Back to Journals » Cancer Management and Research » Volume 10
Prognostic value of ductal carcinoma in situ component in invasive ductal carcinoma of the breast: a Surveillance, Epidemiology, and End Results database analysis
Authors Wu SG, Zhang WW , Sun JY, He ZY
Received 20 October 2017
Accepted for publication 15 January 2018
Published 19 March 2018 Volume 2018:10 Pages 527—534
DOI https://doi.org/10.2147/CMAR.S154656
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
San-Gang Wu,1,* Wen-Wen Zhang,2,* Jia-Yuan Sun,2 Zhen-Yu He2
1Department of Radiation Oncology, Xiamen Cancer Hospital, The First Affiliated Hospital of Xiamen University, Xiamen, People’s Republic of China; 2Department of Radiation Oncology, Sun Yat-sen University Cancer Center, and State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Background: The prognostic implication of concomitant ductal carcinoma in situ (DCIS) in invasive ductal carcinoma (IDC) remains controversial. Our objective was to investigate whether concomitant DCIS affects survival outcomes in patients with IDC.
Materials and methods: Patients with nonmetastatic breast cancer who underwent surgery in 2010–2014 were included from the Surveillance, Epidemiology, and End Results program. Statistical analyses were conducted using Χ2 test, linear-by-linear association, one-way analysis of variance, Kaplan–Meier method, Cox proportional hazards regression model, and propensity score matching (PSM).
Results: A total of 61,745 patients were identified, including 44,630 (72.3%), 13,559 (22.0%), and 3,556 (5.7%) patients with no DCIS component reported (No-DCIS), DCIS <25% (L-DCIS), and ≥25% (H-DCIS), respectively. Patients with H-DCIS were more likely to be younger (p<0.001), have smaller tumors (p<0.001), good/moderate differentiation (p<0.001), human epidermal growth factor receptor 2–positive disease (p<0.001), receive mastectomy (p<0.001), and not receive radiotherapy (p<0.001) and chemotherapy (p<0.001). The median follow-up was 27 months, and the 2-year breast cancer-specific survival (BCSS) in patients with No-DCIS, L-DCIS, and H-DCIS was 97.3%, 98.0%, and 98.5%, respectively (p<0.001). Before PSM, H-DCIS was an independent favorable prognostic factor for BCSS; patients with H-DCIS had better BCSS compared to patients with No-DCIS (hazard ratio [HR] 0.674, 95% CI: 0.528–0.861, p=0.002), while the BCSS between No-DCIS and L-DCIS was similar (HR 0.944, 95% CI: 0.840–1.061, p=0.334). However, this survival advantage disappeared after PSM; there was significantly different BCSS between patients with No-DCIS and H-DCIS (HR 0.923, 95% CI: 0.653–1.304, p=0.650). H-DCIS was not associated with BCSS as compared to No-DCIS in the breast-conserving surgery (p=0.295) and mastectomy (p=0.793) groups.
Conclusion: In breast cancer, patients with H-DCIS have unique clinicopathologic features compared to patients with No-DCIS. Before PSM, H-DCIS was associated with favorable BCSS as compared to No-DCIS. However, the survival advantage disappeared after PSM.
Keywords: breast cancer, ductal carcinoma in situ, invasive ductal carcinoma, survival, prognosis, surgery
Introduction
In breast cancer, ductal carcinoma in situ (DCIS) is recognized as a precursor of invasive ductal carcinoma (IDC) and usually accompanies IDC, which is associated with different clinical courses and treatment strategies.1 Breast IDC is assumed to appear de novo in patients with pure IDC; in patients with IDC with DCIS component (IDC-DCIS), it is postulated that IDC develops from a pre-existing DCIS lesion.2 Patients with breast IDC-DCIS are more likely to be younger, have smaller tumors, and lower probability of lymph node involvement.3–5 In addition, more patients with breast IDC-DCIS have nonpalpable tumors or are screen detected.2,5 Based on mammography screening, DCIS component is present in 30%–60% of breast IDC.6–10 Therefore, patients with breast IDC with DCIS component may have less biologically aggressive disease than patients with pure IDC of the breast. However, the question of whether IDC-DCIS affects the clinical outcome in patients with breast cancer is important, but has not been well studied.
The concordant expression of immunohistochemical11–14 and genomic15–17 markers supports the clonal relationship between breast DCIS and IDC-DCIS. However, survival in patients with DCIS in breast IDC remains uncertain. Several studies have found that the presence of DCIS is often related to favorable clinicopathologic features in invasive breast cancer, but is not an independent prognostic factor in survival outcomes, including locoregional and distant recurrence and disease-specific death.2,4,5 In contrast, other studies have found that breast IDC-DCIS is associated with higher rates of local recurrence due to a higher incidence of positive surgical margins.18–20 In addition, patients with breast IDC-DCIS had better clinical outcomes compared with patients with pure IDC of the breast,3,8,21 which may be due to cell-mediated immunologic response with the presence of DCIS.22 Population heterogeneity and different treatment strategies may be the main causes of the conflicting results. However, there are no prospective studies comparing the clinicopathologic characteristics and survival outcomes of the IDC and IDC-DCIS subtypes in breast cancer. In this study, we analyzed population-based cancer registry data to investigate whether concomitant DCIS affects survival outcomes in patients with IDC.
Materials and methods
Patients
We retrospectively analyzed data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program between 2010 and 2014, which is a collection of de-identified data from 18 cancer registries in the USA covering ~28% of the population.23 The SEER database includes information on patient demographics, clinicopathologic characteristics, first course of treatment, and follow-up for vital status. We received permission to access the SEER database (Authorization No. 11025-Nov2016). We included adult female patients with primary nonmetastatic breast cancer with IDC where the DCIS component was mentioned (ICD-O-3, 8500/3). We excluded patients whose demographic features, clinicopathologic variables, and surgical procedures were unknown. The ethics committee of the First Affiliated Hospital of Xiamen University approved this study.
Demographic and clinicopathologic variables
The following variables were extracted from the SEER database: age, race/ethnicity, tumor grade, tumor (T)-stage, nodal status, breast cancer subtype, radiotherapy, chemotherapy, and surgical procedure, including breast-conserving surgery and mastectomy. The TNM classification was based on the seventh edition of the American Joint Committee on Cancer/Union for International Cancer Control breast cancer staging system. The T-stage was defined as the entire tumor size because the size of invasive component was not stated in the SEER program. Four major subtypes of breast cancer subtypes were defined as follows: hormone receptor (HoR)+/human epidermal growth factor receptor 2 (HER2)−, HoR+/HER2+, HoR−/HER2+, and HoR−/HER2. The HoR-positive disease was defined as estrogen receptor (ER) and/or progesterone receptor (PR) positive. Patients were classified into three groups in accordance with the DCIS component: No-DCIS, no DCIS component reported; L-DCIS, minimal DCIS component present (<25%); and H-DCIS, extensive DCIS component present (≥25%). The patterns of local and distant recurrence were not included in the SEER database. Therefore, we used breast cancer-specific survival (BCSS) as the primary survival outcome. BCSS was defined as the time from initial diagnosis to the date of breast cancer-related death.
Statistical analysis
The χ2 test and linear-by-linear association for frequency distributions and one-way analysis of variance for continuous variables were used to compare differences in DCIS component status. The BCSS among the groups was estimated using the Kaplan–Meier analysis and compared by the log-rank test. Cox proportional hazards regression model analyses were used to identify the risk factors for BCSS. As most variables may not be equally distributed between the groups, we used propensity score matching (PSM) to minimize potential selection bias between the groups.24 The PSM is a useful statistical method that focuses on the relationship between confounding factors and the treatment, allowing the analysis to be performed almost completely without reference to the results variable, avoiding the possibility of the selected statistical method being affected by the outcomes of the analysis to the maximum extent.25–27 In this retrospective study, propensity scores were calculated using a multiple logistic regression model for each patient with an algorithm of 1:1 matching based on the following variables: age, race/ethnicity, tumor grade, T-stage, nodal status, breast cancer subtype, and surgical procedure. Statistical analyses were performed using SPSS version 22 statistical software (IBM Corporation, Armonk, NY, USA). p-value <0.05 was considered statistically significant.
Results
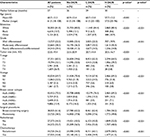
We identified a total 61,745 patients with a median age of 61 years (range, 18–108 years), and a total of 44,630 (72.3%), 13,559 (22.0%), and 3,556 (5.7%) had No-DCIS, L-DCIS, and H-DCIS, respectively. Table 1 lists the patients’ characteristics before PSM. Descriptive and correlational analyses using χ2 test, one-way analysis of variance, or linear-by-linear association indicated that patients with H-DCIS were more likely to be younger (p<0.001), have smaller tumors (p<0.001), early T-stage (p<0.001), well/moderate differentiation (p<0.001), and HER2-positive disease (p<0.001), compared to patients with No-DCIS. Patients with H-DCIS were also more likely to undergo mastectomy (p<0.001) and not receive radiotherapy (p<0.001) and chemotherapy (p<0.001). The median follow-up was 27 months (range, 0–59 months). A total of 2,010 patients died of breast cancer-related disease, and the 2-year BCSS was 97.4%.
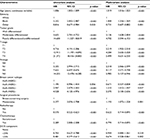
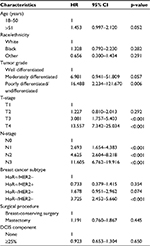
The results of univariate and multivariate analyses before PSM are shown in Table 2. The results indicated that increasing age (p<0.001), black race (p<0.001), higher grade (p<0.001), advanced T-stage (p<0.001) and N-stage (p<0.001), and HoR−/HER2+ (p<0.001) and HoR−/HER2− (p<0.001) subtypes were associated with a higher risk of breast cancer-related death. In addition, patients who received breast-conserving surgery (p<0.001), radiotherapy (p<0.001), and did not receive chemotherapy (p<0.001) had a BCSS advantage. Patients with L-DCIS (p<0.001) and H-DCIS (p<0.001) were also associated with better BCSS compared to patients with No-DCIS. Multivariate Cox regression confirmed that H-DCIS was an independent favorable prognostic factor of BCSS; patients with H-DCIS had better BCSS compared to patients with No-DCIS (hazard ratio [HR] 0.674, 95% CI: 0.528–0.861, p=0.002), while there was no significant difference between No-DCIS and L-DCIS (HR 0.944, 95% CI: 0.840–1.061, p=0.334). The 2-year BCSS in patients with No-DCIS, L-DCIS, and H-DCIS was 97.3%, 98.0%, and 98.5%, respectively (log-rank test, p<0.001; Figure 1A). The other independent prognostic factors were age, race/ethnicity, tumor grade, T-stage, nodal stage, breast cancer subtype, surgical procedure, radiotherapy, and chemotherapy (Table 2).
The survival difference in patients with No-DCIS and H-DCIS was analyzed using PSM. A total of 3,498 pairs were completely matched. Table 3 shows the patients’ characteristics after PSM. Adjustment by age, race/ethnicity, tumor grade, T-stage, nodal stage, breast cancer subtype, and surgical procedure showed no significant difference in BCSS between No-DCIS and H-DCIS (HR 0.923, 95% CI: 0.653–1.304, p=0.650; Table 4). Figure 1B shows the survival curve in the matched group (log-rank test, p=0.731).
The survival difference according to the surgical procedure between No-DCIS and H-DCIS after PSM was further analyzed. The presence of H-DCIS was not associated with survival outcome compared to No-DCIS in the breast-conserving surgery group (p=0.295) and the mastectomy group (p=0.793).
Discussion
The role of IDC-DCIS in the clinical outcome of patients with breast cancer has not been well studied. We used a population-based study to investigate the clinicopathologic features and prognosis of DCIS component in patients with breast IDC. Our findings suggest that patients with H-DCIS have unique clinicopathologic features compared to patients with No-DCIS, and patients with H-DCIS were associated with favorable BCSS as compared to No-DCIS patients before PSM. However, the survival advantage disappeared after PSM.
There is no consensus on the clinical characteristics and prognostic implications of breast IDC with DCIS. It is now widely accepted that patients with breast IDC-DCIS are more likely to be younger, have smaller tumors, and lower incidence of lymph node metastasis.3–5 Our results also showed that patients with accompanying DCIS showed a higher proportion of younger age and had small tumors, while the status of lymph nodes was not significantly different between the groups. Moreover, patients with DCIS component were more likely to have no palpable tumors and be screen detected.2,5 Our results are similar to the concept that patients with tumors originating from in situ lesions may be less biologically aggressive than the patients with entirely invasive tumors.
The expression of ER, PR, HER2, and Ki-67 between No-DCIS and IDC-DCIS of the breast remains controversial.2–4,8,21 Some studies found that patients with accompanying DCIS had a higher expression of ER, PR, and HER2 compared to patients with No-DCIS.2,4 However, several studies also indicated that the expression of ER, PR, and HER2 was not associated with accompanying DCIS in IDC of the breast.3,8 Similarly, there have been several controversial studies regarding the expression of Ki-67 between No-DCIS and IDC-DCIS.2,21 In addition, opposite results were also found with regard to tumor grade between the groups.5,8,28 The results of our study suggested that patients with H-DCIS were more likely to be of lower tumor grade and with HER2-positive disease compared to patients with No-DCIS. The heterogeneity of the population and the difference in sample size may be the main reasons contributing to the contrary results of the above studies.
In breast cancer, extensive DCIS is defined as prominent DCIS occupying at least 25% of the invasive tumor.29 The SEER database recorded the DCIS component data after 2010, classifying the extent of the DCIS component as No-DCIS, L-DCIS, and H-DCIS. Cedolini et al found that invasive cancers with H-DCIS were associated with longer disease-free survival and lower local recurrence rates.21 However, several studies have found that the presence of DCIS was not an independent prognostic factor in survival outcomes, including locoregional and distant recurrence and disease-specific death.2,4,5 The results of this study are similar to those of the study by Cedolini et al,21 indicating the patients with H-DCIS had better BCSS compared to patients with No-DCIS before PSM.
The PSM is becoming an advanced statistical method to control potential confounding effects in any retrospective study. The difference between traditional statistical method and PSM is that PSM relies on the exposure effect model, while traditional statistics method depends on the model being analyzed.30 This study used PSM to assess the association between DCIS component and breast cancer survival. To obtain more reliable outcomes, we used seven covariates including age, race/ethnicity, tumor grade, T-stage, nodal status, breast cancer subtype, and surgical procedure. Before PSM, the survival advantage in patients with H-DCIS may be related to the favorable clinicopathologic features, which could explain why the survival advantage disappeared after PSM. Therefore, DCIS-IDC may not necessarily lead to more favorable survival compared to IDC in breast cancer due to the similar genomic profiles.14–17
It has been reported that patients with breast IDC-DCIS are less likely to undergo breast-conserving surgery,2,4,21 which is similar to our results. Given the diffuse features of DCIS, positive surgical margins are more likely in patients with DCIS-IDC after breast-conserving surgery,18,20,31 which may be associated with a higher rate of local recurrence and potentially worse survival. However, in patients who underwent mastectomy without adjuvant radiotherapy and chemotherapy, IDC-DCIS was an independent prognostic factor of decreased local recurrence compared with IDC alone, but not for distant metastases.3 Due to the limitations of the SEER database, we could not obtain the local and distant recurrence patterns. Nevertheless, we did not find that patients with IDC-DCIS had better BCSS than patients with No-DCIS after breast-conserving surgery or mastectomy.
Despite this being the largest study to assess the prognosis of the coexistence of DCIS in IDC of patients with nonmetastatic breast cancer, our results should be interpreted with caution due to several important limitations. First, there is inherent bias in any retrospective analysis. Second, the tumor samples were not analyzed with central pathologic review. Third, the median follow-up was only 27 months, which is too short to expect significant differences in BCSS; this may be due to the reason that the DCIS component was started to be collected in SEER program after 2010. In addition, the size code about the invasive and in situ components of breast cancer was defined as the entire tumor size because the size of invasive component was not stated. However, the primary strength of this study is that it is the largest population-based cohort to date to investigate whether concomitant DCIS affects survival outcomes in patients with IDC, allowing to minimize the selection and surveillance biases from single academic institutions.
Conclusion
In conclusion, our results suggest that the H-DCIS patients have unique clinicopathologic characteristics compared to patients with No-DCIS of the breast. Before PSM, H-DCIS was associated with favorable BCSS compared to patients with No-DCIS. However, our study also provides compelling evidence that the DCIS component in breast IDC does not affect survival after PSM. More studies are needed to confirm our results.
Acknowledgments
This work was partly supported by the Natural Science Foundation of Fujian Province (No. 2016J01635), the Science and Technology Planning Projects of Xiamen Science and Technology Bureau (No. 3502Z20174070), and Guangdong Medical Research Foundation (No. A2017023).
Disclosure
The authors report no conflicts of interest in this work.
References
Pinder SE, Ellis IO. The diagnosis and management of pre-invasive breast disease: ductal carcinoma in situ (DCIS) and atypical ductal hyperplasia (ADH)–current definitions and classification. Breast Cancer Res. 2003;5(5):254–257. | ||
Wong H, Lau S, Yau T, Cheung P, Epstein RJ. Presence of an in situ component is associated with reduced biological aggressiveness of size-matched invasive breast cancer. Br J Cancer. 2010;102(9):1391–1396. | ||
Dieterich M, Hartwig F, Stubert J, et al. Accompanying DCIS in breast cancer patients with invasive ductal carcinoma is predictive of improved local recurrence-free survival. Breast. 2014;23(4):346–351 | ||
Kim JY, Han W, Moon HG, et al. Grade of ductal carcinoma in situ accompanying infiltrating ductal carcinoma as an independent prognostic factor. Clin Breast Cancer. 2013;13(5):385–391. | ||
Chagpar AB, McMasters KM, Sahoo S, Edwards MJ. Does ductal carcinoma in situ accompanying invasive carcinoma affect prognosis? Surgery. 2009;146(4):561–567; discussion 567–568. | ||
Jo BH, Chun YK. Heterogeneity of invasive ductal carcinoma: proposal for a hypothetical classification. J Korean Med Sci. 2006;21(3):460–458. | ||
Dzierzanowski M, Melville KA, Barnes PJ, MacIntosh RF, Caines JS, Porter GA. Ductal carcinoma in situ in core biopsies containing invasive breast cancer: correlation with extensive intraductal component and lumpectomy margins. J Surg Oncol. 2005;90(2):71–76. | ||
Logullo AF, Godoy AB, Mourão-Neto M, Simpson AJ, Nishimoto IN, Brentani MM. Presence of ductal carcinoma in situ confers an improved prognosis for patients with T1N0M0 invasive breast carcinoma. Braz J Med Biol Res. 2002;35(8):913–919. | ||
Yiu CC, Loo WT, Lam CK, Chow LW. Presence of extensive intraductal component in patients undergoing breast conservative surgery predicts presence of residual disease in subsequent completion mastectomy. Chin Med J (Engl). 2009;122(8):900–905. | ||
Holland R, Connolly JL, Gelman R, et al. The presence of an extensive intraductal component following a limited excision correlates with prominent residual disease in the remainder of the breast. J Clin Oncol. 1990;8(1):113–118. | ||
Leong AS, Sormunen RT, Vinyuvat S, Hamdani RW, Suthipintawong C. Biologic markers in ductal carcinoma in situ and concurrent infiltrating carcinoma. A comparison of eight contemporary grading systems. Am J Clin Pathol. 2001;115(5):709–718. | ||
Wärnberg F, Nordgren H, Bergkvist L, Holmberg L. Tumour markers in breast carcinoma correlate with grade rather than with invasiveness. Br J Cancer. 2001;85(6):869–874. | ||
Steinman S, Wang J, Bourne P, Yang Q, Tang P. Expression of cytokeratin markers, ER-alpha, PR, HER-2/neu, and EGFR in pure ductal carcinomain situ (DCIS) and DCIS with co-existing invasive ductal carcinoma (IDC) of the breast. Ann Clin Lab Sci. 2007;37(2):127–134. | ||
Schorr MC, Pedrini JL, Savaris RF, Zettler CG. Are the pure in situ breast ductal carcinomas and those associated with invasive carcinoma the same? Appl Immunohistochem Mol Morphol. 2010;18(1):51–54. | ||
Iakovlev VV, Arneson NC, Wong V, et al. Genomic differences between pure ductal carcinoma in situ of the breast and that associated with invasive disease: a calibrated aCGH study. Clin Cancer Res. 2008;14(14):4446–4454. | ||
Aubele M, Mattis A, Zitzelsberger H, et al. Extensive ductal carcinoma In situ with small foci of invasive ductal carcinoma: evidence of genetic resemblance by CGH. Int J Cancer. 2000;85(1):82–86. | ||
Castro NP, Osório CA, Torres C, et al. Evidence that molecular changes in cells occur before morphological alterations during the progression of breast ductal carcinoma. Breast Cancer Res. 2008;10(5):R87. | ||
Barentsz MW, Postma EL, van Dalen T, et al. Prediction of positive resection margins in patients with non-palpable breast cancer. Eur J Surg Oncol. 2015;41(1):106–112. | ||
Jimenez RE, Bongers S, Bouwman D, Segel M, Visscher DW. Clinicopathologic significance of ductal carcinoma in situ in breast core needle biopsies with invasive cancer. Am J Surg Pathol. 2000;24(1):123–128. | ||
Sinn HP, Anton HW, Magener A, von Fournier D, Bastert G, Otto HF. Extensive and predominant in situ component in breast carcinoma: their influence on treatment results after breast-conserving therapy. Eur J Cancer. 1998;34(5):646–653. | ||
Cedolini C, Bertozzi S, Londero AP, et al. Impact of the presence and quantity of ductal carcinoma in situ component on the outcome of invasive breast cancer. Int J Clin Exp Pathol. 2015;8(10):13304–13313. | ||
Black MM, Zachrau RE, Hankey BF, Feuer EJ. Prognostic significance of in situ carcinoma associated with invasive breast carcinoma. A natural experiment in cancer immunology? Cancer. 1996;78(4):778–788. | ||
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence–SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2016 Sub (1973–2014 varying)–Linked To County Attributes–Total U.S., 1969–2015 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2017, based on the November 2016 submission. Available from: https://seer.cancer.gov/data. Accessed May 25, 2017. | ||
Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985,39(1):33–38. | ||
Williamson EJ1, Forbes A. Introduction to propensity scores. Respirology. 2014;19(5):625–635. | ||
Kim DH, Pieper CF, Ahmed A, Colón-Emeric CS. Use and interpretation of propensity scores in aging research: a guide for clinical researchers. J Am Geriatr Soc. 2016;64(10):2065–2073. | ||
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. | ||
Matsukuma A, Enjoji M, Toyoshima S. Ductal carcinoma of the breast. An analysis of proportions of intraductal and invasive components. Pathol Res Pract. 1991;187(1):62–67. | ||
Schnitt SJ, Harris JR. Evolution of breast-conserving therapy for localized breast cancer. J Clin Oncol. 2008;26(9):1395–1396. | ||
Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149–1156. | ||
Jung W, Kang E, Kim SM, et al. Factors associated with re-excision after breast-conserving surgery for early-stage breast cancer. J Breast Cancer. 2012;15(4):412–419. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.