Back to Journals » Cancer Management and Research » Volume 12
Prognostic Value of Pretreatment Albumin-to-Alkaline Phosphatase Ratio in Extensive-Disease Small-Cell Lung Cancer: A Retrospective Cohort Study
Authors Zhou S , Wang H, Jiang W, Yu Q, Zeng A
Received 1 February 2020
Accepted for publication 11 March 2020
Published 18 March 2020 Volume 2020:12 Pages 2015—2024
DOI https://doi.org/10.2147/CMAR.S247967
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Eileen O'Reilly
Shaozhang Zhou, Huiling Wang, Wei Jiang, Qitao Yu, Aiping Zeng
Department of Respiratory Oncology, Guangxi Medical University Affiliated Tumor Hospital, Nanning City 530021, Guangxi Zhuang Autonomous Region, People’s Republic of China
Correspondence: Aiping Zeng
Department of Respiratory Oncology, Guangxi Medical University Affiliated Tumor Hospital, No. 71 Heti Road, Nanning City 530021, Guangxi Zhuang Autonomous Region, People’s Republic of China
Tel +86 771 5334955
Fax +86 771 5300613
Email [email protected]
Purpose: Evidence regarding the relationship between albumin-to-alkaline phosphatase ratio (AAPR) and overall survival (OS) in extensive-disease small-cell lung cancer (ED-SCLC) patients is limited. This study aimed to investigate whether AAPR was independently related to OS in ED-SCLC patients after adjusting for potential covariates.
Patients and Methods: This was a retrospective cohort study of 224 patients with ED-SCLC. The target independent and dependent variables were pretreatment AAPR and OS, respectively. Covariates included age; sex; Eastern Cooperative Oncology performance status score; smoking history; existence of metastasis to organs such as the bone, lung, liver, brain, malignant plural effusion and others; sum of organ metastasis (≤ 3, > 3), evaluation of first-line treatment; and sum of treatment lines (< 2, ≥ 2). Student’s t test or chi-squared test was used to analyze the associations between AAPR and clinical characteristics. Kaplan–Meier survival analysis and Cox’s proportional hazards regression model were used to assess the prognostic value of AAPR for OS.
Results: The average patient age was 60.51± 8.73 years, and 87.95% were men. A non-linear relationship between AAPR and OS was detected, with an inflection point of 0.35. The hazard ratios (HRs) of the left (AAPR < 0.35) and right sides (AAPR ≥ 0.35) of inflection point were 0.04 (95% CI=0.00– 0.70, p=0.0268) and 0.52 (95% CI=0.16– 1.64, p=0.2659), respectively. Kaplan–Meier analysis showed a median OS of 9.73 months (95% CI=8.6– 12.33) for AAPR < 0.35 and 13.7 months (95% CI=11.43– 16.37) for AAPR ≥ 0.35 (log-rank p< 0.0001). The Cox proportional hazards model showed that AAPR < 0.35 increased the risk of death after adjusting for potential confounders (HR=1.65, 95% CI=1.11– 2.46). In subgroup analysis, the trends of HRs were increased across all subgroups with AAPR < 0.35 after stratification.
Conclusion: Pretreatment AAPR might be served as an independent prognostic indicator in ED-SCLC patients. Our findings should be further validated in large-scale and prospective clinical trials.
Keywords: AAPR, overall survival, prognosis, extensive-disease small-cell lung cancer
Introduction
Lung cancer remains the leading cause of cancer-related death worldwide.1 While small-cell lung cancer (SCLC) is a less common subtype, accounting for only approximately 10–15% of all lung cancer cases, it tends to be aggressive, thus making it a treatment challenge. The prognosis of SCLC remains poor, particularly in patients with extensive disease (ED). Although the response rate of ED-SCLC in the first-line treatment setting can reach 60–65%, the median overall survival (OS) rarely reaches more than 1 year.2 Thus recently, there has been increasing interest in pre- and posttreatment prognostic predictors. It is crucial to identify prognostic markers in order to predict survival and help in clinical decision-making. Better patient stratification according to treatment outcomes can help physicians provide more optimal treatment.
The TNM staging system of the American Joint Committee on Cancer has been widely used for most cancer types owing to its predictive capabilities3; however, it cannot further discriminate cancer patients who are in the advanced stage. For SCLC, as a distinct entity from non-small cell lung cancer (NSCLC), the Veterans Administration Lung Study Group is more commonly used for staging limited and extensive disease. In addition, it also has been previously established that performance status (PS), tumor burden, or the existence of organ metastasis, such as brain, bone, or liver metastasis, were closely related to unfavorable prognosis.4,5 However, outcomes markedly vary between patients, even between those with similar clinicopathological features.
Blood-based markers, such as the albumin-to-gamma-glutamyl transferase ratio,6 neutrophil-to-lymphocyte ratio,7 and platelet-to-albumin ratio,8 have been well established to be reliable prognostic indicators in an increasing number of cancer types. Further, they can be obtained rapidly, and the tests are cost-effective. As another blood-based marker, the albumin-to-alkaline phosphatase ratio (AAPR) has already been reported to be a novel indicator for the prognosis of hepatocellular carcinoma in 2015.9 Further, it was later found to be associated with the prognosis of several other cancer types.10,14 However, to our best knowledge, its prognostic value in patients with ED-SCLC has never been studied. Therefore, the present study aimed to investigate whether AAPR was independently correlated to OS in patients with ED-SCLC.
Materials and Methods
Study Design and Patients
This was a retrospective cohort study that used AAPR and OS as the target-independent and -dependent variable, respectively. AAPR was obtained when the patients were initially diagnosed before receiving any anti-cancer treatment. The OS information was obtained by regular follow-up and then defined as dichotomous variable (1=dead; 0=alive).
We evaluated consecutive patients with ED-SCLC who were admitted to Guangxi Medical University Affiliated Tumor Hospital, Guangxi Province City, China between March 5, 2009 and August 31, 2018. The inclusion criteria were histologically confirmed diagnosis of SCLC; extensive disease of SCLC identified by positron emission tomography (PET)-computed tomography (CT), or enhanced CT combined with bone emission computed tomography (ECT) scan and then staged according to the staging system of the Veterans Administration Lung Study Group, and availability of clinical electronic data before any anti-cancer treatment. The exclusion criteria were concurrent or secondary malignancies; having a combination of disease, such as hepatitis, cholecystitis, and nephrotic syndrome that may potentially interfere with the outcome analysis; active infection such as obstructive pneumonia requiring anti-biotic treatment; or bone fractures. In total, 224 patients were included in the study. They were further categorized according to an AAPR cutoff of 0.35.
This study was approved by the Ethics Committee of Guangxi Medical University Affiliated Tumor Hospital. The need for informed consent was waived owing to the retrospective nature of the study.
Data Collection
Data were collected from the hospital’s electronic medical record system. All data were anonymized to ensure privacy. The independent variable AAPR was calculated as the ratio of ALB to ALP, information on both of which was obtained from the hospital database at the time of hospitalization. The dependent variable OS was calculated from the date of ED-SCLC diagnosis to the date of death or the last follow-up. The follow-up interval was every 2 months. The last follow-up was on August 31, 2019.
The covariates used in this study were classified as follows: (1) sociodemographic data; (2) clinicopathological data; and (3) variables that may influence AAPR or OS based on previous literatures; Therefore, the following variables were used to construct the fully adjusted model: age, sex, Eastern Cooperative Oncology Group Performance Status (ECOG-PS), smoking history, type of metastasis (bone, lung, liver, brain, adrenal gland, other organs) or malignant pleural effusion, sum of organ metastasis (≤3, >3), efficacy of first-line therapy, and sum of treatment lines (<2, ≥2).
ECOG-PS was used to evaluate the patients’ physical status. Non-smokers were defined as those who had smoked no more than 100 cigarettes in their lifetime and smokers as those who had stopped smoking for <1 year or who are current smokers before diagnosis.
Statistical Analysis
Descriptive statistics were presented as mean ±SD for continuous variables and as frequency (%) for categorical variables. Either Student’s t test or Kruskal Wallis H-test was used depending on whether the continuous variables showed normal or skewed distribution. The Student’s t test and the Pearson’s chi-square test were used to assess the continuous variables and categorical variables, respectively. Fisher’s exact test was applied if theoretical frequency existing in cells of 2x2 tables was less than 5. However, if the frequency in 25% of cells in table >2x2 was less than 5, the Freeman-Halton extension of Fisher’s test was used for the comparisons.15 The relationship between pretreatment AAPR, clinicopathological features and OS were analyzed using logistic regression analyses. The effects of AAPR on OS were evaluated using Kaplan–Meier curves (Log rank test). The hazard ratios (HRs) and 95% confidence intervals (CIs) for the risk of death associated with AAPR and each subgroup as defined according to the AAPR cutoff value (dichotomous variable) were estimated using Cox proportional hazards models with adjustment for pertinent variables. The criteria for selection of variables used for adjustment was that if the change in the effect estimate was more than 10% after an adjusted variable or P value in the univariable analysis was less than 0.05, this variable should be adjusted in Cox proportional hazards models. To address for nonlinearity of AAPR and overall survival, a Cox proportional hazards regression model with cubic spline functions and smooth curve fitting (penalized spline method) was performed.16,17 If nonlinearity was found, we first calculated the inflection point using recursive algorithm, and then constructed a two-piecewise Cox proportional hazards model on both sides of the inflection point. Subgroup analyses were also performed according to the identified covariates. A two-tailed p<0.05 was considered statistically significant in all analyses. Data were analyzed using the statistical package R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria) and Empower (X&Y Solutions, Inc. Boston, Massachusetts).
Results
Clinical Characteristics of Selected Patients
The average patient age was 60.51± 8.73 years, and 87.95% were men. There were no significant differences in age; smoking history; ECOG PS; existence of bone, lung, brain, liver metastasis and malignant pleural effusion; sum of metastatic organs; first-line treatment efficacy, and sum of treatment lines between the patients in the AAPR <0.35 and those in the AAPR ≥0.35 groups (all p>0.05). However, there were more male patients in the AAPR<0.35 group (p=0.034). The patient characteristics are shown in Table 1.
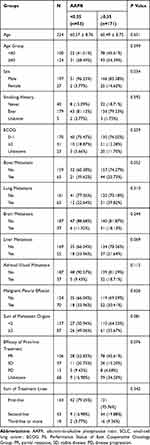 |
Table 1 Baseline Clinicopathological Features in the Present Extensive SCLC Cohort (n=224) |
Nonlinearity of AAPR and Risk of Death in Patients with ED-SCLC
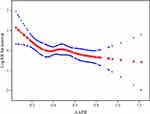
Smooth curve and the result of Cox proportional hazards regression model with cubic spline functions showed a non-linear relationship between AAPR and the risk of death after adjusting for age, sex, ECOG PS, smoking history, existence of metastasis (bone, lung, liver, brain) and malignant plural effusion, sum of organ metastasis (≤3, >3), efficacies of first-line treatment, and sum of treatment lines(<2, ≥2) (Figure 1). Using two-piecewise Cox proportional hazards model and recursive algorithm, we calculated the inflection (cut-off) point to be 0.35. On the left side of the inflection point, the hazard ratio (95% CI) was 0.04 (0.00–0.70) (p=0.0268). On the right side of inflection point, the hazard ratio (95% CI) was 0.52 (0.16–1.64) (p=0.2659).
Univariate Analysis of the Relationship Between AAPR and the Risk of Death in Patients with ED-SCLC
The results of univariate analyses are shown in Table 2. The univariate Cox proportional hazards model showed that age ≥60 years, male sex, smoking history, ECOG PS ≥2, existence of organ metastasis, stable disease (SD) and disease of progression (PD) of first-line treatment, and sum of metastasis organs ≥2 were unfavorable predictors for OS in patients with ED-SCLC. Interestingly, we also noted that female seemed to be a favorable predictor if the AAPR was ≥0.35, with an HR of 0.65 (95% CI=0.37–1.15). However, female was associated with a risk of death in those with AAPR <0.35, with an HR of 3.92 (95% CI=0.89–17.21).
 |
Table 2 Univariate Cox Regression Analysis of Prognostic Factors for Overall Survival in ED-SCLC |
Unadjusted and Adjusted Cox Proportional Hazards Model
We developed three models to identify the independent effects of AAPR on OS (univariate and multivariate Cox proportional hazards model). The HR and 95% CI are listed in Table 3. In the unadjusted model (model 1), the model-based effect size can be explained as the difference in the association with OS according to the AAPR groups (<0.35 and ≥0.35). The HR of 1.55 (95% CI=1.07–2.25, p<0.05) in the unadjusted model indicates that AAPR <0.35 is associated with 55% higher risk of death compared with AAPR ≥0.35. In the fully adjusted model (model 3, adjusted all covariates presented in Table 1), the AAPR <0.35 group had an HR of 1.51 (95% CI=1.04–2.21, p=0.0325), while the APPR ≥0.35 group had an HR of 1.65\ (95% CI=1.11–2.46, p=0.014).
 |
Table 3 Multiple Cox Regression Analysis of AAPR in Patients with Extensive-disease SCLC Unadjusted Model |
Figure 2 shows the Kaplan–Meier OS curves stratified by AAPR cutoff. The median OS in the AAPR <0.35 and the AAPR ≥0.35 groups was 9.73 months (95% CI=8.6–12.33) and 13.7 months (95% CI=11.43–16.37), respectively, with significant difference (log-rank p=0.019).
 |
Figure 2 Kaplan–Meier curves of overall survival in patients with extensive SCLC stratified by AAPR. |
Subgroup Analysis
AAPR was negatively associated with the risk of death in patients with ED-SCLC. To further understand this negative association, subgroup analyses were performed after stratification using potential covariates listed in Table 1. The HR and 95% CI are listed in Table 4. Compared to the AAPR ≥0.35 group, the trends of the HRs were increased across all subgroups after stratification in the AAPR <0.35 group. There were significant differences in the risk of death according to age ≥60, female sex, ECOG PS 0–1, existence of metastasis to the liver or adrenal gland, sum of metastatic organs ≥2, and PD at first-line treatment (all p<0.05). The estimated HRs and its 95% CIs of subgroups were presented using a forest plot (Figure 3).
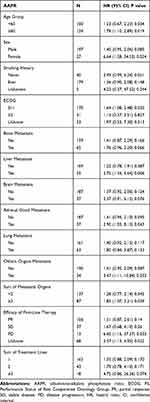 |
Table 4 Subgroup Analysis Using Potential Confounders as the Stratification Variables |
 |
Figure 3 The association between AAPR and the risk of death in various sub-groups in the present extensive SCLC study. |
Discussion
The prognosis of patients with ED-SCLC remains poor, with a median OS of only 9–11 months.2 Identifying the patients with high risk of poor prognosis is crucial in the management of ED-SCLC. Accordingly, exploration and identification of prognostic indicators are important. To the best of our knowledge, this retrospective study was the first to investigate the association between AAPR and OS in patients with ED-SCLC. We found that pretreatment AAPR was an independent factor for prognosis, and thus it may be considered as a prognostic indicator among patients with ED-SCLC in clinical practice.
Albumin and alkaline phosphatase, both of which are important indicators of liver function, have been routinely evaluated at different timepoints during diagnosis, treatment, and follow-ups. Albumin as an indicator for assessing the nutritional status is specifically synthesized by the liver. Hypoalbuminemia not only reflects impaired liver functions but also the protein consuming capacity caused by various diseases, particularly by aggressively growing tumor types.18 Meanwhile, hypoalbuminemia may impair the metabolism and the function of immune cell, stabilization of cell growth, and maintenance of biochemical diversity, which subsequently results in weakened immunity, occurrence of infectious lesions, and a poor response to anti-cancer treatment.19 ALP as a member of hydrolase enzymes is ubiquitously expressed, but at a higher level, in the liver, bile duct, bone, and kidneys. It has been reported that ALP level is closely associated with bone metastasis and liver and kidney diseases.20–22 ALP has also been reported to be a regulator of immune response and inflammatory signaling pathway.23 Additionally, some studies established that cancer cells can express ALP and that ALP is involved in tumor growth regulation, metastasis, and progression.24 Thus, its potential prognostic values in cancer patients beyond bone metastasis or hepatocellular carcinoma (HCC) should be explored.
Chan et al firstly reported that AAPR was an independent prognostic indicator for OS and disease-free survival in HCC patients in 2015.9 Since then, AAPR as a novel prognostic candidate has been reported in various cancer types, such as nasopharyngeal carcinoma,14,25 cholangiocarcinoma,12 non-small cell lung cancer,11 and renal cell carcinoma.10 Consistent with previous studies, we found that decreased AAPR was closely correlated to poor clinical outcomes. Notably, the cut-off point of AAPR for predicting prognosis may differ among different cancer types. The appropriate AAPR cut-off point might even differ according to the disease status for the same disease. For example, Li et al used a cut-off point of 0.61 to analyze the prognostic significance of AAPR in limited-stage SCLC patients who received definitive chemotherapy and radiotherapy.26 Another study found that an AAPR cut-off point of 0.36 was suitable for predicting OS in patients with advanced NSCLC.11 Thus, the optimal cut-off point should be further investigated according to the specific cancer types.
Generally, the optimal cut-off values can be determined by three methods. The first one involves using a biostatistical software to automatically calculate the cut-off values, such as Cutoff Finder and X-tile. The second one entails using the ROC curve to find the optimal cut-off point based on the values for sensitivity and specificity. ROC curve relies on sensitivity and specificity and is widely used in diagnostic research and for the evaluation of efficiency of various predictive models. The third-one requires using the cubic spline functions and smooth curve fitting combined with flection-point calculation, which can directly exhibit the relationship between dependent and independent variables.16 It can show the threshold effect and saturation effect simultaneously. Moreover, smooth curve fitting and threshold/saturation effect can be adjusted by variables. In the present study, we choose the third method to elucidate the relationship between AAPR and OS.
Univariate analysis showed an increased risk of death in patients who are elderly, male, have smoking history, and have organ metastasis regardless of the AAPR value. Meanwhile, female seemed to be a favorable predictor of prognosis if the AAPR was ≥0.35, with an HR 0.65 (95% CI=0.37–1.15). However, when the AAPR was <0.35, femalebecame an unfavorable predictor of the risk of death, with an HR of 3.92 (95% CI=0.89–17.21, p=0.1366). Contrary results were also found in patients with brain metastasis and an AAPR of ≥0.35 (HR=0.94, 95% CI=0.49–1.82, p=0.8581) and in patients without brain metastasis but with an AAPR of <0.35 (HR=1.54, 95% CI=0.54–4.44, p=0.4193). The inconsistent results may be attributed in part to the small number of patients after stratification.
To further identify the prognostic value of AAPR in ED-SCLC, we conducted subgroup analysis according to the identified covariates and found that lower AAPR was associated with poor OS across all subgroups. The tendency remained unchanged after adjusting for the covariates, indicating that AAPR was an independent predictor of prognosis of ED-SCLC. Interestingly, we found that compared with patients without bone metastasis, the risk of death was increased by 71% in patients with bone metastasis. The HR was 1.71, which was lower than in those who had metastasis in other organs beyond the bone, such as the liver, lung, or brain. This indicated that the clinical outcomes differ between those with bone metastasis and those with metastasis to internal organs.
Our study has several strengths. First, to our best knowledge, this is the first study to validate the predictive value of AAPR for OS in patients with ED-SCLC. The findings of this study should be helpful for future research on the establishment of diagnostic or predictive models of OS in these patients. Second, because this was an observational study and therefore susceptible to potential confounding, we used strict statistical adjustment to minimize residual confounders to elucidate the association between AAPR and OS. Third, we conducted a subgroup analysis to verify our results in certain subgroups and to enhance the robustness of the results. Finally, we evaluated ALB and ALP and other covariates that can be easily determined in clinical practice.
However, this study also has some limitations. First, this was a retrospective study conducted in a single institute, and we did not include an independent and prospective cohort to validate the prognostic value of AAPR. Second, we exclusively evaluated patients with ED-SCLC, and thus the generalizability of our findings is limited. Third, because we excluded patients who had concurrent liver or renal diseases as these conditions may influence the ALB or ALP, our study findings also cannot be applied for these patients. Lastly, some variables including comorbidity scores (such as the Charlson comorbidity index) and biochemical markers (such as lactate dehydrogenase, C-reactive protein, and coagulation index), that may also influence OS, were not included for analysis due to missing data. Further studies with larger sample sizes and a multi-center and prospective design are needed.
Conclusion
Our findings indicated that pretreatment AAPR influences OS in patients with ED-SCLC and thus has the potential to be a novel prognostic indicator for these patients.
Abbreviations
AAPR, albumin-to-alkaline phosphatase ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group Performance Status; ECT, emission computer tomography; ED, extensive disease; ED-SCLC, extensive-disease small-cell lung cancer; HRs, hazard ratios; HCC, hepatocellular carcinoma; OS, overall survival; PS, performance status; SCLC, small-cell lung cancer.
Acknowledgment
The authors thank all the staff members in our institution.
Funding
This work was supported by the “139 Talent Planning” granted by Guangxi Health Commission, Guangxi Zhuang Autonomous Zone, China (Granted number: 201903030).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistic, 2017. CA-Cancer J Clin. 2017;1(67):7–30.
2. Van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;9804(378):1741–1755. doi:10.1016/S0140-6736(11)60165-7
3. Sculier JP, Chansky K, Crowley JJ. The impact of additional prognostic factors on survival and their relationship with the anatomical extent of disease expressed by the 6th Edition of the TNM classification of malignant tumors and the proposals for the 7th Edition. J Thorac Oncol. 2008;5(3):457–466.
4. Li J, Dai CH, Chen P. Survival and prognostic factors in small cell lung cancer. Med Oncol. 2010;1(27):73–81. doi:10.1007/s12032-009-9174-3
5. Paesmans M, Sculier JP, Lecomte J. Prognostic factors for patients with small cell lung carcinoma: analysis of a series of 763 patients included in 4 consecutive prospective trials with a minimum follow-up of 5 years. Cancer-Am Cancer Soc. 2000;3(89):523–533.
6. He Z, Duan H, Lin F, et al. Pretreatment neutrophil-to-lymphocyte ratio plus albumin-to-gamma-glutamyl transferase ratio predict the diagnosis of grade III glioma. Ann Transl Med. 2019;7(22):623. doi:10.21037/atm
7. Zer A, Sung MR, Walia P, et al. Correlation of neutrophil to lymphocyte ratio and absolute neutrophil count with outcomes with PD-1 axis inhibitors in patients with advanced non-small-cell lung cancer. Clin Lung Cancer. 2018;19(5):426–434. doi:10.1016/j.cllc.2018.04.008
8. Shirai Y, Shiba H, Haruki K, et al. Preoperative platelet-to-albumin ratio predicts prognosis of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Anticancer Res. 2017;37(2):787–793. doi:10.21873/anticanres.11378
9. Chan AW, Chan SL, Mo FK, et al. Albumin-to-alkaline phosphatase ratio: a novel prognostic index for hepatocellular carcinoma. Dis Markers. 2015;2015:564057. doi:10.1155/2015/564057
10. Xia A, Chen Y, Chen J, Pan Y, Bao L, Gao X. Prognostic value of the albumin-to-alkaline phosphatase ratio on urologic outcomes in patients with non-metastatic renal cell carcinoma following curative nephrectomy. J Cancer. 2019;10(22):5494–5503. doi:10.7150/jca.34029
11. Li D, Yu H, Li W. Albumin-to-alkaline phosphatase ratio at diagnosis predicts survival in patients with metastatic non-small-cell lung cancer. Onco Targets Ther. 2019;12:5241–5249.
12. Xiong JP, Long JY, Xu WY, et al. Albumin-to-alkaline phosphatase ratio: a novel prognostic index of overall survival in cholangiocarcinoma patients after surgery. World J Gastrointest Oncol. 2019;11(1):39–47. doi:10.4251/wjgo.v11.i1.39
13. Tan P, Xie N, Ai J, et al. The prognostic significance of albumin-to-alkaline phosphatase ratio in upper tract urothelial carcinoma. Sci Rep. 2018;8(1):12311. doi:10.1038/s41598-018-29833-5
14. Nie M, Sun P, Chen C, et al. Albumin-to-alkaline phosphatase ratio: a novel prognostic index of overall survival in cisplatin-based chemotherapy-treated patients with metastatic nasopharyngeal carcinoma. J Cancer. 2017;8(5):809–815. doi:10.7150/jca.17536
15. Oster RA, Hilbe JM. An examination of statistical software packages for parametric and nonparametric data analyses using exact methods. Am Stat. 2008;1(62):74–84.
16. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer-Verlag; 2001.
17. Lin L, Changzhong C, Xiaodan Y. Threshold effect analysis using Empower Stats software. Chin J Epidemiol. 2013;11(34):1139–1141.
18. Arroyo V, García-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61:396–407. doi:10.1016/j.jhep.2014.04.012
19. Alwarawrah Y, Kiernan K, MacIver NJ. Changes in nutritional status impact immune cell metabolism and function. Front Immunol. 2018;9:1055. doi:10.3389/fimmu.2018.01055
20. Giljaca V, Stimac D, Gluud C. Are levels of alkaline phosphatases and bilirubin surrogate markers of outcomes of patients with primary biliary cirrhosis? Gastroenterology. 2015;148(4):860. doi:10.1053/j.gastro.2014.11.050
21. Mayne PD, Thakrar S, Rosalki SB, Foo AY, Parbhoo S. Identification of bone and liver metastases from breast cancer by measurement of plasma alkaline phosphatase isoenzyme activity. J Clin Pathol. 1987;40:398–403. doi:10.1136/jcp.40.4.398
22. Al Mamari S, Djordjevic J, Halliday JS, Chapman RW. Improvement of serum alkaline phosphatase to < 1.5 upper limit of normal predicts better outcome and reduced risk of cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol. 2013;58:329–334. doi:10.1016/j.jhep.2012.10.013
23. Rader BA. Alkaline phosphatase, an unconventional immune protein. Front Immunol. 2017;8:897. doi:10.3389/fimmu.2017.00897
24. Rao SR, Snaith AE, Marino D. Tumour-derived alkaline phosphatase regulates tumour growth, epithelial plasticity and disease-free survival in metastatic prostate cancer. Br J Cancer. 2017;2(116):227–236.
25. Kim JS, Keam B, Heo DS, et al. The prognostic value of albumin-to-alkaline phosphatase ratio before radical radiotherapy in patients with non-metastatic nasopharyngeal carcinoma: a propensity score matching analysis. Cancer Res Treat. 2019;51(4):1313–1323. doi:10.4143/crt.2018.503
26. Li X, Li B, Zeng H, et al. Prognostic value of dynamic albumin-to-alkaline phosphatase ratio in limited stage small-cell lung cancer. Future Oncol. 2019;15(9):995–1006. doi:10.2217/fon-2018-0818
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.