Back to Journals » Cancer Management and Research » Volume 10
Prognostic value of primary tumor surgery in minor salivary-gland carcinoma patients with distant metastases at diagnosis: first evidence from a SEER-based study
Authors Shi X , Huang NS, Shi RL, Wei WJ, Wang YL, Ji QH
Received 2 May 2018
Accepted for publication 5 June 2018
Published 20 July 2018 Volume 2018:10 Pages 2163—2172
DOI https://doi.org/10.2147/CMAR.S172725
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Xiao Shi,1,2,* Nai-Si Huang,1,2,* Rong-Liang Shi,1,2,* Wen-Jun Wei,1,2 Yu-Long Wang,1,2 Qing-Hai Ji1,2
1Department of Head and Neck Surgery, Fudan University Shanghai Cancer Center, 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
*These authors contributed equally to this work
Purpose: The prognostic value of primary tumor surgery (PTS) in minor salivary-gland carcinoma (MiSGC) with distant metastasis (DM) at diagnosis has never been investigated. In this study, we aimed to provide the first evidence.
Patients and methods: The Surveillance, Epidemiology, and End Results (SEER) database was employed to identify MiSGC patients with DM at diagnosis. The prognostic value of PTS was evaluated by Kaplan–Meier methods, log-rank analyses, and multivariate Cox proportional-hazard regression models.
Results: Of the 152 eligible patients included in our study, 50 (32.9%) had undergone PTS. Kaplan–Meier analyses showed that the PTS group had >20% increase in 1- and 2-year overall survival (OS) and cancer-specific survival (CSS) compared with their counterparts without PTS (PTS group vs no-PTS group, 1-year OS 66.1% vs 43.9%, 1-year CSS 69.9% vs 44.9%, 2-year OS 56.6% vs 24.2%, 2-year CSS 59.9% vs 25.7%). Compared with the no-PTS group, multivariate analyses also demonstrated a significantly decreased risk of overall mortality (HR 0.601, 95% CI 0.379–0.952; P=0.031) and cancer-specific mortality (HR 0.547, 95% CI 0.336–0.891; P=0.015) in the PTS group. Subgroup multivariate analyses revealed patients with T1–T3 oropharynx, nasal cavity, or paranasal sinus primary MiSGC, especially adenoid cystic carcinoma, might benefit from PTS (all P<0.05).
Conclusion: PTS is associated with improved survival in highly selected MiSGC patients and may be considered in future clinical practice. However, prospective studies with larger sample size are still necessary to validate our findings.
Keywords: minor salivary gland carcinoma, distant metastasis, primary tumor surgery, T stage, primary site, SEER
Introduction
Salivary gland (SG) neoplasms constitute 3%–6% of all head and neck malignancies and incorporate over 20 kinds of histologic subtypes, according to the 2005 World Health Organization classification.1,2 However, the majority of SG cancer (SGC) originates from the major SG,3 whereas minor SGC (MiSGC) accounts for only 20% or even less of the whole SGC entity.4–7
Despite the fact that MiSGCs occur mostly in the oral cavity and oropharynx, they can also arise from MiSGs at almost all regions of the upper aerodigestive tract and paranasal sinuses. Early detection is always difficult, because most of the tumors appear to be painless and slowly growing submucosal swellings.8 For tumors in the paranasal sinus, timely diagnosis is even harder, as apparent symptoms always develop late and are often confused with nasal obstruction.8 Baddour et al reported an 8.3% distant metastasis (DM) rate at diagnosis among MiSGC patients using data from the Surveillance, Epidemiology, and End Results (SEER) database,9 which was higher than that in head and neck squamous cell carcinoma (HNSCC) and major SGC.10,11 Therefore, DM in MiSGC ought to be given more attention.
In the National Comprehensive Cancer Network guidelines, treatment modalities for metastatic MiSGCs are still limited, comprising only chemotherapy, expectant management, supportive care, and selected metastasectomy (not primary tumor [PT] treatment).12 There is a lack of evidence as to whether these therapies can bring improved survival outcomes.6 Primary tumor surgery (PTS) has been proved to play a favorable role in the treatment of multiple cancers with M1 disease at diagnosis, including colorectal, breast, gastric, and bladder cancers, as well as HNSCC.13–19 Our previous work also verified the prognostic significance of PTS in metastatic major SGC.20 However, as far as we know, there are no relevant studies focusing on this subject in the setting of metastatic MiSGC. In the present study, we aimed to provide the first evidence on the prognostic value of PTS in MiSGC patients with DM at diagnosis using data from the SEER database.
Patients and methods
Data source
We explored the National Cancer Institute’s SEER program to identify primary MiSGC cases with DM at diagnosis. As the largest publicly available cancer data set in the world, the SEER program currently covers nearly 30% of the US population and routinely records patients’ demographics, tumor characteristics, treatment, survival time, and annually updated vital status. As SEER is a public database without identified information, this retrospective study was exempted from approval by the Fudan University Shanghai Cancer Center institutional review board.
Cases
Patients were included in our study if the following criteria were met: 1) histologic subtypes identified using the third-edition ICD for Oncology (ICD-O3) codes for adenocarcinoma (8140, 8147, 8290, 8310, 8410, 8440, 8480, 8525, and 8550), mucoepidermoid carcinoma (8430), adenoid cystic carcinoma (8200), mixed subtype (8980 and 8981), and other rare carcinomas (8012, 8041, 8082, 8562, and 8982), which was the same as the methodology of previous studies using the SEER database;3,9,21 2) histologic subtypes confirmed by aspiration cytology, biopsy, or postoperative pathology report; 3) primary site included oral cavity (ICD-O3 topography codes C00.0–C00.9, C02.0–C02.3, C02.8–C02.9, C03.0–C03.9, C04.0–C04.9, C05.0, and C06.0–C06.1), oropharynx (C01.9, C02.4, C05.1–C05.2, and C09.0–C10.9), larynx (C32.0–C32.9), hypopharynx (C12.9–C13.2), nasal cavity (C30.0), and paranasal sinus (C31.0–C31.9). MiSGCs originating from nasopharynx (C11.0–C11.9) were excluded, as none of these patients had undergone PTS, and more importantly almost all these tumors were recorded as lymphoepithelial carcinoma (8082), which was difficult to distinguish from the more common primary nasopharyngeal SCC;2 4) patients diagnosed between January 1, 2004 and June 30, 2014, because some important factors are merely available for patients diagnosed after 2004, such as the American Joint Committee on Cancer (AJCC) TNM staging. In addition, the cutoff date for SEER follow-up information was December 31, 2014, and it is advisable to guarantee a minimum of 6-month follow-up for these metastatic patients; 5) patients diagnosed with DM at initial presentation.
Covariates and outcomes
Demographics of patients (sex, age at diagnosis), characteristics of tumors (AJCC T and N staging, primary site, histopathologic type, tumor grade) and treatment (PTS, radiation, chemotherapy) were retrieved for all cases included in our study cohort. In this study, PTS represented total or at least partial removal of the PT with or without adjacent sites. Local destructive therapies, such as electrocautery and cryosurgery, were excluded from the scope of PTS. Of note, endoscopic laser surgery was not included in this definition, because none of the patients in our study underwent this procedure. Metastasectomy was not incorporated into the analysis because of insufficient cases (n=3). Metastatic organs, such as lung, liver, and bone, were not incorporated in the analysis either, as only a small proportion of patients (diagnosed after 2010) had definite records on this parameter. With regard to the “primary site”, larynx, hypopharynx, nasal cavity, and paranasal sinus were grouped together, due to the small sample. It is also noteworthy that the seventh edition of AJCC T staging was adopted in our study, because some information in the eighth edition was not accessible in the SEER registry.
To simplify our research, we categorized the histopathologic types of MiSGC into low-, intermediate-, and high-risk categories based on three-tiered classification criteria put forward by Therkildsen et al in 1998,22 which was also used in the research by Jouzdani et al.23 In our study, the low-risk category included low-grade mucoepidermoid carcinoma/adenocarcinoma-not otherwise specified (NOS), polymorphous low-grade adenocarcinoma, basal-cell carcinoma, and acinar-cell carcinoma; the intermediate-risk category included intermediate-grade mucoepidermoid carcinoma/adenocarcinoma-NOS, epithelial–myoepithelial carcinoma, oncocytic carcinoma, myoepithelial carcinoma, carcinoma in pleomorphic adenoma, and adenoid cystic carcinoma, whereas the high-risk category included high-grade mucoepidermoid carcinoma/adenocarcinoma-NOS and undifferentiated carcinoma. Mucoepidermoid carcinoma/adenocarcinoma-NOS without known tumor grade was recorded as “unspecified” histology. The end points of our study were overall survival (OS) and cancer-specific survival (CSS). The duration of OS and CSS were calculated as the interval from initial diagnosis to overall mortality (OM; death from any cause) or cancer-specific mortality (CSM; death from MiSGC), respectively.
Statistical analysis
Fisher’s exact test or Pearson’s chi-square test was used to compare differences in categorical baseline parameters. Survival plots were generated by the Kaplan–Meier method and compared by log-rank test. Multivariate Cox proportional-hazard regression analyses were performed to evaluate the impact of covariates on OS and CSS by calculating HR and 95% CI. In order better to illustrate the prognostic significance of PTS in the overall cohort, we separately constructed two multivariate Cox regression models, with one model using the best subsets of covariates identified by the smallest Akaike information criterion (AIC), which indicated minimal loss of prognostic information,24,25 and the other incorporating all the baseline variables. Covariates of subgroup multivariate analyses included all baseline variables, because if we used the AIC method, we would not be able to guarantee the variable of interest (eg, PTS) to be selected in all the subgroup Cox models. All statistical analyses were carried out using SPSS version 24 (IBM, Armonk, NY, USA). Forest plots summarizing the results of subgroup Cox analyses were drawn with Stata version 14.0 (StataCorp, College Station, TX, USA). Survival plots were drawn with GraphPad Prism version 7.0 (GraphPad, San Diego, CA, USA). Two-sided P<0.05 was considered statistically significant.
Results
Patient characteristics
A total of 152 eligible patients were incorporated in our study. Of the overall cohort, only 50 (32.9%) patients had undergone PTS. The median follow-up time was 12 months and median age at diagnosis was 62.5 (range 26–91) years. More than half (n=90, 59.2%) of the patients were male. The vast majority of tumors (n=128, 84.2%) were high-risk pathologies, and ~60% (n=92, 60.5%) of tumors originated from the oral cavity and oropharynx. One hundred and forty-four (93.4%) of the tumors were categorized as intermediate- or high-risk pathology, and adenoid cystic carcinoma (n=62, 40.8%) and adenocarcinoma NOS (n=31, 20.4%) were the two most common histotypes. Eighty-three (25.0%) patients had stage T1–T2 tumors and 48.0% (n=73) had N+ disease. In addition, 55.3% (n=84) and 48.7% (n=74) of the entire cohort had undergone radiation and chemotherapy, respectively. Patient characteristics of the no-PTS and PTS groups are outlined in Table 1. In the overall cohort, metastases of 43 patients (28.3%) were pathologically confirmed, whereas the rest (71.7%) were confirmed by imaging, such as positron-emission tomography/computed tomography.
PTS and survival outcomes in overall cohort
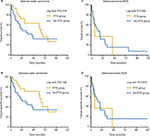
Among the 152 MiSGC cases, Kaplan–Meier analyses demonstrated that the PTS group had >20% higher 1- and 2-year survival rates than their counterparts without PTS (PTS vs no-PTS, 1-year OS 66.1% vs 43.9%, 1-year CSS 69.9% vs 44.9%, 2-year OS 56.6% vs 24.2%, 2-year CSS 59.9% vs 25.7%). Meanwhile, the log-rank tests suggested that all these differences were statistically significant (OS P=0.006, CSS P=0.002; Figure 1). On multivariate analysis for both end points, the smallest AIC value (OM 935.57, CSM 873.30) appeared when we put sex, histopathologic type, and PTS in the Cox regression models. The results revealed that PTS significantly decreased the risk of OM and CSM compared with those absent of surgical intervention (PTS vs no-PTS: HROM 0.601, 95% CI 0.379–0.952, P=0.031; HRCSM 0.547, 95% CI 0.336–0.891, P=0.015; Table 2). Another multivariate Cox regression model incorporating all the baseline covariates also reached similar conclusions. PTS was still an independent positive prognosticator for both end points (PTS vs no-PTS: HROM 0.552, 95% CI 0.326–0.935, P=0.027; HRCSM 0.472, 95% CI 0.272–0.821, P=0.008; Table 3).
  | Figure 1 Kaplan–Meier survival plots of (A) overall and (B) cancer-specific survival according to receipt of PTS (yes vs no) for the overall 152 patients. Abbreviation: PTS, primary tumor surgery. |
  | Table 3 Multivariate Cox analyses of prognostic indicators for overall and cancer-specific mortality in the overall cohort, incorporating all the baseline covariates |
PTS and survival outcomes in patients according to T staging
Subsequently, survival analyses were investigated within each T subgroup. For patients with T1–T2 PTs, Kaplan–Meier methods showed that both 1-year OS and CSS rates of the no-PTS group were 39.1%, whereas the 2-year OS and CSS rate sharply decreased to 21.7%. By contrast, we were surprised to find that the 1-year OS and CSS rates of the PTS group were as high as 81.3%, whereas both the 2-year OS and CSS rates achieved 69.7%. Although the survival difference between the PTS and no-PTS groups did not meet statistical significance (OS P=0.093, CSS; P=0.055; Figure 2), PTS proved to be a positive prognosticator in subgroup multivariate Cox analyses for patients with stage T1–T2 tumors (PTS vs no-PTS: HROM 0.521, 95% CI 0.298–0.911, P=0.022; HRCSM 0.427, 95% CI 0.238–0.767, P=0.004), as summarized in the forest plots (Figure 3). On the other hand, both log-rank tests (OS P=0.003, CSS P=0.006) and subgroup multivariate Cox analyses (PTS vs no-PTS: HROM 0.042, 95% CI 0.004–0.476, P=0.010; HRCSM 0.062, 95% CI 0.005–0.716, P=0.026) revealed the prognostic significance of PTS for patients with stage T3 PTs. However, in neither log-rank tests (PT4a-OS=0.093, PT4a-CSS=0.051; PT4b-OS=0.134, PT4a-CSS=0.062) nor subgroup multivariate analyses (T4a, HROM 0.917, 95% CI 0.316–2.745, P=0.876; HRCSM 0.820, 95% CI 0.276–2.438, P=0.721; T4b, HROM 0.390, 95% CI 0.094–1.628, P=0.197; HRCSM 0.273, 95% CI 0.051–1.465, P=0.130) did we observe any significant difference between the PTS and no-PTS groups for patients with T4a or T4b PTs (Figures 2 and 3).
PTS and survival outcomes according to site of primary lesion
Next, we aimed to explore the prognostic value of PTS according to the primary site of MiSGC. Log-rank tests did not demonstrate significant prognostic differences between the PTS and no-PTS groups for patients with oral cavity (OS P=0.989, CSS P=0.751) or nasal cavity/paranasal sinus MiSGCs (OS P=0.093, CSS P=0.084), and it seemed only patients with oropharyngeal MiSGCs might benefit from PTS (OS P=0.019, CSS P=0.010) (Figure 4).
Subgroup multivariate Cox analyses were also applied. The results of multivariate analyses indicated that PTS was associated with decreased risk of OM and CSM not only in oropharyngeal MiSGCs (HROM 0.153, 95% CI 0.036–0.651, P=0.011; HRCSM 0.098, 95% CI 0.020–0.492, P=0.005) but also in nasal cavity/paranasal sinus MiSGCs (HROM 0.174, 95% CI 0.049–0.600, P=0.006; HRCSM 0.129, 95% CI 0.031–0.529, P=0.004). Similarly, there was no evidence of favorable prognostic impact of PTS in patients with oral cavity tumors (HROM 1.062, 95% CI 0.346–3.265, P=0.916; HRCSM 0.973, 95% CI 0.318–2.981, P=0.962) (Figure 3). It was worth noting that subgroup analyses were not performed in hypopharyngeal/laryngeal MiSGC patients, because there were only three individuals undergoing PTS, which was not sufficient for multivariate analyses.
PTS and survival outcomes for patients with representative histologic subtypes
In our study cohort, adenoid cystic carcinoma (ACC; n=62, 40.8%) and adenocarcinoma-NOS (n=31, 20.4%) were two representative histologic types (Table 1). For ACC or adenocarcinoma NOS MiSGC patients, it seemed that PTS did not confer a survival advantage in univariate log-rank tests (ACC OS P=0.219, CS: P=0.146; adenocarcinoma-NOS OS P=0.928, CSS P=0.816) (Figure 5). However, subgroup multivariate Cox analyses demonstrated that PTS was independently associated with decreased mortality risk for ACC patients (HROM 0.416, 95% CI 0.179–0.971, P=0.042; HRCSM 0.341, 95% CI 0.134–0.871, P=0.025). Nevertheless, we failed to observe any significant beneficial prognostic effect for those with adenocarcinoma-NOS (HROM 0.481, 95% CI 0.125–1.856, P=0.288; HRCSM 0.497, 95% CI 0.127–1.949, P=0.316; Figure 3).
Discussion
For patients with metastatic MiSGC, chemotherapy, selected metastasectomy, and supportive care remain the predominant strategies in current clinical practice. Nevertheless, there is no evidence of a survival advantage associated with these therapies, including the most commonly used chemotherapy.6 Chemotherapy, of which the optimal regimen is not well defined, seems incapable of prolonging survival in patients with end-stage MiSGC. Therefore, chemotherapy is more of a palliative strategy aiming to improve quality of life and alleviate local symptoms.26 Moreover, in spite of the emerging targeted therapies, all these drugs for metastatic SG malignancies are still at the stage of clinical trials.6,27
Once the aforementioned methods fail, there is almost nothing alternative that can help. However, the therapeutic value of PTS in multiple cancers, especially in HNSCC and major SGC,19,20 promotes us to turn our attention to MiSGC. Due to its rare incidence, extensive anatomical distribution, and complicated histology, prospective studies are extremely hard to carry out, let alone for the rarer metastatic disease. What is more, there has been almost no research concentrating on metastatic MiSGCs in the past two decades. Consequently, the US national SEER database may be an optimal tool for investigating this subject. As far as we know, with the use of SEER database, this is the first study to investigate the prognostic value of PTS in the setting of metastatic MiSGC.
Unlike recurrent postoperative disease, detection of metastases at initial diagnosis gives surgeons an opportunity to remove the primary lesion. One of the most important factors for evaluating the value of operations is the resectability of PTs.28 In our study, surgical resection was shown to be of prognostic value for M1 patients with T1–T3 disease. As a result, locoregional resectable PTs might be considered a surgical indication. For their counterparts with moderately or very advanced disease (T4a–T4b tumors), however, aggressive surgical excision was not only unable to bring survival benefits, but was also highly likely to seriously affect the integrity of crucial anatomical structures in the head and neck region.
As mentioned, tumors arising in the nasal cavity and paranasal sinuses are often detected at an advanced stage and are proximal to critical locations, including the skull base, orbit, frontal lobe, and major cranial nerves,3 which sometimes trigger controversy about the balance of clear surgical margins and necessary functional preservation.29 In our study, five of eleven patients (45.5%) with metastatic nasal cavity/paranasal sinus MiSGC who had received PTS underwent only partial tumor removal or so-called “debulking” surgery; however, it was these operations that relieved their local symptoms and simultaneously lengthened their lives. Therefore, if the prognostic value of radiotherapy is not clear and might result in related bony and visual toxicity,30 PTS is worth considering regardless of R0/R1 resection.
The oral cavity remained the most common primary location of distant metastatic MiSGCs. Unfortunately, unlike tumors from other sites, PTS did not confer a survival benefit in patients with oral cavity lesions. We assumed that this was possibly due to different anatomical characteristics. For malignancies of the nasal cavity, paranasal sinuses, and oropharynx, due to their relatively inaccessible anatomical location next to the skull base or respiratory tract,31 advanced tumors are prone to fatal complications when they become seriously infected, ulcerated, and hemorrhagic. PTS might largely delay or even avoid the occurrence of these lethal events. However, for oral cavity neoplasms, it is difficult, given the relative abundance of space in the mouth, for an even larger tumor to cause serious local symptoms, thereby reducing the significance of PTS in oral cavity MiSGC patients. However, further evidence is needed to support these hypotheses.
Some limitations inherent to the use of the SEER database should be acknowledged. First, only a small proportion of patients (2010–2014) had records of metastatic sites in the SEER database; therefore, metastatic sites could not be included in the analysis. Second, there was inevitable bias, due to the lack of information in the SEER registry, such as comorbidities and performance status. For example, healthier patients with better performance status might be more likely to receive PTS. Other similar SEER-based studies have also reported this flaw, concluding that it was unlikely that the significant prognostic effect of PTS in highly-selected groups was due solely to this unadjusted confounding factor.14,16,19,20 Third, although the nationwide SEER database covers nearly 26% of the US population, the sample of this study was still small, due to the low incidence of metastatic MiSGCs. Fourth, for the foreseeable future, it is still very hard to investigate this topic in each histologic subtype of MiSGC, due to its rarity and overcomplicated pathologies. Fifth, this study is also limited by its retrospective nature.
Conclusion
Our study for the first time shows that it is reasonable to consider PTS in highly selected MiSGC patients with DM at diagnosis. Patients with T1–T3 oropharyngeal, nasal cavity, or paranasal sinus neoplasms, especially adenoid cystic carcinoma, may be suitable candidates. However, prospective studies with larger samples are awaited to validate our conclusions.
Acknowledgments
This study was supported by the Shanghai Rising-Star Program (15QA1401100 to YLW) and the National Science Foundation of China (81472498 and 81772851 to YLW and 81572622 to QHJ).
Disclosure
The authors report no conflicts of interest in this work.
References
Adelstein DJ, Koyfman SA, el-Naggar AK, Hanna EY. Biology and management of salivary gland cancers. Semin Radiat Oncol. 2012;22(3):245–253. | ||
Barnes L, Eveson JW, Reichart P, Sidransky D. Pathology and Genetics of Head and Neck Tumours. Lyon: IARC Press; 2005. | ||
Zeidan YH, Pekelis L, An Y, et al. Survival benefit for adjuvant radiation therapy in minor salivary gland cancers. Oral Oncol. 2015;51(5):438–445. | ||
Strick MJ, Kelly C, Soames JV, Mclean NR. Malignant tumours of the minor salivary glands: a 20 year review. Br J Plast Surg. 2004;57(7):624–631. | ||
Spiro RH. The management of salivary neoplasms: an overview. Auris Nasus Larynx. 1985;12 Suppl 2:S122–S127. | ||
Guzzo M, Locati LD, Prott FJ, Gatta G, McGurk M, Licitra L. Major and minor salivary gland tumors. Crit Rev Oncol Hematol. 2010;74(2):134–148. | ||
Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8(3):177–184. | ||
Andry G, Hamoir M, Locati LD, Licitra L, Langendijk JA. Management of salivary gland tumors. Expert Rev Anticancer Ther. 2012;12(9):1161–1168. | ||
Baddour HM, Fedewa SA, Chen AY. Five- and 10-year cause-specific survival rates in carcinoma of the minor salivary gland. JAMA Otolaryngol Head Neck Surg. 2016;142(1):67–73. | ||
Mariano FV, da Silva SD, Chulan TC, de Almeida OP, Kowalski LP. Clinicopathological factors are predictors of distant metastasis from major salivary gland carcinomas. Int J Oral Maxillofac Surg. 2011;40(5):504–509. | ||
Kuperman DI, Auethavekiat V, Adkins DR, et al. Squamous cell cancer of the head and neck with distant metastasis at presentation. Head Neck. 2011;33(5):714–718. | ||
Adelstein D, Gillison ML, Pfister DG, et al. NCCN guidelines insights: head and neck cancers, version 2.2017. J Natl Compr Canc Netw. 2017;15(6):761–770. | ||
Turner N, Tran B, Tran PV, et al. Primary tumor resection in patients with metastatic colorectal cancer is associated with reversal of systemic inflammation and improved survival. Clin Colorectal Cancer. 2015;14(3):185–191. | ||
Tarantino I, Warschkow R, Worni M, et al. Prognostic relevance of palliative primary tumor removal in 37,793 metastatic colorectal cancer patients: a population-based, propensity score-adjusted trend analysis. Ann Surg. 2015;262(1):112–120. | ||
Stillwell AP, Buettner PG, Ho YH. Meta-analysis of survival of patients with stage IV colorectal cancer managed with surgical resection versus chemotherapy alone. World J Surg. 2010;34(4):797–807. | ||
Warschkow R, Güller U, Tarantino I, et al. Improved survival after primary tumor surgery in metastatic breast cancer: a propensity-adjusted, population-based SEER trend analysis. Ann Surg. 2016;263(6):1188–1198. | ||
Warschkow R, Baechtold M, Leung K, et al. Selective survival advantage associated with primary tumor resection for metastatic gastric cancer in a Western population. Gastric Cancer. 2018;21(2):324–337. | ||
Abufaraj M, Dalbagni G, Daneshmand S, et al. The role of surgery in metastatic bladder cancer: a systematic review. Eur Urol. 2018;73(4):543–557. | ||
Patel TD, Marchiano E, Chin OY, et al. Utility of surgery/radiotherapy in distant metastatic head and neck squamous cell carcinoma: a population-based approach. Otolaryngol Head Neck Surg. 2016;154(5):868–874. | ||
Shi X, Dong F, Wei W, et al. Prognostic significance and optimal candidates of primary tumor resection in major salivary gland carcinoma patients with distant metastases at initial presentation: a population-based study. Oral Oncol. 2018;78:87–93. | ||
Goel AN, Badran KW, Braun AP, Garrett AM, Long JL. Minor salivary gland carcinoma of the oropharynx: a population-based analysis of 1426 patients. Otolaryngol Head Neck Surg. 2018;158(2):287–294. | ||
Therkildsen MH, Christensen M, Andersen LJ, Schiødt T, Hansen HS. Salivary gland carcinomas: prognostic factors. Acta Oncol. 1998;37(7–8):701–713. | ||
Jouzdani E, Yachouh J, Costes V, et al. Prognostic value of a three-grade classification in primary epithelial parotid carcinoma: result of a histological review from a 20-year experience of total parotidectomy with neck dissection in a single institution. Eur J Cancer. 2010;46(2):323–331. | ||
Wagenmakers EJ, Farrell S. AIC model selection using Akaike weights. Psychon Bull Rev. 2004;11(1):192–196. | ||
Posada D, Buckley TR. Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst Biol. 2004;53(5):793–808. | ||
de Felice F, de Vincentiis M, Valentini V, et al. Management of salivary gland malignant tumor: the Policlinico Umberto I, “Sapienza” University of Rome Head and Neck Unit clinical recommendations. Crit Rev Oncol Hematol. 2017;120:93–97. | ||
Lagha A, Chraiet N, Ayadi M, et al. Systemic therapy in the management of metastatic or advanced salivary gland cancers. Head Neck Oncol. 2012;4:19. | ||
van der Poorten V, Hunt J, Bradley PJ, et al. Recent trends in the management of minor salivary gland carcinoma. Head Neck. 2014;36(3):444–455. | ||
Lee SY, Shin HA, Rho KJ, et al. Characteristics, management of the neck, and oncological outcomes of malignant minor salivary gland tumours in the oral and sinonasal regions. Br J Oral Maxillofac Surg. 2013;51(7):e142–e147. | ||
Garden AS, Weber RS, Ang KK, et al. Postoperative radiation therapy for malignant tumors of minor salivary glands: outcome and patterns of failure. Cancer. 1994;73(10):2563–2569. | ||
Bradley PJ, Ferris RL. Surgery for malignant sublingual and minor salivary gland neoplasms. Adv Otorhinolaryngol. 2016;78:113–119. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.