guangzhou Women and Children’s Medical Center funds (GCP-2018-001), and Program of Guangzhou Municipal science and technology Bureau (201803010025).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hsu HW, Cheng YJ, Chen LK, et al. Differential analgesic effect of tenoxicam on the wound pain and uterine cramping pain after cesarean section. Clin J Pain. 2003;19(1):55–58. doi:10.1097/00002508-200301000-00007
2. Angle PJ, Halpern SH, Leighton BL, et al. A randomized controlled trial examining the effect of naproxen on analgesia during the second day after cesarean delivery. Anesth Analg. 2002;95(3):741–745. doi:10.1213/00000539-200209000-00038
3. Gadsden J, Hart S, Santos AC. Post-cesarean delivery analgesia. Anesth Analg. 2005;101(5 Suppl):S62–S69. doi:10.1213/01.ANE.0000177100.08599.C8
4. Komatsu R, Ando K, Flood PD. Factors associated with persistent pain after childbirth: a narrative review. Br J Anaesth. 2020;124(3):e117–e130. doi:10.1016/j.bja.2019.12.037
5. Eisenach JC, Pan P, Smiley RM, et al. Resolution of pain after childbirth. Anesthesiology. 2013;118(1):143–151. doi:10.1097/ALN.0b013e318278ccfd
6. Carli F, Mayo N, Klubien K, et al. Epidural analgesia enhances functional exercise capacity and health-related quality of life after colonic surgery: results of a randomized trial. Anesthesiology. 2002;97(3):540–549. doi:10.1097/00000542-200209000-00005
7. Macones GA, Caughey AB, Wood SL, et al. Guidelines for postoperative care in cesarean delivery: Enhanced Recovery After Surgery (ERAS) society recommendations (part 3). Am J Obstet Gynecol. 2019;221(3):241–247. doi:10.1016/j.ajog.2019.04.012
8. Pan PH. Post cesarean delivery pain management: multimodal approach. Int J Obstet Anesth. 2006;15(3):185–188. doi:10.1016/j.ijoa.2006.04.004
9. Dalal PG, Bosak J, Berlin C. Safety of the breast-feeding infant after maternal anesthesia. Paediatr Anaesth. 2014;24(4):359–371. doi:10.1111/pan.12331
10. Cobb B, Liu R, Valentine E, Onuoha O. Breastfeeding after anesthesia: a review for anesthesia providers regarding the transfer of medications into breast milk. Transl Perioper Pain Med. 2015;1(2):1–7.
11. Hirose M, Hara Y, Hosokawa T, Tanaka Y. The effect of postoperative analgesia with continuous epidural bupivacaine after cesarean section on the amount of breast feeding and infant weight gain. Anesth Analg. 1996;82(6):1166–1169. doi:10.1097/00000539-199606000-00011
12. Wu CL, Cohen SR, Richman JM, et al. Efficacy of postoperative patient-controlled and continuous infusion epidural analgesia versus intravenous patient-controlled analgesia with opioids: a meta-analysis. Anesthesiology. 2005;103(5):
13. Block BM, Liu SS, Rowlingson AJ, et al. Efficacy of postoperative epidural analgesia: a meta-analysis. JAMA. 2003;290(18):2455–2463. doi:10.1001/jama.290.18.2455
14. Miao F, Feng K, Feng X, et al. The analgesic effect of different concentrations of epidural ropivacaine alone or combined with sufentanil in patients after cesarean section. Front Pharmacol. 2021;12:631897. doi:10.3389/fphar.2021.631897
15. Fonseca R, Goncalves D, Bento S, Valente E. Postoperative epidural analgesia in cesarean section: comparison of therapeutic schemes. Cureus. 2020;12(12):e12166. doi:10.7759/cureus.12166
16. Sun J, Wu X, Xu X, et al. A comparison of epidural magnesium and/or morphine with bupivacaine for postoperative analgesia after cesarean section. Int J Obstet Anesth. 2012;21(4):310–316. doi:10.1016/j.ijoa.2012.05.006
17. Eltzschig HK, Lieberman ES, Camann WR. Regional anesthesia and analgesia for labor and delivery. New Engl J Med. 2003;348(4):319–332. doi:10.1056/NEJMra021276
18. Liu X, Zhang H, Zhang H, et al. Intermittent epidural bolus versus continuous epidural infusions for labor analgesia: a meta-analysis of randomized controlled trials. PLoS One. 2020;15(6):e234353.
19. Hussain N, Lagnese CM, Hayes B, et al. Comparative analgesic efficacy and safety of intermittent local anaesthetic epidural bolus for labour: a systematic review and meta-analysis. Br J Anaesth. 2020;125(4):560–579. doi:10.1016/j.bja.2020.05.060
20. Heesen M, Bohmer J, Klohr S, et al. The effect of adding a background infusion to patient-controlled epidural labor analgesia on labor, maternal, and neonatal outcomes: a systematic review and meta-analysis. Anesth Analg. 2015;121(1):149–158. doi:10.1213/ANE.0000000000000743
21. Xu J, Zhou J, Xiao H, et al. A systematic review and meta-analysis comparing programmed intermittent bolus and continuous infusion as the background infusion for parturient-controlled epidural analgesia. Sci Rep. 2019;9(1):2583. doi:10.1038/s41598-019-39248-5
22. Yip SK, Sahota D, Pang MW, Chang A. Postpartum urinary retention. Acta Obstet Gynecol Scand. 2004;83(10):881–891. doi:10.1111/j.0001-6349.2004.00460.x
23. Holdcroft A, Snidvongs S, Cason A, Dore CJ, Berkley KJ. Pain and uterine contractions during breast feeding in the immediate post-partum period increase with parity. Pain. 2003;104(3):589–596. doi:10.1016/S0304-3959(03)00116-7
24. Wen L, Hilton G, Carvalho B. The impact of breastfeeding on postpartum pain after vaginal and cesarean delivery. J Clin Anesth. 2015;27(1):33–38. doi:10.1016/j.jclinane.2014.06.010
25. Wong CA, Ratliff JT, Sullivan JT, et al. A randomized comparison of programmed intermittent epidural bolus with continuous epidural infusion for labor analgesia. Anesth Analg. 2006;102(3):904–909. doi:10.1213/01.ane.0000197778.57615.1a
26. Ojo OA, Mehdiratta JE, Gamez BH, Hunting J, Habib AS. Comparison of programmed intermittent epidural boluses with continuous epidural infusion for the maintenance of labor analgesia: a randomized, controlled, double-blind study. Anesth Analg. 2020;130(2):426–435. doi:10.1213/ANE.0000000000004104
27. Sng BL, Zeng Y, de Souza N, et al. Automated mandatory bolus versus basal infusion for maintenance of epidural analgesia in labour. Cochrane Database Syst Rev. 2018;5:D11344.
28. Carvalho B, George RB, Cobb B, McKenzie C, Riley ET. Implementation of programmed intermittent epidural bolus for the maintenance of labor analgesia. Anesth Analg. 2016;123(4):965–971. doi:10.1213/ANE.0000000000001407
29. Capogna G, Camorcia M, Stirparo S, Farcomeni A. Programmed intermittent epidural bolus versus continuous epidural infusion for labor analgesia: the effects on maternal motor function and labor outcome. A randomized double-blind study in nulliparous women. Anesth Analg. 2011;113(4):826–831. doi:10.1213/ANE.0b013e31822827b8
30. Su PP, Peniche A, Clelland E, et al. Comparison of programmed intermittent epidural bolus and continuous epidural infusion for post-operative analgesia after major abdominal surgery: a randomized controlled trial. J Clin Anesth. 2020;64:109850. doi:10.1016/j.jclinane.2020.109850
31. Higashi M, Shigematsu K, Nakamori E, Sakurai S, Yamaura K. Efficacy of programmed intermittent bolus epidural analgesia in thoracic surgery: a randomized controlled trial. BMC Anesthesiol. 2019;19(1):107. doi:10.1186/s12871-019-0780-0
32. Wei K, Min S, Hao Y, Ran W, Lv F. Postoperative analgesia after combined thoracoscopic-laparoscopic esophagectomy: a randomized comparison of continuous infusion and intermittent bolus thoracic epidural regimens. J Pain Res. 2019;12:29–37. doi:10.2147/JPR.S188568
33. Satomi S, Kakuta N, Murakami C, et al. The efficacy of programmed intermittent epidural bolus for postoperative analgesia after open gynecological surgery: a randomized double-blinded study. Biomed Res Int. 2018;2018:6297247. doi:10.1155/2018/6297247
34. Malinovsky J-M, Charles F, Kick O, et al. Intrathecal anesthesia: ropivacaine versus bupivacaine. Anesth Analg. 2000;91(6):1457–1460. doi:10.1097/00000539-200012000-00030
35. Henderson SK, Matthew EB, Cohen H, Avram MJ. Epidural hydromorphone: a double-blind comparison with intramuscular hydromorphone for postcesarean section analgesia. Anesthesiology. 1987;66(6):825–830. doi:10.1097/00000542-198706000-00021
36. Marroquin B, Feng C, Balofsky A, et al. Neuraxial opioids for post-cesarean delivery analgesia: can hydromorphone replace morphine? A retrospective study. Int J Obstet Anesth. 2017;30:16–22. doi:10.1016/j.ijoa.2016.12.008
37. Yang M, Wang L, Chen H, Tang Y, Chen X. Postoperative analgesic effects of different doses of epidural hydromorphone coadministered with ropivacaine after cesarean section: a randomized controlled trial. Pain Res Manag. 2019;2019:9054538. doi:10.1155/2019/9054538
38. Kaynar AM, Shankar KB. Epidural infusion: continuous or bolus? Anesth Analg. 1999;89(2):534. doi:10.1097/00000539-199908000-00063
39. Mowat I, Tang R, Vaghadia H, et al. Epidural distribution of dye administered via an epidural catheter in a porcine model. Br J Anaesth. 2016;116(2):277–281. doi:10.1093/bja/aev432
40. Cole J, Hughey S. Bolus epidural infusion improves spread compared with continuous infusion in a cadaveric porcine spine model. Reg Anesth Pain Med. 2019;44:1080–1083.
41. Ueda K, Ueda W, Manabe M. A comparative study of sequential epidural bolus technique and continuous epidural infusion. Anesthesiology. 2005;103(1):126–129. doi:10.1097/00000542-200507000-00019
42. Capogna G, Stirparo S. Techniques for the maintenance of epidural labor analgesia. Curr Opin Anaesthesiol. 2013;26(3):261–267. doi:10.1097/ACO.0b013e328360b069
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.