Back to Journals » Cancer Management and Research » Volume 10
Prognostic value of serum alkaline phosphatase in the survival of prostate cancer: evidence from a meta-analysis
Authors Li D , Lv H, Hao X, Hu B, Song Y
Received 15 May 2018
Accepted for publication 22 June 2018
Published 30 August 2018 Volume 2018:10 Pages 3125—3139
DOI https://doi.org/10.2147/CMAR.S174237
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Dongyang Li,1,* Hang Lv,2,* Xuanyu Hao,3 Bin Hu,2 Yongsheng Song1
1Department of Urology, Shengjing Hospital of China Medical University, Shenyang, Liaoning 110004, P.R. China; 2Department of Urology, Liaoning Cancer Hospital & Institute, Cancer Hospital of China Medical University, Shenyang, Liaoning 110042, P.R. China; 3Department of Rheumatology and Immunology, Shengjing Hospital of China Medical University, Shenyang, Liaoning 110022, P.R. China
*These authors contributed equally to this work
Background: Many studies have evaluated the relationship between alkaline phosphatase (ALP) and the prognosis for prostate cancer (PCa). But they have not reached a widespread consensus yet. Therefore, we completed a meta-analysis to ascertain the significance of ALP and the prognosis for PCa.
Methods: A literature search was performed in the PubMed, Embase, and Web of Science databases. HRs concerning overall survival (OS), progression-free survival (PFS), and cancer-specific survival (CSS) were extracted to evaluate the impacts of ALP on the prognosis for PCa. Subgroup analyses were conducted on different study types, regions, sample sizes, and cutoff values. Sensitivity analysis was performed by removing one study in sequence.
Results: A total of 63 studies from 54 articles with 16,135 patients were included in this meta-analysis. The pooled results indicated that high baseline ALP was associated with obviously poor OS (HR=1.74, 95% CI: 1.47–2.06) and PFS (HR=1.60, 95% CI: 1.13–2.26) in patients with PCa. The pooled HR for bone-specific ALP and OS was 1.76 (95% CI: 1.45–2.15). However, no association between ALP and CSS (HR=1.002, 95% CI: 0.998–1.005) was found for PCa. The results of subgroup analyses were all in accordance with the main findings. Sensitivity analysis suggested that no single study could affect the stability of the results.
Conclusion: High serum ALP is significantly associated with poor OS and PFS except for CSS in PCa. ALP is an efficient and convenient biomarker for PCa prognosis.
Keywords: prostate cancer, alkaline phosphatase, prognosis, survival, meta-analysis
Introduction
Prostate cancer (PCa) is the most common malignancy in western males.1 It is estimated that 164,690 new PCa cases and 29,430 PCa-related deaths will occur in 2018 in USA.1 So far, prostate-specific antigen (PSA) has been mostly used for early detection and recurrence evaluation as a biomarker. Gleason score is a classical prognostic factor but not sufficient to portray the complexity of clinical prognosis.2 The heterogeneous genomic property of PCa can lead to the difficulty in survival prognosis and therapy monitoring. Therefore, there is an urgent need for novel effective parameters to predict outcomes for treatment decision. Recently, a number of biomarkers about PCa have been investigated and established in patient cohort studies.3–6 In comparison with cancer tissues, serum is an ideal source of biomarkers because of the convenience in routine clinical measurement.7 Scientists have been trying for decades to seek the biomarkers among the different kinds of molecules such as proteins, noncoding RNAs, and chemical compounds.8 Interestingly, we notice that alkaline phosphatase (ALP), a classical parameter, also has a great potential in the prognosis of PCa.
The enzyme ALP can physiologically dephosphorylate compounds under alkaline pH environment.9 Serum ALP level is a widely used parameter for liver disease, bone disease burden, and treatment effects.10 It is acknowledged that the elevation in ALP level is positively related to the rise of bone activity like osteosarcoma.11 Therefore, we speculate that bone metastatic cancer may also lead to the rising of serum ALP, given that bone is the most common metastatic site of PCa. Over 85% patients died from bone metastasis among PCa-related deaths.12 So, can we identify the relationship between ALP and different survival outcomes in patients with PCa?
Up to now, the prognostic performance of ALP in patients with PCa has been discussed in many studies; however, these studies have yielded some conflicting conclusions. The aim of this study was to quantitatively and comprehensively derive a more precise prognostic estimation of ALP in patients with PCa by a meta-analysis.
Methods
Search strategy
This meta-analysis adhered to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).13 A comprehensive literature search in the PubMed, Embase, and Web of Science was conducted from the databases onset to February 5, 2018. The key words were as follows: (“prostate neoplasms[MeSH]” OR “prostate cancer”) AND “alkaline phosphatase” or “ALP” AND (“prognosis[MeSH]” OR “survival” OR “outcome”). The language of studies was not restricted. Additional relevant publications were also manually searched based on the reference lists.
Study selection
Inclusion and exclusion criteria
Studies were included only if they met the following criteria: 1) clinical cohort/trial evaluated the prognostic ability of ALP in PCa; 2) studies compared ALP with other prognostic models and reported survival outcomes such as overall survival (OS), progression-free survival (PFS), and cancer-specific survival (CSS); 3) reported original HR with 95% CI or the HR could be calculated from sufficient data; 4) articles with the most complete information if there were several studies among overlapping cohorts or time periods.
The exclusion criteria were 1) duplicate publications; 2) studies based on less than 20 patients; 3) laboratory studies, animal studies, letters, or review articles.
Assessment of study quality
Two investigators (DL and XH) independently reviewed all the relevant articles, then evaluated the methodological quality of observational studies using Newcastle–Ottawa Quality Assessment Scale (NOS) assessment tool, including selection, comparability, and outcomes.14 The Jadad composite scale was utilized to assess randomized controlled trials (RCTs).15 The NOS score ≥7 or Jadad score ≥4 indicated high quality. Disagreements in data collection and quality assessment were resolved through consensus by involving a third author (HL).
Data extraction
The baseline and outcome data were obtained from each study: first author’s surname, year of publication, study design, country, sample size, age, PSA level, cutoff value, follow-up time, outcomes, and HRs with 95% CI. If the HRs of both univariate and multivariate analysis were available, only the latter was used.
Statistical analysis
HRs with 95% CI from all eligible studies were pooled via a meta-analysis to access the strength of ALP to survival endpoints. The Cochran Q test was used to determine the heterogeneity among studies. A P value <0.10 indicated heterogeneity. The inconsistency (I2) was also calculated to evaluate heterogeneity. An I2 value >50% indicated the presence of statistical heterogeneity. The random-effect model (DerSimonian and Laird method) was used to calculate pooled results when there was heterogeneity among included studies; otherwise, the fixed-effect model was used. To seek deeper relationship between ALP and OS, we conducted subgroup analyses on study type, cutoff value, sample size, and region of study. Furthermore, to test the reliability of the results, sensitivity analysis was conducted by removing each single study in turn. Begg’s test with funnel plots was used to measure publication bias. The P value >0.05 indicated no potential publication bias. The Stata 12.0 software (StataCorp, College Station, TX, USA) was used to perform all the statistical analyses. A two-sided P value <0.05 was considered as statistically significant.
Results
Studies selection and evaluation
The flowchart of articles searching process is shown in Figure 1. A total of 1,107 relevant citations were initially retrieved by the search strategy as described above in PubMed, Embase, and Web of Science. Seven hundred forty duplicate articles were removed. Among the remaining 367 articles, 286 were further excluded for unrelated information and not clinical research articles. Eighty-one potential articles were screened carefully, 27 articles were ruled out because of lack of essential data of survival outcome or overlapping cohorts. If there were multiple outcomes in the same article, we considered them as different studies. Finally, 63 studies from 54 articles16–69 published between 1995 and 2017 encompassing 16,135 patients were included in the meta-analysis, with the sample size ranging from 30 to 1,183 patients (Table 1). The characteristics of the included studies are summarized in Table 1. The median length of follow-up varied from 8.3 to 63.4 months. Prognostic outcomes were quantitatively synthesized, including OS, CSS, and PFS. A total of 36 observational studies and five RCTs had available data for the OS analysis, while seven studies reported HRs for CSS, and nine studies reported HRs for PFS. The quality assessment results of the 54 eligible articles shown in Table S1 revealing the NOS score were equal or greater than 6 in all 48 observational studies and the Jadad score was over 4 in all six RCTs.
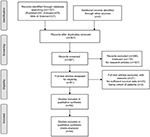  | Figure 1 Flow chart of literature search and study selection. |
Overall analysis
Meta-analysis on OS
There were 33 observational studies presenting the data of ALP and OS. The random effects model was used to analyze the relationship between them. The pooled HR was 1.74 (95% CI: 1.47–2.06, Figure 2A) with significant heterogeneity between studies (I2=96.1%, P<0.001), which demonstrated a significant relationship between ALP and OS. However, the pooled HR was 1.15 (95% CI: 1.02–1.30, Figure 2B), which demonstrates a significant relationship among five RCTs. There were three studies comparing the decrease in serum ALP level and OS, whose pooled HR was 0.56 (95% CI: 0.42–0.75, Figure 3A). Besides, five studies investigated the relationship between bone-specific ALP (BAP) and OS in patients with PCa. The pooled HR for BAP and OS is 1.65 (95% CI: 1.41–1.92, Figure 3B).
  | Figure 3 Forest plot of pooled HR of low ALP (A) or bone-specific ALP (B) and OS prognosis. Abbreviations: ALP, alkane phosphatase; OS, overall survival; ES, effect size. |
Meta-analysis on CSS
Seven studies provided sufficient data on ALP and CSS outcome. The pooled HR was 1.002 (95% CI: 0.998–1.005) via a random effects model, and the potential heterogeneity among studies was observed (I2=75.4%, P<0.001, Figure 4A).
Meta-analysis on PFS
Nine studies reported the data concerning the association between ALP and PFS. Meta-analysis adopting the random effects model revealed that elevated ALP was significantly associated with shorter PFS (HR=1.60, 95% CI: 1.13–2.26) with potential heterogeneity (I2=82.1%, P<0.001, Figure 4B).
Subgroup analyses
Moreover, we conducted a subgroup meta-analysis on different study designs. Although the main results were not affected by different study design, heterogeneity still existed in both prospective cohorts (HR=1.76, 95% CI: 1.42–2.19, Figure S1A) and retrospective studies (HR=1.58, 95% CI: 1.24–2.00, Figure S1B). In epidemiological studies, ethnicity difference was usually recognized as a critical source of bias. Notably, we also found the elevated serum ALP was significantly associated with poor OS among the studies in Asia (Figure S1C), Europe (Figure S1D), and North America (Figure S1E). Furthermore, we performed subgroup analysis in different cutoff values (Figure S1F, G) and sample sizes (Figure S1H, I). To sum up, the pooled HRs indicated that higher ALP was significantly associated with poorer OS in all subgroups of patients with PCa (Table 2).
  | Table 2 Summary of the subgroup analysis results of ALP and OS prognosis for PCa Abbreviations: ALP, alkaline phosphatase; OS, overall survival; PCa, prostate cancer; R, random-effects model. |
Sensitivity analysis
The sensitivity analysis was performed by the sequential deletion of any individual article to measure the effects of each individual study. The results showed that the overall HRs were not significantly influenced by individual study, as shown in Figure 5, indicating the robustness of the results in our meta-analysis.
  | Figure 5 Sensitivity analyses of high ALP and OS prognosis. Notes: (A) Observational cohorts; (B) RCTs. Abbreviations: ALP, alkane phosphatase; OS, overall survival; RCT, randomized controlled trial. |
Assessment of publication bias
Begg’s test was performed to evaluate the publication bias of the inclusion studies (Figure 6). The P-values of Begg’s test for OS (observational studies and RCTs) were 0.747 and 0.086, respectively, indicating that there was no significant publication bias.
Discussion
Serum ALP level is a simple and rapid laboratory test in routine clinical practice. An ideal prognostic biomarker can be used to determine prognosis, monitor response to therapy, and postoperative surveillance.70 The high ALP level has been reported related to the poor survival in colorectal cancer.71 The elevation of ALP is also an independent risk factor in the bone metastasis of gastric cancer and bladder cancer.72,73 However, the underlying mechanisms of ALP in patients with PCa remain unclear. A possible explanation is that when the PCa starts metastasis, ALP reflects bone turnover, osteoblast activity, and the osteoid formation in adjacent bone tissues.11 Thus, ALP may be an indicator of bone metastatic tumor load.
In this meta-analysis, based on the existing data from 63 included studies, the pooled results indicated that high baseline ALP was associated with obviously poor OS and PFS (HR=1.60, 95% CI: 1.13–2.26) in patients with PCa. As presented in Table 1, most included studies used multivariate cox model to explore ALP and survival. After being adjusted for other factors such as tumor stage/grade, PSA, Gleason score, hemoglobin, and metastasis, the original results of ALP were objective and reliable. The meta-analysis on both observational studies (HR=1.74, 95% CI: 1.47–2.06) and RCTs (HR=1.15, 95% CI: 1.02–1.30) reached the consistent conclusions about ALP and OS. In addition, high serum BAP was also significantly related to poor OS (HR=1.76, 95% CI: 1.42–2.15). However, our result revealed that there was no association between ALP and CSS in patients with PCa (HR=1.002, 95% CI: 0.998–1.005). We hypothesize that ALP is more sensitive in reflecting bone metastasis, so, high serum ALP is significantly associated with PFS of PCa. PCa patients with bone metastasis and other underlying diseases may lead to poorer OS. Whereas the seven studies about CSS (Figure 4A) were all retrospective in the study design. The sample size was also relatively smaller for CSS than OS. Thus, we should carefully interpret the result of ALP and CSS. The results of subgroup analyses on different study types, regions, cutoff values, and sample sizes were all in accordance with the main findings. The sensitivity analysis and publication bias tests’ outcomes also supported our results. Therefore, we may recommend ALP as a valuable prognostic marker for PCa treatment decision and adjustment. Compared with the positron emission tomography-computed tomography, ALP combined with bone scintigraphy may also be useful to assess the metastatic burden and survival possibility of PCa with a remarkably less expensive cost.
To our knowledge, this is the first meta-analysis on ALP and the prognosis of PCa. However, there are still a couple of limitations to be stated. First, although the language was not restricted during the searching process, all the included studies were in English, which might lead to language bias. Second, although sensitivity analysis supported the stability of our results, the findings should be cautiously interpreted. Heterogeneity among studies was found in overall and subgroup analyses. It was probably owing to multivariate factors in some included studies. Third, the data of ALP on other prognostic clinical parameters such as metastasis and all-cause mortality are lacking at present. Meanwhile, the retrospective design in 23 included studies (Table 1) may cause potential recall bias. Thus, more large-scale prospective studies are warranted to testify the prognostic ability of ALP in PCa in the future. Moreover, BAP will also be a potential prognostic marker in PCa, which needs verification as well.
Conclusion
In spite of the limitations mentioned above, the results of this study present the conclusion that high serum ALP is significantly associated with poor OS and PFS of PCa, but there is no obvious relation between ALP and CSS. ALP level is an efficient and convenient biomarker for PCa prognosis.
Acknowledgments
This study was supported by the Natural Science Foundation of Liaoning Province (program No.: 2015020275)
Author contributions
YS and BH were involved in project development; DL and HL helped to collect and manage data; DL, HL, and XH analyzed the data; DL wrote/edited the manuscript; YS and BH helped in critical revision of the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. | ||
Liu Y. The context of prostate cancer genomics in personalized medicine. Oncol Lett. 2017;13(5):3347–3353. | ||
Li DY, Hao XY, Ma TM, Dai HX, Song YS. The prognostic value of platelet-to-lymphocyte ratio in urological cancers: a meta-analysis. Sci Rep. 2017;7(1):15387. | ||
Li D, Lv H, Hao X, Dong Y, Dai H, Song Y. Prognostic value of bone scan index as an imaging biomarker in metastatic prostate cancer: a meta-analysis. Oncotarget. 2017;8(48):84449–84458. | ||
Graff RE, Pettersson A, Lis RT, et al. Dietary lycopene intake and risk of prostate cancer defined by ERG protein expression. Am J Clin Nutr. 2016;103(3):851–860. | ||
Cochetti G, Poli G, Guelfi G, Boni A, Egidi MG, Mearini E. Different levels of serum microRNAs in prostate cancer and benign prostatic hyperplasia: evaluation of potential diagnostic and prognostic role. Onco Targets Ther. 2016;9:7545–7553. | ||
Zhang M, Chen L, Yuan Z, et al. Combined serum and EPS-urine proteomic analysis using iTRAQ technology for discovery of potential prostate cancer biomarkers. Discov Med. 2016;22(122):281–295. | ||
Salvi S, Gurioli G, de Giorgi U, et al. Cell-free DNA as a diagnostic marker for cancer: current insights. Onco Targets Ther. 2016;9:6549–6559. | ||
Sharma U, Pal D, Prasad R. Alkaline phosphatase: an overview. Indian J Clin Biochem. 2014;29(3):269–278. | ||
Fan Y, Jin X, Jiang M, Fang N. Elevated serum alkaline phosphatase and cardiovascular or all-cause mortality risk in dialysis patients: A meta-analysis. Sci Rep. 2017;7(1):13224. | ||
Ren H-Y, Sun L-L, Li H-Y, Ye Z-M. Prognostic significance of serum alkaline phosphatase level in osteosarcoma: a meta-analysis of published data. Biomed Res Int. 2015;2015(10):160835. | ||
Groot MT, Boeken Kruger CG, Pelger RC, Uyl-de Groot CA. Costs of prostate cancer, metastatic to the bone, in The Netherlands. Eur Urol. 2003;43(3):226–232. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. | ||
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. | ||
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17(1):1–12. | ||
Halabi S, Lin CY, Small EJ, et al. Prognostic model predicting metastatic castration-resistant prostate cancer survival in men treated with second-line chemotherapy. J Natl Cancer Inst. 2013;105(22):1729–1737. | ||
Goldkorn A, Ely B, Quinn DI, et al. Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: a phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J Clin Oncol. 2014;32(11):1136–1142. | ||
Schellhammer PF, Chodak G, Whitmore JB, Sims R, Frohlich MW, Kantoff PW. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology. 2013;81(6):1297–1302. | ||
Humphrey PA, Halabi S, Picus J, et al. Prognostic significance of plasma scatter factor/hepatocyte growth factor levels in patients with metastatic hormone- refractory prostate cancer: results from cancer and leukemia group B 150005/9480. Clin Genitourin Cancer. 2006;4(4):269–274. | ||
Halabi S, Lin CY, Kelly WK, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2014;32(7):671–677. | ||
Qu Yy, Dai B, Kong YY, et al. Prognostic factors in Chinese patients with metastatic castration-resistant prostate cancer treated with docetaxel-based chemotherapy. Asian J Androl. 2013;15(1):110–115. | ||
Mikah P, Krabbe LM, Eminaga O, et al. Dynamic changes of alkaline phosphatase are strongly associated with PSA-decline and predict best clinical benefit earlier than PSA-changes under therapy with abiraterone acetate in bone metastatic castration resistant prostate cancer. BMC Cancer. 2016;16(1):214. | ||
Klaff R, Varenhorst E, Berglund A, et al. Clinical presentation and predictors of survival related to extent of bone metastasis in 900 prostate cancer patients. Scand J Urol. 2016;50(5):352–359. | ||
Miyamoto S, Ito K, Miyakubo M, et al. Impact of pretreatment factors, biopsy Gleason grade volume indices and post-treatment nadir PSA on overall survival in patients with metastatic prostate cancer treated with step-up hormonal therapy. Prostate Cancer Prostatic Dis. 2012;15(1):75–86. | ||
Kita Y, Shimizu Y, Inoue T, Kamba T, Yoshimura K, Ogawa O. Reduced-dose docetaxel for castration-resistant prostate cancer has no inferior impact on overall survival in Japanese patients. Int J Clin Oncol. 2013;18(4):718–723. | ||
Bilen MA, Hess KR, Subudhi SK, et al. Clinical predictors of survival in patients with castration-resistant prostate cancer receiving sipuleucel-T cellular immunotherapy. Cancer Chemother Pharmacol. 2017;80(3):583–589. | ||
Omlin A, Pezaro C, Mukherji D, et al. Improved survival in a cohort of trial participants with metastatic castration-resistant prostate cancer demonstrates the need for updated prognostic nomograms. Eur Urol. 2013;64(2):300–306. | ||
Nakashima J, Ozu C, Nishiyama T, et al. Prognostic value of alkaline phosphatase flare in patients with metastatic prostate cancer treated with endocrine therapy. Urology. 2000;56(5):843–847. | ||
Templeton AJ, Pezaro C, Omlin A, et al. Simple prognostic score for metastatic castration-resistant prostate cancer with incorporation of neutrophil-to-lymphocyte ratio. Cancer. 2014;120(21):3346–3352. | ||
van Soest RJ, Nieuweboer AJM, de Morrée ES, et al. The influence of prior novel androgen receptor targeted therapy on the efficacy of cabazitaxel in men with metastatic castration-resistant prostate cancer. Eur J Cancer. 2015;51(17):2562–2569. | ||
Sonpavde G, Pond GR, Armstrong AJ, et al. Prognostic impact of the neutrophil-to-lymphocyte ratio in men with metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2014;12(5):317–324. | ||
Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clinic Oncol. 2003;21(7):1232–1237. | ||
Shiota M, Yokomizo A, Adachi T, et al. The oncological outcomes and risk stratification in docetaxel chemotherapy for castration-resistant prostate cancer. Jpn J Clin Oncol. 2014;44(9):860–867. | ||
Oh WK, Miao R, Vekeman F, et al. Patient characteristics and overall survival in patients with post-docetaxel metastatic castration-resistant prostate cancer in the community setting. Med Oncol. 2017; 34(9):160. | ||
Brasso K, Christensen IJ, Johansen JS, et al. Prognostic value of PINP, bone alkaline phosphatase, CTX-I, and YKL-40 in patients with metastatic prostate carcinoma. Prostate. 2006;66(5):503–513. | ||
Chi KN, Kheoh T, Ryan CJ, et al. A prognostic index model for predicting overall survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate after docetaxel. Ann Oncol. 2016;27(3):454–460. | ||
Nozawa M, Hara I, Matsuyama H, et al. Significance of baseline bone markers on disease progression and survival in hormone-sensitive prostate cancer with bone metastasis. World J Urol. 2015;33(9):1263–1268. | ||
Pienta KJ, Redman BG, Bandekar R, et al. A phase II trial of oral estramustine and oral etoposide in hormone refractory prostate cancer. Urology. 1997;50(3):401–407. | ||
Reynard JM, Peters TJ, Gillatt D. Prostate-specific antigen and prognosis in patients with metastatic prostate cancer - a multivariable analysis of prostate cancer mortality. Br J Urol. 1995;75(4):507–515. | ||
Thatai LC, Banerjee M, Lai Z, Vaishampayan U. Racial disparity in clinical course and outcome of metastatic androgen-independent prostate cancer. Urology. 2004;64(4):738–743. | ||
Vesalainen S, Lipponen P, Talja M, Syrjänen K. Biochemical parameters as prognostic factors in prostatic adenocarcinoma. Acta Oncol. 1995;34(1):53–59. | ||
Etchebehere EC, Milton DR, Araujo JC, Swanston NM, Macapinlac HA, Rohren EM. Factors affecting 223Ra therapy: clinical experience after 532 cycles from a single institution. Eur J Nucl Med Mol Imaging. 2016;43(1):8–20. | ||
George DJ, Halabi S, Shepard TF, et al. Prognostic significance of plasma vascular endothelial growth factor levels in patients with hormone-refractory prostate cancer treated on Cancer and Leukemia Group B 9480. Clin Cancer Res. 2001;7(7):1932–1936. | ||
Buttigliero C, Pisano C, Tucci M, et al. Prognostic impact of pretreatment neutrophil-to-lymphocyte ratio in castration-resistant prostate cancer patients treated with first-line docetaxel. Acta Oncol. 2017;56(4):555–562. | ||
Shigeta K, Kosaka T, Kitano S, et al. High absolute monocyte count predicts poor clinical outcome in patients with castration-resistant prostate cancer treated with docetaxel chemotherapy. Ann Surg Oncol. 2016;23(12):4115–4122. | ||
Wyatt RB, Sanchez-Ortiz RF, Wood CG, Ramirez E, Logothetis C, Pettaway CA. Prognostic factors for survival among Caucasian, African-American and Hispanic men with androgen-independent prostate cancer. J Natl Med Assoc. 2004;96(12):1587–1593. | ||
Ramankulov A, Lein M, Kristiansen G, Loening SA, Jung K. Plasma osteopontin in comparison with bone markers as indicator of bone metastasis and survival outcome in patients with prostate cancer. Prostate. 2007;67(3):330–340. | ||
Sonpavde G, Pond GR, Berry WR, et al. Serum alkaline phosphatase changes predict survival independent of PSA changes in men with castration-resistant prostate cancer and bone metastasis receiving chemotherapy. Urol Oncol. 2012;30(5):607–613. | ||
Halabi S, Small EJ, Vogelzang NJ, Barrier RC, George SL, Gilligan TD. Impact of race on survival in men with metastatic hormone-refractory prostate cancer. Urology. 2004;64(2):212–217. | ||
Oh WK, Vargas R, Jacobus S, et al. Elevated plasma tissue inhibitor of metalloproteinase-1 levels predict decreased survival in castration-resistant prostate cancer patients. Cancer. 2011;117(3):517–525. | ||
Izumi K, Mizokami A, Itai S, et al. Increases in bone turnover marker levels at an early phase after starting zoledronic acid predicts skeletal-related events in patients with prostate cancer with bone metastasis. BJU Int. 2012;109(3):394–400. | ||
Hammerich KH, Donahue TF, Rosner IL, et al. Alkaline phosphatase velocity predicts overall survival and bone metastasis in patients with castration-resistant prostate cancer. Urologic Oncology. 2017;35:460.e21–.e28. | ||
Cook RJ, Coleman R, Brown J. Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clin Cancer Res. 2006;12(11):3361–3367. | ||
Park JM, Nam JS, Na W, et al. Prognostic value of body mass index in Korean patients with castration-resistant prostate cancer. Korean J Urol. 2012;53(11):761–765. | ||
Yamada Y, Naruse K, Nakamura K, et al. Investigation of risk factors for prostate cancer patients with bone metastasis based on clinical data. Exp Ther Med. 2010;1(4):635–639. | ||
Kamiya N, Suzuki H, Yano M, et al. Implications of serum bone turnover markers in prostate cancer patients with bone metastasis. Urology. 2010;75(6):1446–1451. | ||
Mohammed AA, El-Tanni H, Ghanem HM, et al. Impact of body mass index on clinico-pathological parameters and outcome in patients with metastatic prostate cancer. J Egypt Natl Canc Inst. 2015;27(3):155–159. | ||
Akimoto S, Inomiya H, Akakura K, Shimazaki J, Ito H. Prognostic factors in patients with prostate cancer refractory to endocrine therapy: univariate and multivariate analyses including doubling times of prostate-specific antigen and prostatic acid phosphatase. Jpn J Clin Oncol. 1997;27(4):258–262. | ||
Koo KC, Park SU, Kim KH, et al. Predictors of survival in prostate cancer patients with bone metastasis and extremely high prostate-specific antigen levels. Prostate Int. 2015;3(1):10–15. | ||
Kato M, Tsuzuki T, Kimura K, et al. The presence of intraductal carcinoma of the prostate in needle biopsy is a significant prognostic factor for prostate cancer patients with distant metastasis at initial presentation. Mod Pathol. 2016;29(2):166–173. | ||
D’Amico AV, Chen MH, Cox MC, Dahut W, Figg WD. Prostate-specific antigen response duration and risk of death for patients with hormone-refractory metastatic prostate cancer. Urology. 2005;66(3):571–576. | ||
Bando Y, Hinata N, Terakawa T, et al. Activity of cabazitaxel in patients with metastatic castration-resistant prostate cancer after treatment with single or dual regimens of novel androgen receptor-targeting agents. Med Oncol. 2017;34(9):163. | ||
Pelger RC, Lycklama A Nijeholt GA, Zwinderman AH, Papapoulos SE, Hamdy NA. The flare in serum alkaline phosphatase activity after orchiectomy: a valuable negative prognostic index for progression-free survival in prostatic carcinoma. J Urol. 1996;156(1):122–126. | ||
Han KS, Hong SJ. Serum alkaline phosphatase differentiates prostate-specific antigen flare from early disease progression after docetaxel chemotherapy in castration-resistant prostate cancer with bone metastasis. J Cancer Res Clin Oncol. 2014;140(10):1769–1776. | ||
Goodman OB, Symanowski JT, Loudyi A, Fink LM, Ward DC, Vogelzang NJ. Circulating tumor cells as a predictive biomarker in patients with hormone-sensitive prostate cancer. Clin Genitourin Cancer. 2011;9(1):31–38. | ||
Matsuyama H, Shimabukuro T, Hara I, et al. Combination of hemoglobin, alkaline phosphatase, and age predicts optimal docetaxel regimen for patients with castration-resistant prostate cancer. Int J Clin Oncol. 2014;19(5):946–954. | ||
Fizazi K, Massard C, Smith M, et al. Bone-related parameters are the main prognostic factors for overall survival in men with bone metastases from castration-resistant prostate cancer. Eur Urol. 2015;68(1):42–50. | ||
Rahbar K, Boegemann M, Yordanova A, et al. PSMA targeted radioligand therapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur J Nucl Med Mol Imaging. 2018;45(1):12–19. | ||
Sartor O, Coleman RE, Nilsson S, et al. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann Oncol. 2017;28(5):1090–1097. | ||
Nowsheen S, Aziz K, Panayiotidis MI, Georgakilas AG. Molecular markers for cancer prognosis and treatment: have we struck gold? Cancer Lett. 2012;327(1–2):142–152. | ||
Liu F, Zhao J, Xie J, et al. Prognostic risk factors in patients with bone metastasis from colorectal cancer. Tumour Biol. 2016;37(12):16127–16134. | ||
Lim SM, Kim YN, Park KH, et al. Bone alkaline phosphatase as a surrogate marker of bone metastasis in gastric cancer patients. BMC Cancer. 2016;16(1):385. | ||
Huang P, Lan M, Peng AF, et al. Serum calcium, alkaline phosphatase and hemoglobin as risk factors for bone metastases in bladder cancer. PLoS One. 2017;12(9):e0183835. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




