Back to Journals » OncoTargets and Therapy » Volume 9
Prognostic value of fever grade combined with neutrophil percentage in hepatocellular carcinoma patients presenting fever as the initial manifestation
Authors Gong ZJ, Guo W, Sun YF, Zhang X, Qiu SJ, Zhou J, Fan J, Yang XR
Received 23 March 2016
Accepted for publication 1 June 2016
Published 13 October 2016 Volume 2016:9 Pages 6281—6290
DOI https://doi.org/10.2147/OTT.S109023
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Li
Zi-Jun Gong,1,2 Wei Guo,1–3 Yun-Fan Sun,1,2 Xin Zhang,1,2 Shuang-Jian Qiu,1,2 Jian Zhou,1,2,4 Jia Fan,1,2,4 Xin-Rong Yang1,2
1Liver Cancer Institute, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 2Key Laboratory of Carcinogenesis and Cancer Invasion (Ministry of Education), Fudan University, Shanghai, People’s Republic of China; 3Department of Laboratory Medicine, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 4Institutes of Biomedical Sciences, Fudan University, Shanghai, People’s Republic of China
Background: Hepatocellular carcinoma (HCC) patients with fever as the initial presentation are extremely rare. Our aim was to investigate the clinical characteristics and prognosis of patients with this disease.
Patients and methods: The clinical features were analyzed in a retrospective study of 63 HCC patients with fever as the first manifestation and 300 HCC patients without fever as the control group.
Results: HCC patients with fever had a higher neutrophil percentage, larger tumor size, worse tumor differentiation, advanced Barcelona Clinic Liver Cancer stage, and more hilar lymph node metastasis than HCC patients without fever (all P<0.05). Compared with HCC patients without fever, patients presenting with fever had shorter overall survival (OS, median: 13 months, P<0.001) and time to recurrence (TTR, median: 7.5 months, P<0.001). In addition, HCC patients with fever also had shorter OS and TTR than those without fever in all clinical subgroups with aggressive features (all P<0.05). Multivariate analysis showed that neutrophil percentage >70%, fever grade ≥38.5°C, tumor size >5 cm, and hilar lymph node metastasis were independent factors for OS and TTR. A positive correlation was observed between body temperature and serum neutrophil percentage (r=0.527, P<0.001). Patients with a fever grade ≥38.5°C had more incomplete encapsulation and larger tumor size, while those with a neutrophil percentage >70% presented with more incomplete encapsulation, vascular invasion, and worse tumor differentiation. Patients with a fever grade ≥38.5°C combined with a neutrophil percentage >70% had worse OS and TTR than other groups.
Conclusion: HCC patients presenting with fever have poorer prognosis than those without fever; however, their prognosis could be improved by timely surgical intervention. Patients with a neutrophil percentage >70% and a fever grade ≥38.5°C represent a rare HCC subgroup with an extremely dismal outcome and more aggressive clinical course.
Keywords: hepatocellular carcinoma, fever, neutrophils, prognosis, hepatectomy
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide, and its incidence has increased in recent years.1,2 HCC patients with fever as the initial presentation are extremely rare and only a few cases have been reported.3,4 These HCC patients present with symptoms that mimic liver abscess, as both share similar clinical symptoms and laboratory findings, which makes differential diagnosis a challenge.5,6 Due to its rarity and diagnostic difficulties, most HCC patients with fever are already at an advanced stage at the time of diagnosis, and their prognosis is extremely dismal compared with those without fever, so they may represent a clinically distinct type of HCC.4 Thus, it is necessary to clarify the clinical features of these patients and to explore the related risk factors influencing their prognosis in order to improve patient outcomes.
Although the reasons for fever may be varied, it has been reported that tumor progression is extremely rapid in HCC patients presenting with fever, with a mean survival of only 2.35–3.5 months.3,4 Furthermore, these patients exhibit clinical characteristics that are distinct from other HCC patients. At this time, only limited experience based on a few cases has shown that surgical treatment might prolong the survival time of HCC patients with fever.5 However, detailed analyses of a large cohort evaluating the prognosis of this HCC subgroup after surgery and the related predictive factors are not available.
In this study, the data of 363 cases were retrospectively analyzed, including 63 consecutive HCC patients presenting fever as the initial manifestation and 300 HCC patients without fever who underwent resection at our institute during the past 10 years. We evaluated the efficiency of surgical management of this HCC clinical subgroup as well as the prognostic factors influencing disease recurrence and survival after surgery.
Patients and methods
Patients
Between February 2004 and January 2013, 63 HCC patients with fever underwent curative hepatic resection at the Department of Surgery, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China. Ethical approval for this study and the use of human subjects was obtained from the research ethics committee of Zhongshan Hospital, consistent with the ethical guidelines of the 1975 Declaration of Helsinki, and written informed consent was obtained from all patients. The definitive pathological diagnosis of HCC was based on the World Health Organization criteria. Tumor differentiation was defined according to the Edmondson grading system.7 Tumor stage was determined according to the Barcelona Clinic Liver Cancer (BCLC) staging system.8 Liver function was assessed by the Child-Pugh classification.
The enrollment criteria for all patients with fever in this study were as follows: 1) body temperature >38.0°C; 2) no prior anticancer treatment; 3) complete surgical resection, defined as resection of all tumor nodules with the cut surface being free of cancer by histologic examination;9 4) availability of suitable formalin-fixed, paraffin-embedded tissues; and 5) availability of complete clinicopathologic and follow-up data. Patients with specific factors underlying the fever, such as extrahepatic infection, septicemia, and hepatolithiasis, were excluded. All patients underwent serological testing within 3 days before surgery to determine serum biochemistry. In addition, 300 HCC patients without fever were selected as the control group by random sampling stratified by age, sex, and year of diagnosis from the database within the same observational period.
Follow-up and recurrence treatment
Survival data, including overall survival (OS) and time to recurrence (TTR), were collected until November 30, 2015. The median follow-up was 47 months. OS and TTR were defined as the interval between the date of surgery and death (or the last observation point taken), or any diagnosed relapse (intrahepatic recurrence and extrahepatic metastasis), respectively.10–12 After surgery, one to three courses of prophylactic transcatheter arterial chemoembolization (doxorubicin, cisplatin, 5-fluorouracil, and iodized oil) were given to those patients with a high risk of recurrence, as evidenced by clinical features such as vascular invasion and microsatellite lesions.13
Patient follow-up was performed every 2 months during the first postoperative year and then at least every 3–4 months. Serum α-fetoprotein (AFP) level, abdomen ultrasonography, and chest X-ray were performed to prospectively monitor recurrence every 1–6 months depending on the postoperative time. A computed tomography scan of the abdomen was performed every 6 months. A bone scan or magnetic resonance imaging was done if localized bone pain was reported. If recurrence was suspected, a computed tomography scan or magnetic resonance imaging was done immediately. Patients with an elevated serum AFP level and typical imaging appearance on computed tomography or magnetic resonance imaging were diagnosed with recurrence and received further treatment. Re-resection, radio frequency ablation, or percutaneous ethanol injection were considered if the recurrent tumor was localized. Transcatheter arterial chemoembolization was administered if the recurrent tumor was multiple or diffused. External radiotherapy was given if lymph node or bone metastasis was found; otherwise, symptomatic treatment was provided.14,15
Statistical analysis
SPSS version 18 (SPSS Inc., Chicago, IL, USA) was used to analyze the data. A chi-square test, Fisher’s exact test, and Student’s t-test were used for comparisons between the groups as appropriate. OS and recurrence rates were calculated by the Kaplan–Meier method. The differences in survival between the groups were compared using the log-rank test. Univariate and multivariate analyses were performed using the Cox proportional hazard regression model. All tests were two-tailed, and P-values <0.05 were considered significant.
Results
Clinical characteristics of HCC patients presenting with fever and their prognosis
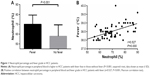
A total of 63 HCC patients presenting fever as the initial manifestation, including 56 males and seven females, were consecutively enrolled in this study. The median body temperature was 38.5°C, which was used as a cutoff for further analysis. Clinically, HCC patients with fever have symptoms that are similar to those associated with liver abscess, such as leukocytosis. In particular, neutrophil percentage in the peripheral blood increases in response to fever and is commonly used as an indicator for systemic inflammation from infectious or noninfectious causes. In addition, we found that the neutrophil percentage was higher in HCC patients with fever than in those without fever (70.1%±9.3% vs 57.9%±11.5%, P=0.001; Figure 1A). Therefore, for patients with fever, a neutrophil percentage >70% was used as cutoff for further study. The relationship between the neutrophil percentage and fever grade was further analyzed, and we found that body temperature and serum neutrophil percentage were positively correlated (r=0.527, P=0.000; Figure 1B).
Next, we compared the clinical characteristics of HCC patients with fever (n=63) to those of HCC patients without fever (n=300; Table 1). We found that HCC patients presenting with fever were more likely to have increased serum levels of γ-glutamyl transferase (GGT, P=0.000), larger tumor size (P=0.000), worse tumor differentiation (P=0.045), more advanced BCLC stage (P=0.000), and a higher likelihood of hilar lymph node metastasis (P=0.005). There were no significant differences in other clinical characteristics between the two groups (Table 1).
The prognosis of HCC patients with fever was dismal. Kaplan–Meier analyses showed that HCC patients with fever had shorter OS (median: 13.0 months vs 58.3 months, P=0.000) and TTR (7.5 months vs 40.5 months, P=0.000) after operation compared with those without fever (Figure 2A and B). The 1-, 3-, 5-, and 7-year survival rates were 66.2%, 39.7%, 16.1%, and 16.1% in HCC patients with fever compared with 85.9%, 70.6%, 59.1%, and 56.9% in the control group, respectively. The 1-, 3-, 5-, and 7-year relapse rates were 55.8%, 77.2%, 85.8%, and 91.6% in HCC patients with fever, compared with 24.7%, 38.8%, 47%, and 56.7% in those without fever, respectively. Only three of 63 (4.8%) HCC patients with fever survived more than 5 years without tumor recurrence.
The OS and TTR between HCC patients with/without fever in different subgroups were further investigated using the Kaplan–Meier method (Table 2). The results showed that the prognosis for HCC with fever was worse than HCC without fever in all subgroups with aggressive features such as no tumor encapsulation, presence of vascular invasion, AFP >20 ng/mL, GGT levels >54 U/L, BCLC stage B + C, tumor size >5 cm, and tumor differentiation III–IV (all P<0.05).
Factors affecting the prognosis of HCC patients presenting with fever
The prognostic factors of HCC patients with fever were further identified. Univariate analysis showed that tumor size >5 cm, lack of encapsulation, hilar lymph node metastasis, neutrophil percentage >70%, and fever grade ≥38.5°C were related to tumor recurrence of HCC patients with fever after operation. Multivariate analysis using a Cox regression model showed that the independent factors for tumor recurrence were tumor >5 cm (P=0.036, hazard ratio [HR]: 2.584, 95% confidence interval [95% CI]: 1.066–6.263), the presence of hilar lymph node metastasis (P=0.037, HR: 2.939, 95% CI: 1.069–8.081), neutrophil percentage >70% (P=0.026, HR: 2.204, 95% CI: 1.097–4.429), and fever grade ≥38.5°C (P=0.025, HR: 2.059, 95% CI: 1.093–3.878; Table 3).
Univariate analysis showed that AFP >20 ng/mL, tumor size >5 cm, presence of hilar lymph node metastasis, lack of encapsulation, neutrophil percentage >70%, and fever grade ≥38.5°C were associated with shorter patient survival. Multivariate analysis showed that the independent factors for survival were the presence of hilar lymph node metastasis (P=0.004, HR: 6.140, 95% CI: 1.802–20.917), larger tumor size (P=0.022, HR: 3.610, 95% CI: 1.208–10.788), neutrophil percentage >70% (P=0.013, HR: 2.674, 95% CI: 1.232–5.804), and fever grade ≥38.5°C (P=0.012, HR: 2.922, 95% CI: 1.266–6.774; Table 3).
Next, we compared OS and TTR in the patients with fever according to neutrophil percentage >70% and fever grade ≥38.5°C. Patients with neutrophil percentage >70% had shorter OS (median: 6.8 months vs 16 months; P=0.000) and TTR (median: 3.8 months vs 9 months; P=0.004) than those with neutrophil percentage ≤70%. The 1-, 3-, and 5-year OS rates were 48.1%, 26.7%, and 0% in patients with neutrophil percentage >70% compared with 77.9%, 48.5%, and 25.1% in those with neutrophil percentage ≤70%, respectively. The 1-, 3-, and 5-year relapse rates were 78.2%, 88.4%, and 100% in patients with neutrophil percentage >70% compared with 41.6%, 70.8%, and 79.6% in those with ≤70%, respectively (Figure 2C and D).
Those with fever grade ≥38.5°C had shorter OS (median: 7 months vs 19.6 months, P=0.000) and TTR (median: 5.6 months vs 12.1 months; P=0.000) than those with fever grade <38.5°C. The 1-, 3-, and 5-year OS rates were 48%, 17.2%, and 6.5% in patients with fever grade ≥38.5°C compared with 88%, 68.3%, and 27.7% in those with fever grade <38.5°C, respectively. The 1-, 3-, and 5-year relapse rates were 75.7%, 90.9%, and 95.4% in patients with fever grade ≥38.5°C compared with 34.9%, 58.7%, and 73.4% in those with fever grade <38.5°C, respectively (Figure 2E and F).
Prognostic significance of the combination of neutrophil percentage and fever grade in HCC patients presenting with fever
As the neutrophil percentage and fever grade were independent factors for survival in HCC patients presenting with fever, as well as the most easily available indices in clinical practice, the power of their combination for predicting patient outcome was further evaluated. Patients with fever were classified into three subgroups: group I (neutrophil percentage ≤70% and fever grade <38.5°C, n=22); group II (neutrophil percentage >70% and fever grade <38.5°C or neutrophil percentage ≤70% and fever grade ≥38.5°C, n=24); and group III (neutrophil percentage >70% and fever grade ≥38.5°C, n=17). Those in group III had the shortest OS (median: 5.1 months in group III vs 11.5 months in group II and 19.8 months in group I) and TTR (3 months in group III vs 7.2 months in group II and 11.5 months in group I) compared with those in group I and II (all P<0.05, Figure 2G and H). Among the 17 patients in group III, only five patients survived for more than 1 year, and of those patients only two survived for more than 2 years after surgery. Furthermore, 14 of these patients had tumor recurrence within 1 year after surgery. In addition, multivariate analysis confirmed that group III was an independent prognosis indicator for both OS and TTR (all P-values <0.05, Table 3).
Correlation of neutrophil percentage and fever grade with clinicopathologic features
The clinical features of patients with fever according to neutrophil percentage >70% and fever grade ≥38.5°C were analyzed. Those with fever grade ≥38.5°C (n=34) presented with more incomplete encapsulation (P=0.020) and larger tumor size (P=0.007) compared with those with fever <38.5°C (n=29; Table 4). Patients with neutrophil percentage >70% (n=24) presented with more incomplete encapsulation (P=0.000), vascular invasion (P=0.015), and worse tumor differentiation (P=0.039) than those with neutrophil levels ≤70% (n=39; Table 4).
Those with neutrophil percentage >70% combined with fever grade ≥38.5°C had the worst prognosis, and we found that these patients had more incomplete encapsulation (P=0.000) and vascular invasion (P=0.028) than any other combination (all P-values <0.05; Table 4).
Discussion
HCC with fever is an uncommon subgroup that has different clinicopathologic features and poorer prognosis than HCC without fever.4 Our study showed that HCC patients with fever had a poor prognosis compared with those without fever. The median OS in patients with fever was only 13 months. However, this was higher than the median OS found in previous reports, which was only 2–3 months without surgery.3,5 Thus, the longer survival time obtained in our study cohort may be due to timely surgical resection for these patients. Although surgical resection could improve the prognosis of this special subgroup of HCC, most of the patients presenting with fever died from tumor recurrence after surgery, and there were only three of 63 (4.8%) patients who survived for more than 5 years. Thus, a reasonable assessment of the prognosis of patients prior to surgery is necessary in order to tailor the most effective therapy according to characteristics of the individual tumors. Our data imply that the fever grade and neutrophil percentage could meet these requirements, as both show prognostic significance in predicting the clinical outcome for HCC patients presenting with fever and can be easily obtained before surgery.
We found that HCC patients with fever had a propensity for large tumor size, poor tumor differentiation, advanced tumor stage, and lymph node metastasis, which is indicative of the malignant nature of these patients. Furthermore, we found that even in the subgroups with malignant features, the OS and TTR of HCC with fever were worse than those of HCC without fever, suggesting that the worse prognosis of patients with fever may be mainly caused by other factors. We found that the neutrophil percentage was higher in the HCC patients presenting with fever (70.1% vs 57.9%, P=0.001) compared with those without fever. The cause of fever remains unclear. One explanation for neoplasm-associated fever and leukocytosis is the production of granulopoietin and pyrogens, including interleukin-1, interleukin-6, and tumor necrosis factor, by tumor cells.16–21 These pyrogens have been associated with an increased incidence of neoplastic fever and with advanced stage in renal cell carcinomas and lymphomas.22–24 These cytokines activate the anterior preoptic nuclei of the hypothalamus and raise the threshold of body temperature via prostaglandin E2.25 In our study, patients with fever were characterized by large tumors, which have a risk of spontaneous liquefied necrosis within the tumor26 and they may produce more humoral factors, contributing to fever and leukocytosis. In addition, patients with liquefied necrosis combined with fever and leukocytosis are often misdiagnosed as having liver abscess, which causes them to miss the best opportunity for resection and makes them more likely to be at an advanced stage at the time of eventual diagnosis.27–29
Host inflammatory conditions play important roles in tumor development and progression.30 We found that the neutrophil percentage was higher in the HCC patients presenting with fever compared with those without fever, which indicated the activation of an inflammatory state in these patients. More importantly, shorter OS and TTR were observed in patients with fever with a neutrophil percentage >70% than in those with a neutrophil percentage ≤70%. Neutrophils, which are the primary inflammatory cells, can enhance tumor cell survival, proliferation, and metastasis by secreting immunoreactive molecules, such as neutrophil elastase, β2-integrins, oncostatin M, or hepatocyte growth factor.31–34 Thus, the activation of an inflammatory state might contribute to the aggressive clinical course in HCC patients with fever.
We found that a neutrophil percentage >70% was an independent risk factor for the OS and TTR of HCC with fever, which confirmed that neutrophil-associated inflammation was involved in the poor prognosis of patients with fever. Meanwhile, we also found that body temperature was positively correlated with the neutrophil percentage, and fever grade ≥38.5°C was also an independent risk factor for OS and TTR of HCC with fever after resection. Because fever grade and leukocytosis are direct indices for host inflammatory status and are readily available in the clinic, we combined these two factors to predict the prognosis of HCC patients with fever undergoing resection. We found that the median survival was only 5.1 months in patients presenting with fever grade ≥38.5°C combined with neutrophil percentage >70%, and most of these patients (14/17) died from tumor recurrence even after tumor resection, which was similar to the survival of patients without surgery in previous reports (median survival: 2–3 months). Thus, it seems that surgical resection is not effective for these patients, and novel nonsurgical approaches need to be further explored in order to improve their prognosis, as they have aggressive disease and a rapidly deteriorating clinical course.
Limitations
The limitations of this study are its relatively small cohort size, retrospective design, and procurement of data from a single study center. Additionally, C-reactive protein is not routinely measured in our daily practice, and we did not include C-reactive protein in this study. Finally, the precise mechanism of fever and leukocytosis in these HCC patients needs to be further explored.
Conclusion
To our knowledge, this is the first report to demonstrate comprehensively the clinical characteristics of this rare clinical HCC subgroup with the largest sample size in the world. Our study indicates that HCC patients presenting with fever as the initial manifestation have a poorer prognosis than those without fever, while their prognosis would be improved by timely surgical intervention. Patients with a neutrophil percentage >70% and a fever grade of ≥38.5°C have an extremely dismal outcome and more aggressive clinical course. Inflammatory processes and endogenous pyrogen-related cytokines may mediate the development and progression of cancer in these patients.
Acknowledgments
The authors thank Pingting Gao, Bo Hu, and Ao Huang for their clinical help and support in this study. This study was supported by grants from the National Natural Science Foundation of China (81572064, 81372317, 81472676, and 81572823), the Projects from the Shanghai Science and Technology Commission (13140901900, 134119a1201, 14DZ1940300, 14411970200, and 14140902301), and the Specialized Research Fund for the Doctoral Program of Higher Education and Research Grants Council Earmarked Research Grants Joint Research Scheme (20130071140008).
Disclosure
The authors report no conflicts of interest in this work.
References
De Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62(4):1190–1200. | ||
Kaseb AO, Morris JS, Iwasaki M, et al. Phase II trial of bevacizumab and erlotinib as a second-line therapy for advanced hepatocellular carcinoma. Onco Targets Ther. 2016;9:773–780. | ||
Yeh TS, Jan YY, Jeng LB, Chen TC, Hwang TL, Chen MF. Hepatocellular carcinoma presenting as pyogenic liver abscess: characteristics, diagnosis, and management. Clin Infect Dis. 1998;26(5):1224–1226. | ||
Okuda K, Kondo Y, Nakano M, et al. Hepatocellular carcinoma presenting with pyrexia and leukocytosis: report of five cases. Hepatology. 1991;13(4):695–700. | ||
Li C, Li G, Miao R, et al. Primary liver cancer presenting as pyogenic liver abscess: characteristics, diagnosis, and management. J Surg Oncol. 2012;105(7):687–691. | ||
Chong VH, Lim KS. Pyogenic liver abscess as the first manifestation of hepatobiliary malignancy. Hepatobiliary Pancreat Dis Int. 2009;8(5):547–550. | ||
Wittekind C. Pitfalls in the classification of liver tumors. Pathologe. 2006;27(4):289–293. | ||
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. | ||
Poon RTP, Ng IOL, Lau C, et al. Tumor microvessel density as a predictor of recurrence after resection of hepatocellular carcinoma: a prospective study. J Clin Oncol. 2002;20(7):1775–1785. | ||
Yang X-R, Xu Y, Shi G-M, et al. Cytokeratin 10 and cytokeratin 19: predictive markers for poor prognosis in hepatocellular carcinoma patients after curative resection. Clin Cancer Res. 2008;14(12):3850–3859. | ||
Llovet JM, Di Bisceglie AM, Bruix J, et al. Panel of Experts in HCC-Design Clinical Trials. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100(10):698–711. | ||
Yang X-R, Xu Y, Yu B, et al. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut. 2010;59(7):953–962. | ||
Choi D, Lim HK, Rhim H, et al. Percutaneous radiofrequency ablation for recurrent hepatocellular carcinoma after hepatectomy: long-term results and prognostic factors. Ann Surg Oncol. 2007;14(8):2319–2329. | ||
Li T, Fan J, Qin L-X, et al. Risk factors, prognosis, and management of early and late intrahepatic recurrence after resection of primary clear cell carcinoma of the liver. Ann Surg Oncol. 2011;18(7):1955–1963. | ||
Hu B, Yang X-R, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. | ||
Conti B, Tabarean I, Andrei C, Bartfai T. Cytokines and fever. Front Biosci. 2004;9:1433–1449. | ||
Netea MG, Kullberg BJ, Van der Meer JW. Circulating cytokines as mediators of fever. Clin Infect Dis. 2000;31(suppl 5):S178–S184. | ||
Nakagawa H, Umemura A, Taniguchi K, et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell. 2014;26(3):331–343. | ||
Ryu J, Kang M, Lee MS, et al. Cross talk between the TM4SF5/focal adhesion kinase and the interleukin-6/STAT3 pathways promotes immune escape of human liver cancer cells. Mol Cell Biol. 2014;34(16):2946–2960. | ||
Kohga K, Tatsumi T, Tsunematsu H, et al. Interleukin-1beta enhances the production of soluble MICA in human hepatocellular carcinoma. Cancer Immunol Immunother. 2012;61(9):1425–1432. | ||
Su B, Luo T, Zhu J, et al. Interleukin-1beta/Iinterleukin-1 receptor-associated kinase 1 inflammatory signaling contributes to persistent Gankyrin activation during hepatocarcinogenesis. Hepatology. 2015;61(2):585–597. | ||
Seymour JF, Talpaz M, Cabanillas F, Wetzler M, Kurzrock R. Serum interleukin-6 levels correlate with prognosis in diffuse large-cell lymphoma. J Clin Oncol. 1995;13(3):575–582. | ||
Blay JY, Rossi JF, Wijdenes J, et al. Role of interleukin-6 in the paraneoplastic inflammatory syndrome associated with renal-cell carcinoma. Int J Cancer. 1997;72(3):424–430. | ||
Blay JY, Negrier S, Combaret V, et al. Serum level of interleukin-6 as a prognosis factor in metastatic renal-cell carcinoma. Cancer Res. 1992;52(12):3317–3322. | ||
Kurzrock R. The role of cytokines in cancer-related fatigue. Cancer. 2001;92(6):1684–1688. | ||
Lin YT, Liu CJ, Chen TJ, et al. Pyogenic liver abscess as the initial manifestation of underlying hepatocellular carcinoma. Am J Med. 2011;124(12):1158–1164. | ||
Shimizu Y, Kaneko F, Motoori T, Yokomori H. Undifferentiated hepatocellular carcinoma difficult to distinguish from liver abscess. Intern Med. 2011;50(5):519–520. | ||
Inagaki Y, Sugimoto K, Shiraki K, et al. Sarcomatous hepatocellular carcinoma with remittent fever. Intern Med. 2012;51(21):3025–3029. | ||
Kruth J, Michaely H, Trunk M, et al. A rare case of fever of unknown origin: inflammatory myofibroblastic tumor of the liver. Case report and review of the literature. Acta Gastroenterol Belg. 2012;75(4):448–453. | ||
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. | ||
Wislez M, Rabbe N, Marchal J, et al. Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: role in tumor progression and death. Cancer Res. 2003;63(6):1405–1412. | ||
Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65(19):8896–8904. | ||
Strell C, Lang K, Niggemann B, Zaenker KS, Entschladen F. Neutrophil granulocytes promote the migratory activity of MDA-MB-468 human breast carcinoma cells via ICAM-1. Exp Cell Res. 2010;316(1):138–148. | ||
Houghton AM, Rzymkiewicz DM, Ji H, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16(2):219–223. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.