Back to Journals » Cancer Management and Research » Volume 13
Prognostic Value of Inflammatory Markers in Nasopharyngeal Carcinoma Patients in the Intensity-Modulated Radiotherapy Era
Authors Li Q , Yu L, Yang P, Hu Q
Received 14 March 2021
Accepted for publication 23 July 2021
Published 31 August 2021 Volume 2021:13 Pages 6799—6810
DOI https://doi.org/10.2147/CMAR.S311094
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Qian Li, Lushi Yu, Pengcheng Yang, Qinyong Hu
Cancer Center, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, 430060, People’s Republic of China
Correspondence: Qinyong Hu
Cancer Center, Renmin Hospital of Wuhan University, No. 238 Jie Fang Road, Wuchang District, Wuhan, 430060, Hubei, People’s Republic of China
Tel +86-13986123799
Email [email protected]
Purpose: Inflammatory markers have been widely used in various cancers, but rarely in nasopharyngeal carcinoma (NPC). Here, we evaluated the prognostic value of pretreatment neutrophil-to-lymphocyte ratio (NLR), platelet-lymphocyte-ratio (PLR), systemic immune index (SII), and systemic inflammation response index (SIRI) on NPC in the intensity-modulated radiotherapy (IMRT) era.
Methods: We retrospectively analyzed data from NPC patients from the Renmin Hospital of Wuhan University, between January 2012 and July 2020. We used Chi-square test or Fisher’s exact test to compare the baseline characteristics, then applied Kaplan–Meier (K-M) survival analysis to compare the overall survival (OS) and progression-free survival (PFS) rates. Multivariate Cox proportional risk models were applied to identify independent prognostic factors.
Results: We enrolled a total of 342 NPC patients and found optimal cut-off values of 2.65, 184.91, 804.08, and 1.34 for NLR, PLR, SII, and SIRI, respectively. K-M survival analysis revealed that high NLR, PLR, SII, and SIRI were significantly associated with worse OS and PFS relative to those in the low groups. Results from univariate Cox analysis showed that clinical, T, and M stages, as well as NLR, PLR, SII, and SIRI were associated with OS, whereas age, alongside the aforementioned parameters, was associated with PFS. Moreover, multivariate Cox analysis showed that age ≥ 49 years (HR=2.48, 95% CI=1.21– 5.05, P=0.013) and M1 stage (HR=3.84, 95% CI=1.52– 9.73, P=0.013) were independent prognostic factors for OS, whereas SIRI ≥ 1.34 (HR=1.91, 95% CI=1.05– 3.47, P=0.034) and M1 stage (HR=2.91, 95% CI=1.44– 5.86, P=0.003) were independent prognostic factors for PFS.
Conclusion: Overall, our findings indicated that high NLR, PLR, SII, and SIRI were significantly associated with poor OS and PFS in NPC patients. High SIRI may be an independent risk factor for PFS of NPC patients in the IMRT era.
Keywords: nasopharyngeal carcinoma, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, systemic immune inflammation index, systemic inflammatory response index, prognosis
Introduction
Nasopharyngeal carcinoma (NPC), which arises from nasopharyngeal epithelial tissue, is one of the most common malignancies in the head and neck. Previous evidence has shown that an estimated 60,600 people were diagnosed with NPC in 2015 alone, with about 34,100 of them dying from the disease.1 However, more than 70% of all NPC patients are diagnosed at locally advanced stage because of the concealed location of the nasopharynx and the lack of obvious symptoms in the early period, leading to a relatively poor prognosis.2
Recent improvements in radiotherapy (RT) technology coupled with development of systematic therapy have significantly improved the efficacy of NPC treatment. Particularly, intensity-modulated radiotherapy (IMRT) has significantly improved the local control rate compared to conventional radiotherapy, and has therefore become the standard RT method for NPC treatment.3 However, a previous study revealed that about 12–14% of all NPC patients developed recurrence and/or metastasis within 5 years of IMRT.4 Nowadays, distant metastasis after initial treatment has become the main mode of treatment failure of NPC patients.5
It is widely recognized that the TNM stage system and the Epstein-Barr virus (EBV) DNA play important roles in guiding NPC treatment, evaluation of curative efficacy, as well as prognosis prediction.6 However, clinical outcomes significantly vary even among NPC patients with the same TNM stage and similar therapeutic regimens.7 Moreover, EBV-DNA testing is a complex detection process, and is further characterized by the high cost of testing, and huge discrepancies in results, which consequently limit its promotion and practical application.8 Therefore, exploration of cheaper, as well as more convenient and stable markers is required to supplement TNM stage and enhance the prognostic prediction of NPC patients.
The relationship between inflammation and tumor was first proposed by Virchow in 1863.9 For decades, numerous studies have confirmed that inflammation plays an important role in cancer initiation, progression and metastasis.10 Furthermore, inflammatory markers, such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune inflammation index (SII), and systemic inflammation response index (SIRI), have been employed to predict the prognosis of breast,11 lung,12 pancreatic,13 and cervical14 cancers, among others. Previous studies have also suggested that high NLR, PLR, SII, and SIRI are significantly associated with poor prognosis in various malignancies. More importantly, all of the aforementioned inflammatory markers were derived from patients’ routine blood tests, which are cheap, convenient, and stable, and have a great prospect in clinical promotion.
In recent years, application of inflammatory markers in the prognosis of tumors has gradually become a focus in tumor research. However, only a handful of similar evidence has been described in NPC, while the obtained conclusions are inconsistent. With regards to the IMRT era, explorations of all TNM stages in patients aimed at elucidating the relationship between SIRI and the prognosis of NPC are lacking. Therefore, the purpose of this study was to evaluate the prognostic value of NLR, PLR, SII, and SIRI in all TNM stage NPC patients.
Materials and Methods
Patients Recruitment and Selection Criteria
We retrospectively collected data of patients, who were diagnosed with NPC between January 2012 and July 2020 at the Renmin Hospital of Wuhan University. Patients were enrolled in the study if they met the following criteria: 1) NPC diagnosis was confirmed via histopathological examination; 2) they underwent complete baseline examination, including nasopharynx and neck magnetic resonance imaging (MRI), chest computed tomography (CT), abdominal CT or ultrasonography, and whole-body bone scanning; 3) they had hematological data within 7 days prior to treatment, including blood routine and biochemistry tests; and 4) they received radical irradiation with IMRT. Conversely, patients were excluded from the study if they: 1) manifested other serious diseases, including severe heart disease and/or liver and renal dysfunction; 2) exhibited other malignant tumors; 3) had acute infectious diseases and blood system diseases; 4) lacked complete medical records or did not complete the prescribed treatment; or 5) were without complete follow-up information. Finally, a total of 342 participants met the aforementioned inclusion eligible and were included in this study.
Treatment and Data Collection
All patients completed baseline assessment prior to treatment, including collection of their medical history inquiry, routine physical and imaging examinations, as well as laboratory tests. Disease staging was performed according to the 8th edition of the American Joint Committee on Cancer (AJCC) system, whereas values of inflammatory markers were calculated according to the results obtained from blood routine tests. NLR was calculated as neutrophil count/lymphocyte count, PLR was taken as platelet count/lymphocyte count, SII was calculated as platelet count*neutrophil count/lymphocyte count, while SIRI was calculated as monocyte count*neutrophil count/lymphocyte count. Treatment was administered according to the guidelines of the National Comprehensive Cancer Network (NCCN), with all patients in the study receiving radical IMRT. Stage I patients received IMRT alone, whereas those at stages II–IV mainly received concurrent chemoradiotherapy with or without induction and/or adjuvant chemotherapy. All chemotherapy regimens were platinum-based.
Patient information, including gender, age, clinical stage and TNM stage, treatment methods and outcomes, as well as NLR, PLR, SII, and SIRI were collected.
Follow-Up and Study Endpoints
After treatment, patients were followed up every 3 months during the first 2 years, every 6 months between 3–5 years, and annually after 5 years. During the follow-up period, patients were subjected to regular physical, laboratory, and imaging examinations, including palpation of cervical lymph nodes, nasopharyngoscopy, nasopharynx and neck MRI, chest and abdominal imaging examination, as well as whole-body bone scans, among others. Patients with recurrence and/or distant metastasis required specific treatment. The last follow-up date was January 31, 2021. The primary endpoint for this study was overall survival (OS), which was defined as the time between the date of diagnosis and the date of last follow-up or death from any cause. On the other hand, the secondary endpoint was progression-free survival (PFS), defined as the time between the date of diagnosis and the date of progression, including death, or last follow-up.
Statistical Analysis
Most previous studies have selected the cutoff values of inflammatory markers based on receiver operating characteristics (ROC) curves,15,16 which intuitively represents the sensitivity and specificity of various cutoff values. In the present study, we generated ROC curves, then selected the optimal cutoff values based on the Youden Index. Baseline characteristics in patients between low and high NLR, PLR, SII, and SIRI groups were compared using the chi-square tests or Fisher exact tests. Moreover, we generated Kaplan–Meier (K-M) curves to compare differences in survival of NPC patients between low and high NLR, PLR, SII, and SIRI groups. Furthermore, we applied univariate and multivariate Cox regression to identify independent prognostic factors in NPC. All statistical analyses were conducted in SPSS 26.0 software, with data followed by P<0.05 considered statistically significant.
Results
A summary of basic characteristics of the 342 NPC patients is displayed in Table 1. Summarily, 247 (72.2%) and 95 (27.8%) were male and female, respectively, while the male:female ratio was approximately 2.6:1. The median age for the entire study group was 49 (range=16–83) years. With regard to clinical stage, 10 (2.9%) patients were at stage I, while 44 (12.9%), 182 (53.2%), and 106 (31.0%) were at stages II, III, and IV, respectively. A total of 23 (6.7%) patients suffered distant metastasis at initial diagnosis. With regard to therapy, 21 (6.1%) patients received IMRT alone, while 321 (93.9%) patients received IMRT plus chemotherapy. The median follow-up time was 66 (range=3–110) months. During follow-up, 63 patients (18.4%) experienced relapse or metastasis after initial treatment, of which 10 and 5 had local and regional recurrence, respectively, whereas 48 developed bone, lung, liver, or brain metastases. The median progression time for these patients was 25 months after treatment. Unfortunately, 41 (12.0%) of them died during the follow-up period.
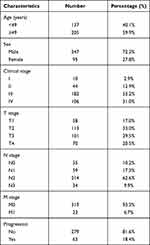 |
Table 1 Baseline Characteristics of Patients with Nasopharyngeal Carcinoma |
Cut-off Values and Baseline Characteristics
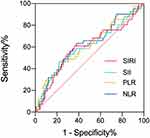
A summary of cut-off values, according to ROC curves, are shown in Figure 1. Briefly, the area under curve (AUC) of the ROC for NLR, PLR, SII, and SIRI were 0.619, 0.608, 0.599, and 0.595, respectively, whereas the cut-off values for NLR, PLR, SII, and SIRI were 2.65, 184.91, 804.08, and 1.34, respectively. Baseline characteristics were generally balanced except for gender and T stage (Table 2 and 3). Moreover, patients with progression after initial treatment tended to have higher SII and SIRI compared to those without progression.
 |
Table 2 Baseline Characteristics According to NLR and PLR |
 |
Table 3 Baseline characteristics according to SII and SIRI |
Survival Analysis
Kaplan Meier curves revealed that patients with low NLR, PLR, SII, and SIRI levels showed significantly superior OS than those in high group (Figure 2). Similarly, those with low NLR, PLR, SII, and SIRI exhibited better PFS than their high-group counterparts (Figure 3). A summary of 1-, 3-, and 5-year OS and PFS rates is displayed in Tables 4 and 5, respectively.
 |
Table 4 1-, 3-, and 5-Year OS for Patients with Nasopharyngeal Carcinoma |
 |
Table 5 1-, 3-, and 5-Year PFS for Patients with Nasopharyngeal Carcinoma |
Identification of Significant Prognostic Factors
We used Cox regression analysis to identify the prognostic factors for NPC. Results from univariate analysis revealed that high NLR (HR=1.72, 95% CI=1.10–2.69, P=0.016), PLR (HR=1.93, 95% CI= 1.22–3.07, P=0.005), SII (HR=2.15, 95% CI=1.36–3.41, P=0.001), and SIRI (HR=2.17, 95% CI=1.39–3.39, P=0.001) were significantly associated with poor PFS (Table 6). Similarly, high NLR (HR=2.66, 95% CI=1.40–5.02, P=0.003), PLR (HR=2.84, 95% CI=1.53–5.27, P=0.001), SII (HR=3.07, 95% CI=1.65–5.68, P<0.001), and SIRI (HR=2.75, 95% CI=1.48–5.13, P=0.001) were associated with worse OS. Besides, T4 (HR=2.19, 95% CI=1.02–4.70, P=0.044) was significantly associated with poor PFS, while T3 (HR=2.77, 95% CI=1.40–16.26, P=0.012) and age≥49 years (HR=2.08, 95% CI=1.04–4.16, P=0.038) were significantly corrected with poor OS. Moreover, patients with stage III–IV exhibited worse PFS (HR=3.78, 95% CI=1.38–10.35, P=0.010) than those with stage I–II NPC.
 |
Table 6 Univariate Cox Analysis of PFS and OS |
Next, we incorporated the statistically significant variables, namely age, clinical, T, N, and M stages, as well as NLR, PLR, SII, and SIRI into a multivariate Cox regression model for identification of independent prognostic factors. Results revealed that SIRI≥1.34 (HR=1.91, 95% CI=1.05–3.47, P=0.034) and M1 stage (HR=2.91, 95% CI=1.44–5.86, P=0.003) were independent prognostic factors for PFS, while higher age (≥49 years) (HR=2.48, 95% CI=1.21–5.05, P=0.013) and M1 stage (HR=3.84, 95% CI=1.52–9.73, P=0.013) were independent prognostic factors for OS (Table 7).
 |
Table 7 Multivariate Cox Analysis of PFS and OS |
Discussion
In this study, we retrospectively investigated 342 NPC patients from Renmin hospital at Wuhan University, then explored the value of pretreatment NLR, PLR, SII, and SIRI in predicting prognosis of these patients in the IMRT era. Results showed that patients with high NLR, PLR, SII, and SIRI levels exhibited poor OS and PFS. Particularly, high SIRI (≥1.34) was considered an independent risk factor for the PFS in NPC. To our knowledge, this is the first study incorporating all TNM stages of patients during exploration of the relationship between SIRI and the prognosis of NPC patients who underwent IMRT. Our results further showed that higher age (≥49 years) was significantly associated with worse OS, while distant metastasis was an independent risk factor for both PFS and OS in this group of patients.
Since the discovery of the relationship between inflammation and tumors by Virchow,9 numerous evidences have affirmed the important role played by inflammatory cells in the occurrence and development of tumors. Numerous studies have also shown that neutrophils can promote the progression and metastasis of tumors,17 mainly by secreting pro-angiogenic factors that promote tumor angiogenesis, and also acting on the extracellular matrix EGF, TGF-β, and PDGF to enhance tumor growth. In addition, further evidence has shown that monocytes and platelets were also important protumor factors.18 In fact, lymphocytes are reportedly the main mediators of a host’s antitumor immunity, with reduction of peripheral lymphocytes shown to impair antitumor immunity in the host and thereby accelerating tumor development.7 In addition, cytokines, such as IFN-γ and TNF-α, secreted by circulating lymphocytes, reportedly control tumor growth and improve the prognosis of cancer patients.19 Over the years, the aforementioned inflammatory markers have been widely used as prognostic factors for various malignancies, either alone or in combination.20 Currently, it is generally believed that upregulation of NLR, PLR, SII, and SIRI is significantly associated with poor prognosis of malignant tumors, hence can be used as independent prognostic factors for predicting many malignant tumors.21,22 However, only a handful of studies have described the role of the biomarkers, especially SII and SIRI, in NPC, and no consensus has been reached yet.
Results from a previous retrospective study by Lu et al,23 comprising 140 patients with non-metastatic NPC, revealed that patients with high NLR and PLR exhibited poor OS, while high NLR was an independent prognostic factor for OS. On the other hand, Ye et al24 reported that high pretreatment NLR and PLR were independent risk factors for OS in non-metastasis NPC patients treated with IMRT. Besides, results from another prospective study also showed that NPC patients with high pretreatment NLR and PLR exhibited relatively shorter disease‐specific survival (DSS) compared to those in the low group.25 Results of the present study partly corroborated these results, as evidenced by K-M curves which revealed that patients with higher NLR and PLR levels exhibited significantly poor OS and PFS, while NLR and PLR were not independent prognostic factors for NPC after multivariate analysis.
SII is a novel inflammatory marker, that has been widely proven to be an independent prognostic factor in many cancers.26 However, only a small number of studies have demonstrated its importance in NPC. For example, results from a previous retrospective study comprising 327 patients with non-metastatic NPC indicated that high SII was an independent prognostic factor for NPC, while the resulting prognostic value was found to be superior to PLR and NLR,27 that were corroborated by Oei et al.28 In the present study, K-M curves and results from univariate Cox regression revealed that high SII was significantly associated with poor OS and PFS. However, SII was not an independent prognostic factor for both OS and PFS in NPC patients, which was consistent with the results of recent studies.7,18
SIRI, first proposed by Qi et al29 for use as a biomarker for predicting survival of patients with pancreatic cancer, has since shown that patients with SIRI≥1.8 exhibit shorter time to progression (TTP) and OS relative to those with SIRI<1.8. Thereafter, Chen et al30 also found that NPC patients with high SIRI had poor OS. Similarly, a recent retrospective study also confirmed that high SIRI was an independent risk factor for OS and PFS in patients with NPC,7 although this was not the case in a subsequent study.18 In contrast to the above studies, we enrolled NPC patients with all TNM stages, and all of whom had underwent IMRT. Notably, our findings were similar to the aforementioned studies, with high SIRI patients were found to exhibit significantly worse OS and PFS, hence SIRI could be considered as an independent risk factor for PFS of NPC patients who received IMRT.
Interestingly, we observed two additional findings emerged from this study. Firstly, NPC patients aged≥49 years exhibited an increase in all-cause death rate, which was 1.48-fold higher than those aged below 49 years, which was consistent with findings from previous studies.31,32 Secondly, M stage could be a risk factor for NPC patients, as evidence by a 2.84-fold increased all-cause death rate relative to that observed in non-metastatic NPC patients. This was also consistent with the results from previous studies that reported that NPC patients with distant metastasis exhibited worse survival outcome.33,34
Despite the above findings, we did not acquire complete EBV-DNA data in this study. Therefore, we could not evaluate its prognostic value for patients with NPC. However, some recent studies have considered the effect of EBV-DNA on prognosis when discussing the prognostic value of inflammatory markers in NPC. For example, Jiang et al35 found that both EBV-DNA and PLR could be used as independent prognostic factors for NPC, while PLR could complement EBV-DNA to predict the prognosis of NPC patients. Similarly, Li et al36 found that both EBV-DNA and lymphocyte-to-monocyte ratio (LMR) were independent prognostic factors for NPC, while results from a recent study also found that both EBV-DNA and SII could be used as prognostic factors for NPC patients.37 In order to comprehensively elucidate the prognostic value of these factors in patients with NPC, it is necessary to conduct higher-level clinical studies aimed at exploring both EBV-DNA and inflammatory markers.
This study had some limitations. Firstly, being a retrospective study, we had a relatively low sample size (342 patients), which might have affected the level of evidence. Secondly, we did not have complete data for lactate dehydrogenase (LDH), hence we could not compare the prognostic value of inflammatory biomarkers with this parameter. Finally, we did not use uniform treatment approaches during the study, including chemotherapy regimens, the number of cycles of chemotherapy, as well as the use of immunotherapy and targeted therapy, which may have affected the observed outcomes.
In conclusion, the findings of this retrospective study confirmed that NLR, PLR, SII, and SIRI played important roles in predicting the prognosis of NPC patients. Notably, only SIRI was the independent predictor for PFS in NPC patients after IMRT therapy. Despite these breakthroughs, further prospective studies, using larger sample sizes, are needed to validate our findings.
Data Sharing Statement
All datasets supporting the conclusions of this article are available from the authors upon request.
Ethical Approval
This study was approved by the Ethics Committee of Renmin Hospital of Wuhan University.
Consent to Participate
Written informed consent was waived by our ethics committee because of the retrospective nature of the study. However, the study was conducted in accordance with the ethical standards of the Helsinki Declaration. All patient data accessed complied with adherence to the rules of data protection and privacy regulations.
Consent for Publication
All authors read and approved the final version of the manuscript prior to submission for publication.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81670144).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
2. Chen Y, Liu MZ, Liang SB, et al. Preliminary results of a prospective randomized trial comparing concurrent chemoradiotherapy plus adjuvant chemotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma in endemic regions of china. Int J Radiat Oncol Biol Phys. 2008;71(5):1356–1364. doi:10.1016/j.ijrobp.2007.12.028
3. Peng G, Wang T, Yang KY, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012;104(3):286–293. doi:10.1016/j.radonc.2012.08.013
4. Ng WT, Lee MC, Hung WM, et al. Clinical outcomes and patterns of failure after intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2011;79(2):420–428. doi:10.1016/j.ijrobp.2009.11.024
5. Zhang MX, Li J, Shen GP, et al. Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: a 10-year experience with a large cohort and long follow-up. Eur J Cancer. 2015;51(17):2587–2595. doi:10.1016/j.ejca.2015.08.006
6. Gihbid A, Benzeid R, Faouzi A, et al. Circulating cell-free epstein-barr virus DNA levels and clinical features in Moroccan patients with nasopharyngeal carcinoma. Infect Agent Cancer. 2021;16(1):15. doi:10.1186/s13027-021-00353-8
7. Feng Y, Zhang N, Wang S, et al. Systemic inflammation response index is a predictor of poor survival in locally advanced nasopharyngeal carcinoma: a propensity score matching study. Front Oncol. 2020;10:575417. doi:10.3389/fonc.2020.575417
8. Trevisiol C, Gion M, Vaona A, et al. The appropriate use of circulating EBV-DNA in nasopharyngeal carcinoma: comprehensive clinical practice guidelines evaluation. Oral Oncol. 2021;114:105128. doi:10.1016/j.oraloncology.2020.105128
9. Schmidt A, Weber OF. In memoriam of Rudolf virchow: a historical retrospective including aspects of inflammation, infection and neoplasia. Contrib Microbiol. 2006;13:1–15. doi:10.1159/000092961
10. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi:10.1038/nature01322
11. Wang L, Zhou Y, Xia S, et al. Prognostic value of the systemic inflammation response index (SIRI) before and after surgery in operable breast cancer patients. Cancer Biomark. 2020;28(4):537–547. doi:10.3233/CBM-201682
12. Hu M, Xu Q, Yang S, et al. Pretreatment systemic inflammation response index (SIRI) is an independent predictor of survival in unresectable stage III non-small cell lung cancer treated with chemoradiotherapy: a two-center retrospective study. Ann Transl Med. 2020;8(20):1310. doi:10.21037/atm-20-6484
13. Pacheco-Barcia V, Mondejar SR, France T, et al. A systemic inflammation response index (SIRI) correlates with survival and predicts oncological outcome for mFOLFIRINOX therapy in metastatic pancreatic cancer. Pancreatology. 2020;20(2):254–264. doi:10.1016/j.pan.2019.12.010
14. Chao B, Ju X, Zhang L, Xu X, Zhao Y. A novel prognostic marker Systemic Inflammation Response Index (SIRI) for operable cervical cancer patients. Front Oncol. 2020;10:766. doi:10.3389/fonc.2020.00766
15. Bulut G, Ozdemir ZN. Prognostic significance of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in metastatic colorectal cancer. J Gastrointest Cancer. In Press 2021. doi:10.1007/s12029-021-00616-y
16. Wolfe AR, Siedow M, Nalin A, et al. Increasing neutrophil-to-lymphocyte ratio following radiation is a poor prognostic factor and directly correlates with splenic radiation dose in pancreatic cancer. Radiother Oncol. 2021;158:207–214. doi:10.1016/j.radonc.2021.02.035
17. Mollinedo F. Neutrophil degranulation, plasticity, and cancer metastasis. Trends Immunol. 2019;40(3):228–242. doi:10.1016/j.it.2019.01.006
18. Zeng X, Liu G, Pan Y, Li Y. Development and validation of immune inflammation-based index for predicting the clinical outcome in patients with nasopharyngeal carcinoma. J Cell Mol Med. 2020;24(15):8326–8349. doi:10.1111/jcmm.15097
19. Ferrone C, Dranoff G. Dual roles for immunity in gastrointestinal cancers. J Clin Oncol. 2010;28(26):4045–4051. doi:10.1200/JCO.2010.27.9992
20. Zhang Y, Liu F, Wang Y. Evidence of the prognostic value of pretreatment systemic inflammation response index in cancer patients: a pooled analysis of 19 cohort studies. Dis Markers. 2020;2020:8854267. doi:10.1155/2020/8854267
21. Topkan E, Selek U, Kucuk A, et al. Prechemoradiotherapy systemic inflammation response index stratifies stage iIIB/C non-small-cell lung cancer patients into three prognostic groups: a propensity score-matching analysis. J Oncol. 2021;2021:6688138. doi:10.1155/2021/6688138
22. Chuang HC, Tsai MH, Lin YT, et al. The clinical impacts of pretreatment peripheral blood ratio on lymphocytes, monocytes, and neutrophils among patients with laryngeal/hypopharyngeal cancer treated by chemoradiation/radiation. Cancer Manag Res. 2020;12:9013–9021. doi:10.2147/CMAR.S275635
23. Lu A, Li H, Zheng Y, et al. Prognostic significance of neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio, and platelet to lymphocyte ratio in patients with nasopharyngeal carcinoma. Biomed Res Int. 2017;2017:3047802. doi:10.1155/2017/3047802
24. Ye L, Oei RW, Kong F, et al. Prognostic values of hematological biomarkers in nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. Eur Arch Otorhinolaryngol. 2018;275(5):1309–1317. doi:10.1007/s00405-018-4956-x
25. Li XH, Chang H, Xu BQ, et al. An inflammatory biomarker-based nomogram to predict prognosis of patients with nasopharyngeal carcinoma: an analysis of a prospective study. Cancer Med. 2017;6(1):310–319. doi:10.1002/cam4.947
26. Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic value of systemic immune-inflammation index in cancer: a meta-analysis. J Cancer. 2018;9(18):3295–3302. doi:10.7150/jca.25691
27. Jiang W, Chen Y, Huang J, et al. Systemic immune-inflammation index predicts the clinical outcome in patients with nasopharyngeal carcinoma: a propensity score-matched analysis. Oncotarget. 2017;8(39):66075–66086. doi:10.18632/oncotarget.19796
28. Oei RW, Ye L, Kong F, et al. Prognostic value of inflammation-based prognostic index in patients with nasopharyngeal carcinoma: a propensity score matching study. Cancer Manag Res. 2018;10:2785–2797. doi:10.2147/CMAR.S171239
29. Qi Q, Zhuang L, Shen Y, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. 2016;122(14):2158–2167. doi:10.1002/cncr.30057
30. Chen Y, Jiang W, Xi D, et al. Development and validation of nomogram based on SIRI for predicting the clinical outcome in patients with nasopharyngeal carcinomas. J Investig Med. 2019;67(3):691–698. doi:10.1136/jim-2018-000801
31. Huang SJ, Tang YY, Liu HM, et al. Impact of age on survival of locoregional nasopharyngeal carcinoma: an analysis of the surveillance, epidemiology, and end results program database, 2004–2013. Clin Otolaryngol. 2018;43(5):1209–1218. doi:10.1111/coa.13124
32. Wu SG, Liao XL, He ZY, et al. Demographic and clinicopathological characteristics of nasopharyngeal carcinoma and survival outcomes according to age at diagnosis: a population-based analysis. Oral Oncol. 2017;73:83–87. doi:10.1016/j.oraloncology.2017.08.006
33. Li AC, Xiao WW, Shen GZ, et al. Distant metastasis risk and patterns of nasopharyngeal carcinoma in the era of IMRT: long-term results and benefits of chemotherapy. Oncotarget. 2015;6(27):24511–24521. doi:10.18632/oncotarget.4312
34. Loong HH, Ma BB, Chan AT. Update on the management and therapeutic monitoring of advanced nasopharyngeal cancer. Hematol Oncol Clin North Am. 2008;22(6):1267–1278. doi:10.1016/j.hoc.2008.08.012
35. Jiang R, Zou X, Hu W, et al. The elevated pretreatment platelet-to-lymphocyte ratio predicts poor outcome in nasopharyngeal carcinoma patients. Tumour Biol. 2015;36(10):7775–7787. doi:10.1007/s13277-015-3505-0
36. Li JP, Chen SL, Peng SG, et al. Prognostic nomogram for patients with nasopharyngeal carcinoma incorporating hematological biomarkers and clinical characteristics. Int J Biol Sci. 2018;14(5):549–556. doi:10.7150/ijbs.24374
37. Xiong Y, Shi LL, Zhu LS, et al. Prognostic efficacy of the combination of the pretreatment systemic immune-inflammation index and epstein-barr virus DNA status in locally advanced nasopharyngeal carcinoma patients. J Cancer. 2021;12(8):2275–2284. doi:10.7150/jca.52539
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.