Back to Journals » OncoTargets and Therapy » Volume 11
Prognostic value of platelet-to-lymphocyte ratio in pancreatic cancer: a comprehensive meta-analysis of 17 cohort studies
Authors Zhou Y, Cheng S, Fathy AH, Qian H, Zhao Y
Received 15 October 2017
Accepted for publication 9 January 2018
Published 5 April 2018 Volume 2018:11 Pages 1899—1908
DOI https://doi.org/10.2147/OTT.S154162
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Yongping Zhou,1 Sijin Cheng,2 Abdel Hamid Fathy,2 Haixin Qian,3 Yongzhao Zhao1,2
1Department of Hepatobiliary, Wuxi Second Hospital, Nanjing Medical University, Wuxi, China; 2Tongji University School of Medicine, Shanghai, China; 3Department of Hepatobiliary, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China
Background and aims: Several studies were conducted to explore the prognostic value of platelet-to-lymphocyte ratio (PLR) in pancreatic cancer and have reported contradictory results. This study aims to summarize the prognostic role of PLR in pancreatic cancer.
Materials and methods: Embase, PubMed and Cochrane Library were completely searched. The cohort studies focusing on the prognostic role of PLR in pancreatic cancer were eligible. The overall survival (OS) and progression-free survival (PFS) were analyzed.
Results: Fifteen papers containing 17 cohort studies with pancreatic cancer were identified. The results showed patients that with low PLR might have longer OS when compared to the patients with high PLR (hazard ratio=1.28, 95% CI=1.17–1.40, P<0.00001; I2=42%). Similar results were observed in the subgroup analyses of OS, which was based on the analysis model, ethnicity, sample size and cut-off value. Further analyses based on the adjusted potential confounders were conducted, including CA199, neutrophil-to-lymphocyte ratio, modified Glasgow Prognostic Score, albumin, C-reactive protein, Eastern Cooperative Oncology Group, stage, tumor size, nodal involvement, tumor differentiation, margin status, age and gender, which confirmed that low PLR was a protective factor in pancreatic cancer. In addition, low PLR was significantly associated with longer PFS when compared to high PLR in pancreatic cancer (hazard ratio=1.27, 95% CI=1.03–1.57, P=0.03; I2=33%).
Conclusion: In conclusion, it was found that high PLR is an unfavorable predictor of OS and PFS in patients with pancreatic cancer, and PLR is a promising prognostic biomarker for pancreatic cancer.
Keywords: platelet-to-lymphocyte ratio, pancreatic cancer, prognostic, progression-free survival, overall survival, biomarker
Introduction
It was estimated that 53,670 cases would be newly diagnosed with pancreatic cancer and 43,090 cases would die from pancreatic cancer in the USA in 2017.1 Although great development of the diagnosis and treatment of pancreatic cancer has been made in recent years, the prognosis of patients with pancreatic cancer remains disappointing.2,3 In 2016, there were an estimated 53,070 patients newly diagnosed with pancreatic cancer and an estimated 41,780 deaths from pancreatic cancer in the USA.4
In view of the poor prognosis outcome of patients with various cancers, more and more attention was paid to explore the predictive factors of cancers.5–8 Regarding pancreatic cancer, several factors might be involved in the prognosis of patients, including mRNA, protein, clinical index and so on.9–12
In recent years, several studies have reported that inflammatory pathways might play an important role in the tumorigenesis and metastasis.13–16 Meanwhile, inflammatory biomarkers are expected to be a prognostic index of pancreatic cancer, including neutrophil-to-lymphocyte ratio (NLR), C-reactive protein (CRP), platelet-to-lymphocyte ratio (PLR) and so on.17–19 Yang et al performed a meta-analysis and found that high peripheral blood PLR suggested a poor prognosis for patients with pancreatic cancer.19 However, there was no consistent conclusion on the role of PLR in pancreatic cancer. Kishi et al analyzed 65 patients with pancreatic cancer and drew the conclusion that PLR was not associated with the prognosis of these patients.20 Nevertheless, other researchers focusing on pancreatic cancer found opposite results, which indicated that patients with low PLR might have longer overall survival (OS) when compared to the patients with high PLR.20–22 On account of these controversies, we performed this meta-analysis to explore the prognostic value of PLR in pancreatic cancer.
Materials and methods
Literature search strategy
The Cochrane Library, PubMed and Embase database were comprehensively searched up to May 2, 2017. The search terms included “pancreatic neoplasm”, “pancreatic cancer”, “PLR”, “platelet-to-lymphocyte ratio”, “platelet lymphocyte ratio” and “platelet-lymphocyte ratio”. The relevant conference papers were also carefully assessed. All the retrieved papers were carefully checked. After scanning the abstracts or titles, the distinctly irrelevant articles were excluded. For the remaining papers, the full text was carefully reviewed.
Inclusion criteria
The study would be included into this meta-analysis if it met all the following criteria: 1) cohort study; 2) focusing on the prognostic value of PLR in pancreatic cancer; 3) enough data to obtain the hazard ratio (HR) for OS, along with their 95% CIs or P-values and 4) published in English.
Exclusion criteria
The exclusion criteria were as follows: 1) comments, reviews, case reports and expert opinions; 2) data deficiencies of the HR; 3) not focusing on the prognostic value of PLR in pancreatic cancer; 4) lacking key information for further analysis; 5) duplicate publications; 6) reporting the overlapping data and 7) non-human research.
Data extraction
Two investigators evaluated and extracted the data independently. For each included study, the following data were abstracted: the first author, year of publication, country of the study, ethnicity, number of patients, percentage of males, cut-off value, survival outcomes and analysis model. It should be noted that patients in each original study were divided into two groups based on the cut-off value of PLR: high PLR group and low PLR group. The HRs of prognostic outcomes obtained directly or indirectly from the published articles were integrated in the meta-analysis according to the study conducted by Tierney et al.23 If the HR was assessed with both multivariate analysis and univariate analysis, the results of multivariate analysis were applied in the current study. The quality of the included studies was assessed using the Newcastle–Ottawa Scale (NOS). Also, studies with NOS score ≥6 were considered to be of high quality. Any other disputes were discussed with the third investigator.
Statistical analysis
The main results of this meta-analysis were analyzed by Review Manager Version 5.3 software. The prognosis outcomes were assessed using the HR, along with the corresponding 95% CI or P-values. The main prognostic outcome was the OS. Cochran’s Q test and Higgins I2 were applied to evaluate the heterogeneity among included studies. Heterogeneity should be considered if I2>50%, and the random-effect model was applied; if not, the fixed-effect model was applied. In addition, to explore the publication bias, the funnel plot was drawn using Review Manager Version 5.3 software. To validate the credibility of outcomes in this meta-analysis, the sensitivity analysis was performed using Stata 12.0. Nonetheless, the subgroup analysis was carried out to further explore the association between the PLR and prognosis of patients with pancreatic cancer. The difference was considered to be statistically significant when the P-value was <0.05.
Results
Literature search
As shown in Figure 1, the literature search process is summarized in a flow diagram according to Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA).24 One hundred seventy-one papers were initially retrieved from the Cochrane Library, PubMed and Embase database. One hundred thirty-six papers remained after duplicates were removed. For the remaining 136 papers, 112 papers with significantly diverse topics were directly excluded by scanning the titles or abstracts. Among the rest of the papers, eight papers were excluded for not focusing on the topic and one paper was excluded for inefficient data. Therefore, 15 papers containing 17 cohort studies were finally included in this meta-analysis.20–22,25–36
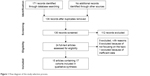  | Figure 1 Flow diagram of the study selection process. |
Characteristics of included studies
The clinical details of the included studies are presented in Table 1. Regarding the 17 included cohort studies, 5 were performed in China,30,32,34,36 5 in Japan,20,21,26,28,35 2 in USA,22,25 2 in Austria,33 1 in Australia,311 in Singapore27 and 1 in South Korea.29 Besides, sample size of the included cohort studies varied from 37 to 440. As for the gender information in the included studies, the percentage of males among the cohort studies varied from 39.5% to 88.8%. As for clinical outcomes, all the included cohort studies reported the OS,20–22,25–36 two studies reported the disease-free survival (DFS),20,21 one study presented the recurrence-free survival (RFS)22 and one study covered the progression-free survival (PFS).29 Moreover, the OS of 10 cohort studies was assessed with univariate analysis;20,25,28–30,33,34,36 however, the other studies evaluated it with multivariate analysis.21,22,26,27,31,32,35 Besides, the NOS score of each included study was ≥6, which meant that the included studies were of relatively high quality. Regarding the seven studies assessed with multivariate analysis, the main adjusted potential confounders included CA199, NLR, modified Glasgow Prognostic Score (mGPS), albumin, CRP, Eastern Cooperative Oncology Group (ECOG), stage, tumor size, nodal involvement, tumor differentiation, margin status, age and gender, and the details are listed in Table S1.
The meta-analysis of OS
All the included studies covered the OS of patients with pancreatic cancer. Therefore, 17 cohort studies were finally gathered into the meta-analysis of OS. As shown in Figure 2, in view of low heterogeneity (I2=42%), fixed-effect model was used. And the results indicated that there was statistically significant relationship between the PLR and prognosis of patients with pancreatic cancer, and patients with low PLR might have longer OS when compared to the patients with high PLR (HR=1.28, 95% CI=1.17–1.40, P<0.00001). Furthermore, funnel plot was conducted to explore the bias among the included studies, and the results demonstrated that no obvious publication bias was detected (Figure 3). Furthermore, sensitivity analysis conducted by Stata 12.0 confirmed the robustness of the results (Figure S1).
  | Figure 2 Meta-analysis of overall survival. |
  | Figure 3 Funnel plot of overall survival. |
To further explore the prognostic value of PLR in pancreatic cancer, subgroup analyses were performed based on the analysis model, ethnicity, sample size and cut-off value. The main results were presented in Table 2. In terms of analysis model, when only the studies with multivariate analysis were included into the meta-analysis, significant association between the PLR and OS was observed, with low heterogeneity (HR=1.76, 95% CI=1.45–2.14, P<0.00001; I2=9%). Similarly, when only considering the studies with univariate analysis, obvious correlation was detected between the PLR and prognosis of pancreatic cancer (HR=1.18, 95% CI=1.07–1.30, P=0.001; I2=0%). Subgroup analyses by ethnicity revealed that negative predictor of high PLR for OS was found both in Asian cases (HR=1.35, 95% CI=1.14–1.61, P=0.0006; I2=52%) and in Caucasian populations (HR=1.25, 95% CI=1.08–1.45, P=0.004; I2=17%). Regarding sample size, distinct association between the PLR and OS was detected not only when the sample size ≤150 (HR=1.49, 95% CI=1.14–1.94, P=0.003; I2=52%) but also when the sample size >150 (HR=1.26, 95% CI=1.11–1.42, P=0.0003; I2=31%). Additionally, considering different cut-off values, PLR was a negative prognostic biomarker for the cut-off value ≤150 (HR=1.19, 95% CI=1.02–1.40, P=0.002; I2=29%) and the cut-off value >150 (HR=1.39, 95% CI=1.23–1.56, P<0.00001; I2=43%). As for treatment, obvious association between the PLR and OS was detected not only when the treatment was surgery (HR=1.86, 95% CI=1.22–2.84, P=0.004; I2=63%) but also when the treatment was not surgery (HR=1.24, 95% CI=1.13–1.36, P<0.0001; I2=22%).
The further analysis was conducted based on the 7 studies assessed with multivariate analysis. As listed in Table 3, obvious relationship between the PLR and OS in pancreatic cancer was detected based on the subgroup analyses regarding the main adjusted potential confounders, including CA199, NLR, mGPS, albumin, CRP, ECOG, stage, tumor size, nodal involvement, tumor differentiation, margin status, age and gender.
The meta-analysis of PFS
Among the included studies, two studies reported the DFS, one study presented the RFS and one study covered the PFS. Also, these four studies were finally included into the meta-analysis of PFS. As shown in Figure 4, the results indicated that low PLR was significantly associated with longer PFS when compared to high PLR, with low heterogeneity (HR=1.27, 95% CI=1.03–1.57, P=0.03; I2=33%). In addition, funnel plot indicated that there was no obvious bias of included studies (Figure 5).
  | Figure 4 Meta-analysis of progression-free survival. |
  | Figure 5 Funnel plot of progression-free survival. |
Discussion
Inflammation is a hallmark of various cancers. Increasing evidences have shown that systemic inflammatory response was involved in tumorigenesis, malignant transformation and metastasis.37,38 Platelet has been proved to be associated with the diagnosis and treatment of cancers.39,40 Besides, lymphocyte infiltration in advanced stages is lower than that in the early stages of pancreatic cancer. In recent years, more and more researchers have started to pay attention to the prognostic role of PLR in various cancers, including liver cancer,41 lung cancer42 and colorectal cancer,43 as well as esophageal cancer.44 Similarly, the prognostic value of PLR in pancreatic cancer was also being investigated; however, the results were controversial.21,26,28,31
In the current study, the results demonstrated that PLR was significantly associated with the OS of patients with pancreatic cancer, which was similar to the conclusion in other cancers.42,44–48 Patients with low PLR had longer OS when compared to the patients with high PLR, indicating that low PLR might be a protective factor for pancreatic cancer. Besides, the subgroup analysis based on analysis model, ethnicity, sample size and cut-off value also presented similar results. It is worth mentioning that we conducted further analysis based on the adjusted potential confounders, including CA199, NLR, mGPS, albumin, CRP, ECOG, stage, tumor size, nodal involvement, tumor differentiation, margin status, age and gender, which was not reported in the earlier similar meta-analysis.19,44 Also, the results confirmed that low PLR was associated with longer OS in pancreatic cancer. We further explored the association between PLR, PFS, RFS and DFS; the results similarly demonstrated that low PLR was a protective factor for patients with pancreatic cancer. Zhou et al performed a meta-analysis to explore the prognostic value of PLR in various cancers and found that PLR was not associated with OS in pancreatic cancer, which was inconsistent with our study.49 It should be noted that only three studies were included in the meta-analysis of OS in Zhou et al’s study.49 Nevertheless, 17 cohort studies were finally included in the meta-analysis of OS; therefore, our conclusion was more convincing.
In spite of the conclusion arrived at in our study, the underlining mechanism of the prognostic value of PLR in pancreatic cancer remains unclear. Platelets might promote tumor growth, angiogenesis, metastasis and cancer-associated thrombosis.50,51 Moreover, Abiko et al reported that interferon-gamma from the lymphocytes induced PD-L1 expression and promoted the progression of ovarian cancer.52 Besides, He et al declared that lymphocyte promoted tumorigenesis by activating gene-3, an important immune checkpoint in cancer.53 Xu et al found that circulating CD3+CD8+ T lymphocytes might be used as a prognostic biomarker for lung cancer.54 Based on the platelet and lymphocyte counts, PLR might be related to the prognosis of patients with pancreatic cancer.
To the best of our knowledge, this is the first meta-analysis to explore the prognostic value of PLR in pancreatic cancer. Besides, our study contained 17 cohort studies involving 3,182 patients; therefore, the conclusion was convincing. Nonetheless, our meta-analysis is not without any limitation. First, all the data were obtained from the published articles and the original data of included patients were unavailable. Second, patients in the meta-analysis received several therapies and we cannot get the details, which may lower the applicability of this study. Third, the cut-off value of the included studies varied a lot, which might increase the heterogeneity. Fourth, some included studies lacked assessment of the confounding factors.
Conclusion
PLR could be used as a prognostic predictor in patients with pancreatic cancer. High PLR was associated with poor prognosis, especially shorter OS. In contrast, low PLR obviously was correlated with favorable OS in pancreatic cancer. More studies should be carried out to investigate the underlying mechanism.
Author contributions
Study concepts and design: YZZ, HXQ, YPZ; literature search: YPZ, SJC; data extraction: YZZ, YPZ; manuscript preparation and revision: AHF, SJC, YPZ, HXQ. All authors have participated sufficiently in the study and approved the final version. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. | ||
Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22:9694–9705. | ||
Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. | ||
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. | ||
Huang J, Zhao Y, Xu Y, et al. Comparative effectiveness and safety between oxaliplatin-based and cisplatin-based therapy in advanced gastric cancer: a meta-analysis of randomized controlled trials. Oncotarget. 2016;7:34824–34831. | ||
Wang J, Zhao Y, Qi R, et al. Prognostic role of podocalyxin-like protein expression in various cancers: a systematic review and meta-analysis. Oncotarget. 2016;8(32):52457–52464. | ||
Zhao Y, Si G, Zhu F, et al. Prognostic role of platelet to lymphocyte ratio in hepatocellular carcinoma: a systematic review and meta-analysis. Oncotarget. 2017;8:22854–22862. | ||
Zhao Y, Wang H, Shi Y, et al. Comparative effectiveness of combined therapy inhibiting EGFR and VEGF pathways in patients with advanced non-small-cell lung cancer: a meta-analysis of 16 phase II/III randomized trials. Oncotarget. 2017;8:7014–7024. | ||
Cheng H, Luo G, Lu Y, et al. The combination of systemic inflammation-based marker NLR and circulating regulatory T cells predicts the prognosis of resectable pancreatic cancer patients. Pancreatology. 2016;16:1080–1084. | ||
Leppanen J, Helminen O, Huhta H, et al. High toll-like receptor (TLR) 9 expression is associated with better prognosis in surgically treated pancreatic cancer patients. Virchows Arch. 2017;470:401–410. | ||
Li BS, Liu H, Yang WL. Reduced miRNA-218 expression in pancreatic cancer patients as a predictor of poor prognosis. Genet Mol Res. 2015;14:16372–16378. | ||
Zhang Y, Jiang H, Qin M, Su X, Cao Z, Wang J. TNIK serves as a novel biomarker associated with poor prognosis in patients with pancreatic cancer. Tumour Biol. 2016;37:1035–1040. | ||
Dai D, Zhang CF, Williams S, Yuan CS, Wang CZ. Ginseng on cancer: potential role in modulating inflammation–mediated angiogenesis. Am J Chin Med. 2017;45:13–22. | ||
Hung RJ, Ulrich CM, Goode EL, et al. Cross cancer genomic investigation of inflammation pathway for five common cancers: lung, ovary, prostate, breast, and colorectal cancer. J Natl Cancer Inst. 2015;107. | ||
Kimura Y, Nagai N, Tsunekawa N, et al. IL-17A-producing CD30(+) Vdelta1 T cells drive inflammation-induced cancer progression. Cancer Sci. 2016;107:1206–1214. | ||
Shigdar S, Li Y, Bhattacharya S, et al. Inflammation and cancer stem cells. Cancer Lett. 2014;345:271–278. | ||
Asaoka T, Miyamoto A, Maeda S, et al. Prognostic impact of preoperative NLR and CA19-9 in pancreatic cancer. Pancreatology. 2016;16:434–440. | ||
Stevens L, Pathak S, Nunes QM, et al. Prognostic significance of pre-operative C-reactive protein and the neutrophil-lymphocyte ratio in resectable pancreatic cancer: a systematic review. HPB (Oxford). 2015;17:285–291. | ||
Yang JJ, Hu ZG, Shi WX, Deng T, He SQ, Yuan SG. Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: a meta-analysis. World J Gastroenterol. 2015;21:2807–2815. | ||
Kishi T, Nakamura A, Itasaka S, et al. Pretreatment C-reactive protein level predicts outcome and patterns of failure after chemoradiotherapy for locally advanced pancreatic cancer. Pancreatology. 2015;15:694–700. | ||
Shirai Y, Shiba H, Sakamoto T, et al. Preoperative platelet to lymphocyte ratio predicts outcome of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Surgery. 2015;158:360–365. | ||
Spolverato G, Maqsood H, Kim Y, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after resection for hepato-pancreatico-biliary malignancies. J Surg Oncol. 2015;111:868–874. | ||
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. | ||
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. | ||
Alagappan M, Pollom EL, von Eyben R, et al. Albumin and neutrophil-lymphocyte ratio (NLR) predict survival in patients with pancreatic adenocarcinoma treated with SBRT. Am J Clin Oncol. Epub 2016 Jan 13. | ||
Asari S, Matsumoto I, Toyama H, et al. Preoperative independent prognostic factors in patients with borderline resectable pancreatic ductal adenocarcinoma following curative resection: the neutrophil-lymphocyte and platelet-lymphocyte ratios. Surg Today. 2016;46:583–592. | ||
Goh BK, Tan DM, Chan CY, et al. Are preoperative blood neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios useful in predicting malignancy in surgically-treated mucin-producing pancreatic cystic neoplasms? J Surg Oncol. 2015;112:366–371. | ||
Inoue D, Ozaka M, Matsuyama M, et al. Prognostic value of neutrophil-lymphocyte ratio and level of C-reactive protein in a large cohort of pancreatic cancer patients: a retrospective study in a single institute in Japan. Jpn J Clin Oncol. 2015;45:61–66. | ||
Lee JM, Lee HS, Hyun JJ, et al. Prognostic value of inflammation-based markers in patients with pancreatic cancer administered gemcitabine and erlotinib. World J Gastrointest Oncol. 2016;8:555–562. | ||
Liu Z, Jin K, Guo M, et al. Prognostic value of the CRP/Alb ratio, a novel inflammation-based score in pancreatic cancer. Ann Surg Oncol. 2017;24:561–568. | ||
Martin HL, Ohara K, Kiberu A, et al. Prognostic value of systemic inflammation-based markers in advanced pancreatic cancer. Intern Med J. 2014;44:676–682. | ||
Qi Q, Geng Y, Sun M, Wang P, Chen Z. Clinical implications of systemic inflammatory response markers as independent prognostic factors for advanced pancreatic cancer. Pancreatology. 2015;15:145–150. | ||
Stotz M, Gerger A, Eisner F, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109:416–421. | ||
Wang DS, Luo HY, Qiu MZ, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol. 2012;29:3092–3100. | ||
Watanabe J, Otani S, Sakamoto T, et al. Prognostic indicators based on inflammatory and nutritional factors after pancreaticoduodenectomy for pancreatic cancer. Surg Today. 2016;46:1258–1267. | ||
Yu SL, Xu LT, Qi Q, et al. Serum lactate dehydrogenase predicts prognosis and correlates with systemic inflammatory response in patients with advanced pancreatic cancer after gemcitabine-based chemotherapy. Sci Rep. 2017;7:45194. | ||
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. | ||
Munkley J, Elliott DJ. Hallmarks of glycosylation in cancer. Oncotarget. 2016;7:35478–35489. | ||
Hanze J, Jakubowski P, Heers H, et al. Assessing blood platelets as RNA biomarker source for prostate cancer. Biomarkers. 2016;21:653–659. | ||
Holmes CE, Levis JE, Schneider DJ, et al. Platelet phenotype changes associated with breast cancer and its treatment. Platelets. 2016;27:703–711. | ||
Ma W, Zhang P, Qi J, et al. Prognostic value of platelet to lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. Sci Rep. 2016;6:35378. | ||
Ding N, Pang Z, Shen H, Ni Y, Du J, Liu Q. The prognostic value of PLR in lung cancer, a meta-analysis based on results from a large consecutive cohort. Sci Rep. 2016;6:34823. | ||
Chen N, Li W, Huang K, et al. Increased platelet-lymphocyte ratio closely relates to inferior clinical features and worse long-term survival in both resected and metastatic colorectal cancer: an updated systematic review and meta-analysis of 24 studies. Oncotarget. 2017;8(19):32356–32369. | ||
Yodying H, Matsuda A, Miyashita M, et al. Prognostic significance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2016;23:646–654. | ||
Qiang G, Liang C, Xiao F, et al. Prognostic significance of platelet-to-lymphocyte ratio in non-small-cell lung cancer: a meta-analysis. Onco Targets Ther. 2016;9:869–876. | ||
Tan D, Fu Y, Su Q, Wang H. Prognostic role of platelet-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Medicine (Baltimore). 2016;95:e3837. | ||
Zhang H, Gao L, Zhang B, Zhang L, Wang C. Prognostic value of platelet to lymphocyte ratio in non-small cell lung cancer: a systematic review and meta-analysis. Sci Rep. 2016;6:22618. | ||
Zhao QT, Yuan Z, Zhang H, et al. Prognostic role of platelet to lymphocyte ratio in non-small cell lung cancers: a meta-analysis including 3,720 patients. Int J Cancer. 2016;139:164–170. | ||
Zhou X, Du Y, Huang Z, et al. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One. 2014;9:e101119. | ||
Mezouar S, Frere C, Darbousset R, et al. Role of platelets in cancer and cancer-associated thrombosis: experimental and clinical evidences. Thromb Res. 2016;139:65–76. | ||
Menter DG, Tucker SC, Kopetz S, et al. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev. 2014;33:231–269. | ||
Abiko K, Matsumura N, Hamanishi J, et al. IFN-gamma from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112:1501–1509. | ||
He Y, Rivard CJ, Rozeboom L, et al. Lymphocyte-activation gene-3, an important immune checkpoint in cancer. Cancer Sci. 2016;107:1193–1197. | ||
Xu L, Chen D, Lu C, et al. Advanced lung cancer is associated with decreased expression of perforin, CD95, CD38 by circulating CD3+CD8+ T lymphocytes. Ann Clin Lab Sci. 2015;45:528–532. |
Supplementary materials
  | Figure S1 Sensitivity analysis of overall survival. |
References
Kishi T, Nakamura A, Itasaka S, et al. Pretreatment C-reactive protein level predicts outcome and patterns of failure after chemoradiotherapy for locally advanced pancreatic cancer. Pancreatology. 2015;15:694–700. | ||
Shirai Y, Shiba H, Sakamoto T, et al. Preoperative platelet to lymphocyte ratio predicts outcome of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Surgery. 2015;158:360–365. | ||
Spolverato G, Maqsood H, Kim Y, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after resection for hepato-pancreatico-biliary malignancies. J Surg Oncol. 2015;111:868–874. | ||
Alagappan M, Pollom EL, von Eyben R, et al. Albumin and neutrophil-lymphocyte ratio (NLR) predict survival in patients with pancreatic adenocarcinoma treated with SBRT. Am J Clin Oncol. Epub 2016 Jan 13. | ||
Asari S, Matsumoto I, Toyama H, et al. Preoperative independent prognostic factors in patients with borderline resectable pancreatic ductal adenocarcinoma following curative resection: the neutrophil-lymphocyte and platelet-lymphocyte ratios. Surg Today. 2016;46:583–592. | ||
Goh BK, Tan DM, Chan CY, et al. Are preoperative blood neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios useful in predicting malignancy in surgically-treated mucin-producing pancreatic cystic neoplasms? J Surg Oncol. 2015;112:366–371. | ||
Inoue D, Ozaka M, Matsuyama M, et al. Prognostic value of neutrophil-lymphocyte ratio and level of C-reactive protein in a large cohort of pancreatic cancer patients: a retrospective study in a single institute in Japan. Jpn J Clin Oncol. 2015;45:61–66. | ||
Lee JM, Lee HS, Hyun JJ, et al. Prognostic value of inflammation-based markers in patients with pancreatic cancer administered gemcitabine and erlotinib. World J Gastrointest Oncol. 2016;8:555–562. | ||
Liu Z, Jin K, Guo M, et al. Prognostic value of the CRP/Alb ratio, a novel inflammation-based score in pancreatic cancer. Ann Surg Oncol. 2017;24:561–568. | ||
Martin HL, Ohara K, Kiberu A, et al. Prognostic value of systemic inflammation-based markers in advanced pancreatic cancer. Intern Med J. 2014;44:676–682. | ||
Qi Q, Geng Y, Sun M, Wang P, Chen Z. Clinical implications of systemic inflammatory response markers as independent prognostic factors for advanced pancreatic cancer. Pancreatology. 2015;15:145–150. | ||
Stotz M, Gerger A, Eisner F, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109:416–421. | ||
Wang DS, Luo HY, Qiu MZ, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol. 2012;29:3092–3100. | ||
Watanabe J, Otani S, Sakamoto T, et al. Prognostic indicators based on inflammatory and nutritional factors after pancreaticoduodenectomy for pancreatic cancer. Surg Today. 2016;46:1258–1267. 21. Shirai Y, Shiba H, Sakamoto T, et al. Preoperative platelet to lymphocyte ratio predicts outcome of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Surgery. 2015;158:360–365. | ||
Yu SL, Xu LT, Qi Q, et al. Serum lactate dehydrogenase predicts prognosis and correlates with systemic inflammatory response in patients with advanced pancreatic cancer after gemcitabine-based chemotherapy. Sci Rep. 2017;7:45194. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




