Back to Journals » OncoTargets and Therapy » Volume 7
Prognostic value of preoperative inflammatory markers in Chinese patients with breast cancer
Authors Yao M, Liu Y, Jin H, Liu X, Lv K, Wei H, Du C, Wang S, Wei B, Fu P
Received 19 June 2014
Accepted for publication 16 July 2014
Published 26 September 2014 Volume 2014:7 Pages 1743—1752
DOI https://doi.org/10.2147/OTT.S69657
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Minya Yao,1 Yu Liu,1 Hailong Jin,2 Xiaojiao Liu,1 Kezhen Lv,1 Haiyan Wei,1 Chengyong Du,1 Shuqian Wang,1 Bajin Wei,1 Peifen Fu1
1Department of Breast Center, 2Gastrointestinal Surgery, First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang Province, People's Republic of China
Abstract: Cancer-associated inflammation is a key determinant of disease progression and survival in most cancers. The aim of our study was to assess the predictive value of preoperative inflammatory markers, such as the neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio, red cell distribution width (RDW), and mean platelet volume, for survival in breast cancer patients. In total, 608 breast cancer patients operated on between January 2009 and December 2011 were included in this observational study. The association between preoperative inflammatory markers and survival outcomes was analyzed. Patients with high NLR (>2.57) or high RDW (>13.45%) showed a significantly lower overall survival rate than those with lower NLR (≤2.57) or lower RDW (≤13.45%). NLR and RDW, along with node stage and molecular subtypes, were independent prognostic factors. There was a significant survival difference according to NLR in the luminal A and triple-negative subtypes (93.3% versus 99.3%, P=0.001; 68.8% versus 95.1%, P=0.000, respectively). The triple-negative subtype was the only subtype in which higher RDW patients showed significantly poor prognosis (81.3% versus 95.5%, P=0.025). Pre-operation NLR and RDW is a convenient, easily measured prognostic indicator for patients with breast cancer, especially in patients with the triple-negative subtype.
Keywords: neutrophil-to-lymphocyte ratio, NLR, red cell distribution width, RDW, overall survival
Introduction
Breast cancer is the most common cancer among females, and the incidence has increased greatly in recent years. Despite advances in treatment and the fact that mortality has dropped since 1990, breast cancer remains the leading cause of cancer death in females worldwide.1,2 Patient age, lymph node stage, tumor size, histological traits, hormonal receptors, human epidermal growth factor receptor 2 (HER2) status, and molecular typing are used for the stratification of breast cancer patients for prognostic purposes and for determining the appropriate treatment strategy.3,4 Nevertheless, some patients present with combinations of features/markers and thus have very different clinical outcomes.
It is now widely recognized that outcomes in patients with cancer are not determined by tumor characteristics alone, but patient-related factors are also key factors.5 Cancer-associated inflammation is a key determinant of disease progression and survival in most cancers.6,7 The inflammatory response involves systemic alterations triggered by circulating cytokines and chemokines, such as an increase in neutrophil count or a slight increase in platelet count.8 In addition, there are other parameters like red cell distribution width (RDW) and mean platelet volume (MPV) that are routine and easy-to-measure inflammatory markers.9–11 Studies have shown that the neutrophil–lymphocyte ratio (NLR) and platelet–lymphocyte ratio (PLR) may be predictive of mortality in various cancer populations, including breast, lung, colon, stomach, liver, pancreatic, and esophageal cancer,12–20 but there is no study regarding these markers in breast cancer patients from the People’s Republic of China. On the other hand, a few studies regarding RDW and MPV have suggested that they might be associated with cancer prognosis. Thus, the aim of this study was to determine the prognostic value of NLR, PLR, RDW, and MPV in Chinese breast cancer patients.
Materials and methods
Patients and methods
A retrospective analysis was conducted of 608 female patients who were diagnosed with primary breast cancer and who were operated on at the First Affiliated Hospital of Zhejiang University School of Medicine (Hangzhou, People’s Republic of China) from January 2009 to December 2011.
Medical records were reviewed, and each patient’s medical history, age, and pathologic results (such as tumor size, lymph node status, hormonal status, HER2, and laboratory data) were obtained.
Estrogen receptor (ER) and progesterone receptor (PR) status were obtained from immunohistochemistry, and a value ≥10% was considered positive. HER2 status was obtained from immunohistochemistry or fluorescent in situ hybridization (FISH). The C-erbB-2 scores of three in immunohistochemistry or with a ≥2.2-fold increase in HER2 gene amplification, as determined by FISH, were considered to be positive for HER2. The C-erbB-2 scores of two in immunohistochemistry without FISH were considered to be uncertain for HER2.
Molecular subtype was determined using the following criteria: luminal A, ER-positive and/or PR-positive and HER2-negative; luminal B, ER-positive and/or PR-positive and HER2-positive; HER2 enriched, ER- and PR-negative with positive HER2; and triple-negative, ER-negative, PR-negative, and HER2-negative.12
Complete blood count (CBC) test results were obtained within 1 week prior to surgery. The NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count, and PLR was defined as the absolute platelet count divided by the absolute lymphocyte count. RDW and MPV values were obtained directly from the CBC test (normal range: 11.5%–14.5% and 7.4–12.5 fl).
Patients with any inflammatory signs or conditions, hematological disease, coronary artery disease, end-stage renal disease, heart failure, cerebrovascular disease, peripheral arterial disease, or a lack of information pertaining to pathologic or laboratory results were excluded. We also excluded patients undergoing neoadjuvant chemotherapy, those who were lost to follow-up, or those who died from causes other than breast cancer.
Patients were followed up every 3–6 months for the first 2 years after the operation, then annually. The last follow up was in March 2014.
The study was approved by the Ethical Committees of the First Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, People’s Republic of China.
Statistical analysis
Disease-free survival (DFS) and overall survival (OS) were calculated from the date of operation to the date of disease recurrence and death, respectively. The cutoff values of NLR, PLR, RDW, and MPV were determined using receiver operating characteristic (ROC) curve analysis, and the dependent variable was the DFS for 2 years. The optimal cutoff levels for NLR, PLR, RDW, and MPV were established at 2.57, 107.64, 13.45, and 9.05, respectively, and these cutoff values were used to categorize high and low NLR, PLR, RDW, or MPV groups. ROC curves were also plotted to verify the accuracy of NLR and RDW for OS prediction. The association between each marker and DFS and OS was analyzed using the Kaplan–Meier method with the log-rank test. Frequency distributions between categorical variables among the groups were compared using the chi-square test. If the expected frequency was <5, Fisher’s exact test was used. Univariate analysis was used to assess significant differences in clinical characteristics, and variables with P<0.10 were entered into multivariate analyses. A P-value of <0.05 was considered statistically significant. Analyses were performed using SPSS (IBM Corporation, Armonk, NY, USA) software, version 19.0.
Results
Baseline characteristics and factors affecting prognosis
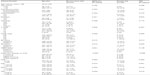
Among the 608 patients in this study, the age range was 26–86 years, and the mean age was 52.4±10.8 years. The follow-up time ranged from 8 to 62 months, and the median follow-up time was 42 months. The baseline characteristics of the study subjects are shown in Table 1.
The median levels of NLR, PLR, RDW, and MPV were 1.75 (0.55–7.22), 117.28 (13.33–428.29), 13% (11.6%–20.2%), and 11 fl (6.6–14.9 fl), respectively.
To identify factors for breast cancer DFS and OS, 15 potential variables of interest were analyzed by univariate analysis, as listed in Table 1. T (tumor) stage, node stage, ER status, PR status, and molecular subtype were significantly associated with DFS and OS. HER2 status was only significantly associated with DFS. We found that none of NLR, PLR, RDW, or MPV were associated with DFS. However, NLR and RDW were significantly associated with OS, and PLR might be associated with OS. Patients with an NLR higher than 2.57 showed a significantly lower 5-year OS rate than did patients with an NLR ≤2.57 (90.2% versus 97.4%, respectively) (Figure 1). Patients with a RDW higher than 13.45% showed significantly lower 5-year OS rate than did patients with RDW ≤13.45% (93.3% versus 97.5%, respectively) (Figure 2).
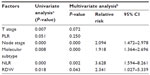
Next, a Cox proportional multivariate hazard model for OS was performed. We found that NLR and RDW, along with node stage and molecular subtype, were independent prognostic factors (Table 2). The hazard ratios for NLR and RDW were 3.628 and 2.341, respectively.
The ROC curve for OS prediction revealed an area under the curve of 0.643 (95% confidence interval [CI]: 0.518–0.767, P=0.018) for NLR and 0.624 (95% CI: 0.507–0.740, P=0.040) for RDW. Therefore, NLR was superior to RDW as a predictive factor in patients with breast cancer (Figure 3).
Table 3 shows that NLR >2.57 was associated with diabetes and infiltrative carcinoma, while RDW >13.45% was associated with increased T stage and premenopausal status.
OS stratified by molecular subtype according to NLR and RDW
Both luminal A and triple-negative subtypes in which the NLR was higher than 2.57 showed significantly lower 5-year survival rate than did those types with an NLR ≤2.57 (93.3% versus 99.3%, P=0.001; 68.8% versus 95.1%, P=0.000, respectively). There was no significant survival difference according to NLR in the luminal B and HER2-enriched subtypes (Figure 4).
The triple-negative subtype was the only subtype in which RDW >13.45% showed significantly lower 5-year survival rate than did an RDW ≤13.45% (81.3% versus 95.5%, respectively, P=0.025); there was no significant survival difference according to RDW in the other subtypes (Figure 5).
Discussion
Our study demonstrated that an elevated NLR pre-operation was an independent factor of poor survival in breast cancer patients. This finding is consistent with those from previous reports regarding breast cancer.12–14 However, our results showed only a trend of higher mortality with elevated PLR (P=0.051). In this study, the cutoff value for NLR was 2.57, which is approximately equal to 2.5 in the Noh et al study13 and lower than 3.3 in the Azab et al studies.12,14 It is interesting to note that patients in Noh et al’s study13 and our study were Asian, while in the Azab et al study,12,14 the subjects were American and the authors had divided patients into four groups; therefore, these differences might be attributed to population differences.
Although there are a great many studies showing an association between high NLR and poor prognosis in cancer, the exact mechanisms underlying this relationship are unclear. One possible hypothesis is that cancer-associated inflammation, as a chronic systemic inflammatory response, impacts patient survival.21 The inflammatory response involves systemic alterations triggered by circulating cytokines and chemokines, such as an increase in neutrophil and platelet counts, and a decline in lymphocyte counts. Tumor-related neutrophils play important roles in enhanced angiogenesis, tumor growth, and metastasis.8 Some studies have established systemic inflammation-based prognostic scores before surgery.22,23 For instance, a study by Proctor et al23 showed that systemic inflammation-based scores, including the modified Glasgow Prognostic Score, NLR, PLR, Prognostic Index, and Prognostic Nutritional Index, have prognostic value for a variety of cancers. NLR without other inflammatory markers, on the other hand, may not provide clinicians with information about the inflammatory condition of the patient.24 RDW and MPV are also routine inflammatory markers.11
The exact mechanisms of how inflammation influences RDW levels are unknown, but potential mechanisms include impairing iron metabolism, inhibiting the response to erythropoietin, and decreasing red blood cell survival via the production of inflammatory markers.25 A study by Seitanides et al26 suggested that elevated RDW is significantly correlated with disseminated solid malignancies to the bone marrow. Another study by Koma et al27 indicated that high levels of RDW are associated with poor survival in lung cancer. A breast cancer study by Seretis et al28 showed that elevated RDW could be a useful biomarker to distinguish between benign or malignant breast tumors, and elevated RDW is significantly correlated with primary tumor diameter and the absolute number of the infiltrated axillary lymph nodes.
To our knowledge, our study is the first to analyze the relationship between RDW or MPV and prognosis in breast cancer patients. We found a significant association between high RDW and poor breast cancer prognosis, and it was an independent factor of poor survival. However, compared with NLR, ROC curves verified that NLR was superior to RDW as a predictive factor in patients with breast cancer.
We did not identify any predictive value for MPV. However, MPV measurements are complex, and the delay in time between sample collection and laboratory analysis might have affected our results.29
In this study, we also focused on the relationship between NLR or RDW and the prognosis according to subtype. In contrast to the study by Noh et al,13 our results showed that elevated NLR was significantly associated with poor prognosis not only for luminal A, but for triple-negative subtypes. Moreover, it demonstrated a better discrimination for the triple-negative subtype in terms of P-value than did the luminal A subtype (P=0.001 versus P=0.000, respectively). Our results also showed that elevated RDW is significantly associated with poor prognosis for the triple-negative subtype only. Triple-negative breast cancer is well known to have a poor prognosis compared with other subtypes, which is a hot topic in breast cancer research. Thus, the triple-negative subtype may be more influenced by a chronic systemic inflammatory response. A prospective study by Retsky et al30 showed that the nonsteroidal anti-inflammatory drug ketorolac used perioperatively suppresses early breast cancer relapse, which has particular relevance to the triple-negative subtype. On the other hand, inflammation may regulate the host’s immune reaction. It is plausible that host cell-mediated immunity continues to exert important destructive effects on any residual tumor cells and micrometastases.12 Engel et al31 found that triple-negative breast cancer cells stimulate a significantly stronger natural killer cell immune response than ER-positive breast cancer cells, and infiltration of immunosuppressive T-regs (CD4+ T-cells, CD8+ T-cells, and forkhead box P3-positive [Foxp3] regulatory T-cells) increased in human triple-negative breast cancer specimens. Many studies report that high levels of tumor-infiltrating lymphocytes are associated with a good outcome in patients with triple-negative breast cancer.32–34 The precise mechanism in which NLR and RDW exert their effect, especially in triple-negative subtypes, should be further studied. Our study had a short mean follow-up duration and was a single-center retrospective study. Results with longer follow-up should be performed in the future, and a multicentric perspective study is warranted.
Conclusion
Preoperative NLR and RDW is a convenient, easily measured prognostic indicator in patients with breast cancer, especially those with the triple-negative subtype. These results suggest that cancer-associated inflammation in triple-negative breast cancer may play a greater role in promoting breast cancer progression than the other subtypes. However, further validation studies are required.
Acknowledgment
We thank the Health and Family Planning Commission of Zhejiang Province for medical and health research projects funding (2012KYA071).
Disclosure
The authors report no conflicts of interest in this work.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. | |
DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64(1):52–62. | |
Schnitt SJ. Classification and prognosis of invasive breast cancer: from morphology to molecular taxonomy. Mod Pathol. 2010;23 Suppl 2:S60–S64. | |
Rakha EA, Ellis IO. Modern classification of breast cancer: should we stick with morphology or convert to molecular profile characteristics. Adv Anat Pathol. 2011;18(4):255–267. | |
Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–230. | |
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. | |
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. | |
Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. | |
Demirkol S, Balta S, Unlu M, et al. Evaluation of the mean platelet volume in patients with cardiac syndrome X. Clinics (Sao Paulo). 2012;67(9):1019–1022. | |
Demirkol S, Balta S, Cakar M, Unlu M, Arslan Z, Kucuk U. Red cell distribution width: a novel inflammatory marker in clinical practice. Cardiol J. 2013;20:209. | |
Balta S, Demirkol S, Kucuk U, Sarlak H, Kurt O, Arslan Z. Neutrophil to lymphocyte ratio may predict mortality in breast cancer patients. J Breast Cancer. 2013;16(3):354–355. | |
Azab B, Bhatt VR, Phookan J, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19(1):217–224. | |
Noh H, Eomm M, Han A. Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J Breast Cancer. 2013;16(1):55–59. | |
Azab B, Shah N, Radbel J, et al. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med Oncol. 2013;30(1):432. | |
Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137(2):425–428. | |
Urrejola GI, Bambs CE, Espinoza MA, et al. [An elevated neutrophil/lymphocyte ratio is associated with poor prognosis in stage II resected colon cancer]. Rev Med Chil. 2013;141(5):602–608. Spanish. | |
Lee S, Oh SY, Kim SH, et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. 2013;13:350. | |
Lai Q, Castro Santa E, Rico Juri JM, Pinheiro RS, Lerut J. Neutrophil and platelet-to-lymphocyte ratio as new predictors of dropout and recurrence after liver transplantation for hepatocellular cancer. Transpl Int. 2014;27(1):32–41. | |
Stotz M, Gerger A, Eisner F, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109(2):416–421. | |
Feng JF, Huang Y, Chen QX. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factoring patients with esophageal squamous cell carcinoma. World J Surg Oncol. 2014;12:58. | |
McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12(3):223–226. | |
Mohri Y, Tanaka K, Ohi M, Yokoe T, Miki C, Kusunoki M. Prognostic significance of host- and tumor-related factors in patients with gastric cancer. World J Surg. 2010;34:285–290. | |
Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47(17):2633–2641. | |
Balta S, Demirkol S, Cakar M, Arslan Z, Unlu M, Celik T. Other inflammatory markers should not be forgotten when assessing the neutrophil-to-lymphocyte ratio. Clin Appl Thromb Hemost. 2013;19(6):693–694. | |
de Gonzalo-Calvo D, de Luxán-Delgado B, Rodríguez-González S, et al. Interleukin 6, soluble tumor necrosis factor receptor I and red blood cell distribution width as biological markers of functional dependence in an elderly population: a translational approach. Cytokine. 2012;58(2):193–198. | |
Seitanides B, Giakoumakis G, Tsakona C. Increased red cell volume distribution width in patients with bone marrow metastases. J Clin Pathol. 1988;41(11):1246. | |
Koma Y, Onishi A, Matsuoka H, et al. Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PLoS One. 2013;8(11):e80240. | |
Seretis C, Seretis F, Lagoudianakis E, Gemenetzis G, Salemis NS. Is red cell distribution width a novel biomarker of breast cancer activity? Data from a pilot study. J Clin Med Res. 2013;5(2):121–126. | |
Varol E. Mean platelet volume and neutrophil-to-lymphocyte ratio in patients with Henoch-Schonlein purpura. Rheumatol Int. Epub April 20, 2014. | |
Retsky M, Rogers R, Demicheli R, et al. NSAID analgesic ketorolac used perioperatively may suppress early breast cancer relapse: particular relevance to triple negative subgroup. Breast Cancer Res Treat. 2012;134(2):881–888. | |
Engel JB, Honig A, Kapp M, et al. Mechanisms of tumor immune escape in triple-negative breast cancers (TNBC) with and without mutated BRCA 1. Arch Gynecol Obstet. 2014;289(1):141–147. | |
Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. | |
Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14(2):R48. | |
Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860–867. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.