Back to Journals » OncoTargets and Therapy » Volume 8
Prognostic value of Bcl-2 expression in patients with non-small-cell lung cancer: a meta-analysis and systemic review
Authors Zhang J, Wang S, Wang L, Wang R , Chen S, Pan B, Sun Y, Chen H
Received 26 May 2015
Accepted for publication 6 August 2015
Published 18 November 2015 Volume 2015:8 Pages 3361—3369
DOI https://doi.org/10.2147/OTT.S89275
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Faris Farassati
Jie Zhang,1–3,* Shengfei Wang,1–3,* Lei Wang,1,2,* Rui Wang,1,2,* Sufeng Chen,1,2 Bin Pan,1,2 Yihua Sun,1,2 Haiquan Chen1,4
1Department of Thoracic Surgery, Fudan University Shanghai Cancer Center, Shanghai, People’s Republic of China; 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, People’s Republic of China; 3Department of Oncology, Longhua Hospital, Shanghai, People’s Republic of China; 4Department of Thoracic Surgery, Shanghai Chest Hospital, Jiao Tong University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Objective: B-cell-lymphoma-2 (Bcl-2) is a proto-oncogene that plays an important role in the regulation of apoptosis and cell survival. However, there are much conflicting data in the literature concerning the association between Bcl-2 and prognosis in non-small-cell lung cancer (NSCLC). There is little in the way of meta-analysis focused on Bcl-2 and its effect on NSCLC prognosis. This study was performed to provide an assessment of whether expression levels of Bcl-2 are associated with prognosis in patients with NSCLC.
Materials and methods: We searched PubMed, the Cochrane Library, and China National Knowledge Infrastructure for all eligible studies. The combined hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) in terms of overall survival were evaluated.
Results: Fifty published studies including 6,863 patients with lung cancer were included in this meta-analysis. Overall, Bcl-2 was expressed in 33% of the NSCLC tumors studied. Our analysis indicates that NSCLC patients with Bcl-2-positive expression have a better prognosis than those with Bcl-2-negative expression in both Asian and non-Asian study populations (HR 0.79, 95% CI 0.72–0.87, P<0.00001). However, Bcl-2-positive expression seems to have no significant impact on survival of stage I NSCLC patients.
Conclusion: Our results indicated that Bcl-2 might be a useful prognostic marker for NSCLC generally. Larger clinical trials are needed to confirm the prognostic value of Bcl-2 in stage I NSCLC.
Keywords: Bcl-2, non-small-cell lung cancer, meta-analysis, prognosis
Introduction
Lung cancer is the most common cause of cancer-related death worldwide. Non-small-cell lung cancer (NSCLC) accounts for >85% of primary lung cancers, and approximately two-thirds of NSCLC patients are diagnosed at an advanced stage.1 Pathological features, such as pathological stage, histological type, and lymph node metastasis, have been independent prognostic markers predicting the development of metastasis.2 However, they are imperfect, represent only crude measures of the biological behavior of a tumor, and cannot predict the optimal therapeutic course for the individual patient. Thus, it is important to identify biological markers which can predict survival.
The ability of cancer cells to avoid apoptosis and continue to proliferate is one of the fundamental hallmarks of cancer. B-cell-lymphoma-2 (Bcl-2) is a key regulator of the mitochondrial apoptotic pathway promoting survival by inhibition of adapters necessary for the activation and cleavage of caspases.3 The Bcl-2 gene was discovered in a follicular B-cell lymphoma, and its tumorigenic potential has been shown in animal models.4 Bcl-2 is overexpressed in a variety of human tumors including lung cancer.3,5
In lung cancer, the prognostic value of Bcl-2 expression has been analyzed by several groups, but results have been conflicting and controversial. One group investigated Bcl-2 expression in a meta-analysis of nine studies with a total of 673 small-cell lung cancer patients and concluded that Bcl-2 expression was associated with a better prognosis, but this did not reach significance.6 Also, although there are a large number of studies investigating the prognostic value of Bcl-2 expression in NSCLC survival, no consensus has been reached. These conflicting results have been reported from different laboratories. We, therefore, carried out a meta-analysis of data from published studies to quantitatively review the effect of Bcl-2 overexpression in tumor tissue on overall survival (OS) in patients with NSCLC.
Materials and methods
Search strategy and study selection
The search was performed by consulting the electronic database PubMed, the Cochrane Library, and China National Knowledge Infrastructure. Searches included the terms “non-small cell lung cancer” and “Bcl-2”. The keywords hit 553 citations. Manual selection of relevant studies was carried out based on the summary analysis. The citation lists of all retrieved articles were scanned to identify other potentially relevant reports.
The following criteria for eligibility among studies were set before collecting articles: 1) Bcl-2 expression was evaluated in primary NSCLC tissue. 2) Survival information at specific times was reported in the article. 3) Follow-up time exceeded 5 years. 4) Articles were published in English and in Chinese. 4) When several articles were published by the same authors or group, the newest or most informative single article was selected. Exclusion criteria were the following: 1) No information on survival was provided, or the hazard ratio (HR) of OS could not be calculated based on the given information. 2) Letters to editor, reviews, and articles published in a book or papers published in non-English. 3) Studies with radiotherapy or concurrent chemoradiotherapy treatment investigating response rates only.
Two authors (SW and JZ) did the search and identification independently, and selection of an article was reached by consensus. The following information was extracted from each report by the two authors independently: year of publication, patient size, time period of patient enrollment, patient source, histology, disease stage, test method, cutoff value, and survival data. If data from any of the above categories were not reported in the primary study, items were treated as “not applicable”.
Quality assessment
Quality assessment of each study was performed using the Newcastle–Ottawa Quality Assessment Scale for cohort studies. This scale is an 8-item instrument that allows for assessment of patient population and selection, study comparability, follow-up, and outcome of interest. The scale was recommended by the Cochrane Non-Randomized Studies Methods Working Group. Two investigators (JZ and SW) performed quality assessment independently. Disagreement was resolved by consensus.
Statistical analysis
Analysis of variance was used to compare the means of quality scores between different groups. For quantitative aggregation of survival results, HR and their 95% confidence intervals (CIs) were combined to give the effective value. The HR was calculated from the reported data directly by number of events. If data were presented in the form of Kaplan–Meier survival curve, we extracted them from the survival rates at specified times in order to reconstruct the HR estimate and its variance using methods reported by Parmar et al.7 Data were entered into the Cochrane Collaboration software, RevMan Version 5.0 for Windows (the Cochrane Collaboration, Oxford, UK). The Cochran’s test was used to assess the heterogeneity of included studies. For heterogeneity tests, P-value <0.05 was considered to indicate significance. If the test of heterogeneity was significant (P<0.05; I2>50%), the random-effect model was used. Otherwise, the fixed-effect model was used. By convention, an observed HR of <1 implied a better survival for the group with positive Bcl-2 expression. This impact of Bcl-2 on survival was considered as statistically significant if the 95% CI for the overall HR did not overlap.
Results
Studies selection and characteristics
Five hundred and fifty-three potentially relevant citations were reviewed (Figure 1). Among them, six studies were based on the same patient cohorts. Thus, the less informative ones were excluded (studies excluded were the following: Fontanini et al (1996), Laudanski et al (1995), and O’Byrne et al (2001); studies included were the following: Fontanini et al,53 Laudanski et al,35 and Cox et al37). Forty papers were review articles, 354 were not clinical studies including signal transduction, cell lines, animals, or pharmacogenomic studies, 13 were case reports, and the other 16 papers were designed without lung cancer. Ultimately, 50 studies3,8–56 that reported the prognostic value of Bcl-2 status for OS were analyzed. The total number of patients included was 6,863, ranging from 24 to 534 patients per study (median, 137). The major characteristics are shown in Table 1. These 50 studies included all lung cancer subtypes (n=46), adenocarcinomas only (n=2), or squamous cell carcinomas (SCCs) only (n=2). Twenty-nine studies had information for stages I–III, eight for advanced-stage (III–IV) disease, and 13 for all stages, I–IV. Forty-eight studies used immunohistochemistry to evaluate Bcl-2 expression, one used reverse transcription polymerase chain reaction, and one used Western blot.
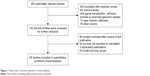  | Figure 1 Flow chart of article selection in meta-analysis. |
  | Table 1 Baseline characteristics of the 50 trials used in the meta-analysis |
In 50 studies evaluating Bcl-2 expression, the proportion of patients exhibiting Bcl-2 overexpression in individual studies ranged from 5% to 71%. Twenty-six out of the 50 studies identified Bcl-2 overexpression as an indicator of positive prognosis; Bcl-2 expression in six studies was significantly associated with poor prognosis. Eighteen studies showed no statistically significant impact of Bcl-2 overexpression on survival.
Quality assessment
We used the Newcastle–Ottawa Scale to perform quality assessment of all 50 studies. Studies that fulfill five or more of the eight criteria were higher quality studies. Overall, the total quality score of the included studies ranged from 5 to 8 (Table 1).
Impact of Bcl-2-positive expression on OS of NSCLC
The effect of Bcl-2 expression on OS was evaluated in 50 studies with a total of 6,863 patients. Overall, Bcl-2 was expressed in 33% of the NSCLCs studied. HRs were calculated from the reported data directly by number of events (25 out of 50), or data reading from Kaplan–Meier survival curve from the survival curves reading (25 out of 50). The combined HR was calculated using a random-effect model, and a value was obtained that was statistically significant (HR 0.79, 95% CI 0.72–0.87, P<0.00001), indicating that Bcl-2-positive expression was an indicator of better prognosis.
The data extracted were adequate to aggregate the studies of stage I NSCLC. When we aggregated the eight studies which reported data from 1,432 patients, no heterogeneity was found. The combined HR was not statistically significant (HR 0.93, 95% CI 0.80–1.07, P=0.50). Thus, no relationship between Bcl-2 and survival was observed for stage I NSCLC (Figure 2A).
When 29 studies containing 4,390 patients who had received radical surgery (I–III) were considered, highly significant heterogeneity was detected (χ2=92.48, P<0.00001; I2=70%). The random-effect model was used to perform meta-analysis, and the result was significant in favor of patients with positive Bcl-2 expression (HR 0.77, 95% CI 0.69–0.89, P<0.0001) (Figure 2B).
The advanced-stage subgroup included eight studies comprising 425 patients. Because no heterogeneity was found in this subgroup (χ2=7.77, P=0.35; I2=10%), the fixed-effect model was used to perform meta-analysis. The aggregated survival data also showed a good survival prognosis where there was Bcl-2-positive expression (HR 0.74, 95% CI 0.59–0.94, P=0.01) (Figure 2C).
Thirteen studies comprising 2,048 patients were included in the all-stage subgroup. The result of the test for heterogeneity was significant (χ2=52.56, P<0.0001; I2=77%), and then, the combined HR was calculated using a random-effect model. Bcl-2 expression was not significantly associated with OS (HR 0.84, 95% CI 0.69–1.02, P=0.08) (Figure 2D). There were not adequate data to aggregate studies of stage II or III disease.
The data extracted were also adequate to aggregate the studies of SCC and adenocarcinoma for subgroup analyses. When we aggregated seven studies that reported results for SCC, the combined HR was statistically significant (HR 0.54, 95% CI 0.32–0.90, P=0.02) (Figure 2E). We observed no statistically significant effect of Bcl-2 expression on survival in patients with adenocarcinoma, however (HR 0.92, 95% CI 0.56–1.51, P=0.73) (Figure 2F).
There were 32 studies from Europe and USA and 18 from East Asia. The combined HRs of East Asian studies and non-East Asian studies were 0.78 (95% CI 0.63–0.96, P<0.0001) and 0.82 (95% CI 0.72–0.92, P<0.0001), respectively. In both East Asian and European/USA populations, Bcl-2 expression was an indicator of better prognosis.
Discussion
Identification of prognostic factors allows the definition of high-risk groups of patients for whom further specific therapy might be necessary and stratification should thus be performed in randomized trials.
A previous meta-analysis showed an association between Bcl-2 positivity and better survival of patients with lung cancer.57 This analysis included 18 NSCLC, three neuroendocrine, and four small-cell lung cancer trials reported from 1993 to 1999. However, data were insufficient to evaluate the prognostic value of Bcl-2 in surgical cases. The effect of Bcl-2-positive expression on specific stage, such as stage I, advanced stages, and adenocarcinomas, was not assessed. We have improved upon that previous meta-analysis by including more recent studies and by generally using a more comprehensive search strategy.
In this meta-analysis, 50 published studies including 6,863 patients with lung cancer were included. Our analysis indicates that NSCLC patients with Bcl-2-positive expression have a better prognosis than those with Bcl-2-negative expression in both East Asian and non-East Asian study populations (HR 0.79, 95% CI 0.72–0.87, P<0.00001). However, Bcl-2 expression seems to have no significant impact on survival of stage I NSCLC patients.
The mechanisms through which Bcl-2 might exert its protective effect in NSCLC are unclear. One group demonstrated that Bcl-2 expression showed an association with biologic features, such as the absence of c-erb-B2 and mutant p53 expression, which define a better prognosis.58 Therefore, although bcl-2 is a proto-oncogene involved in oncogenesis, because of its ability to prolong cell survival through the inhibition of apoptosis, its expression may be associated with other features that define a more favorable prognosis. Bcl-2 may also suppress the proliferative activity of tumor cells. It has been reported that the proliferative activity of Bcl-2-positive tumors tended to be lower than that in negative tumors.51 Furthermore, the process of apoptosis involves many proteins such as antiapoptotic proteins (Bcl-2, Bcl-X) and proapoptotic proteins (Bax, Bak, Bad). Thus, it remains to be clarified if other proteins or bcl homologs potentiate the tumor suppressor role of Bcl-2 in NSCLC. Recently, Bcl-2 was found to inhibit DNA replication and DNA repair.59,60 Due to its dual function, NSCLC patients with Bcl-2-positive expression had a better prognosis than those with Bcl-2-negative expression in both Asian and non-Asian study populations in this analysis.
Some potentially important methodologic biases need to be discussed. When the analysis was limited to eight advanced-stage studies, or eight studies assessing Bcl-2 in stage I subgroup, heterogeneity was not detected. However, heterogeneity was detected when analyses were limited to the 18 East Asian studies or the seven studies including only SCC. Therefore, patient type and histologic type were not a major source of heterogeneity. The heterogeneity in this study could be explained by the stage or by differences in the method used to detect Bcl-2 status. Small sample size was also a source of bias. When the analysis was limited to >100 patients in studies in surgical group, no significant heterogeneity was detected (χ2=35.29, P=0.01; I2=46%).
Additional biases could be introduced by the methodology used. We performed a methodological assessment of the studies to avoid selection biases where possible. The comparison of the scores of the three groups (positive, nonsignificant, negative studies) showed no statistically significant difference, allowing a meaningful data aggregation.
In conclusion, Bcl-2-positive expression was associated with a better prognosis in patients with NSCLC, so Bcl-2 might be a useful prognostic marker.61 However, Bcl-2-positive expression seems to have no significant impact on survival of stage I patients as determined in our meta-analysis. These results should be confirmed by an adequately designed prospective study. Because there was only limited number of patients to test for Bcl-2 expression in platinum-based chemotherapy, these results need to be confirmed by well-designed prospective studies.
Acknowledgments
The authors acknowledge support from the National Natural Science Foundation of China (grant numbers 81101761 and 81172218), Shanghai science and technology commission foundation key project, Longhua Medicial Project (D25), Ministry of Education Returned Scientific Research Foundation, key project from the Shanghai Municipal Science and Technology Commission Foundation (14JC1401400), Trans-Century Training Programme Foundation for the Talents by the State Education Commission (to JZ), and key technology support project of the field of medicine and agriculture science from Shanghai Municipal Science and Technology Commission (15411951602).
Author contributions
JZ, LW, RW, and HC were involved in concept and design of the study. LW and JZ drafted the manuscript. All authors participated in acquisition, analysis, and interpretation of data, revised the manuscript, and read and approved the final version.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–229. | ||
Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–271. | ||
Anagnostou VK, Lowery FJ, Zolota V, et al. High expression of BCL-2 predicts favorable outcome in non-small cell lung cancer patients with non squamous histology. BMC Cancer. 2010;10:186. | ||
Choi J, Choi K, Benveniste EN, et al. Bcl-2 promotes invasion and lung metastasis by inducing matrix metalloproteinase-2. Cancer Res. 2005;65:5554–5560. | ||
Callagy GM, Webber MJ, Pharoah PD, Caldas C. Meta-analysis confirms BCL2 is an independent prognostic marker in breast cancer. BMC Cancer. 2008;8:153. | ||
Lawson MH, Cummings NM, Rassl DM, et al. Bcl-2 and beta1-integrin predict survival in a tissue microarray of small cell lung cancer. Br J Cancer. 2010;103:1710–1715. | ||
Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. | ||
Cakir E, Yilmaz A, Demirag F, et al. Prognostic significance of micropapillary pattern in lung adenocarcinoma and expression of apoptosis-related markers: caspase-3, bcl-2, and p53. APMIS. 2011;119:574–580. | ||
Grimminger PP, Schneider PM, Metzger R, et al. The prognostic role of Bcl-2 mRNA expression in curatively resected non-small cell lung cancer (NSCLC). Lung Cancer. 2010;70:82–87. | ||
Jeong SH, Jung JH, Han JH, et al. Expression of Bcl-2 predicts outcome in locally advanced non-small cell lung cancer patients treated with cisplatin-based concurrent chemoradiotherapy. Lung Cancer. 2010;68:288–294. | ||
Porebska I, Kosacka M, Wyrodek E, et al. [Expression of p53, bcl-2 and nm 23 proteins in squamous cell lung cancer]. Pneumonol Alergol Pol. 2009;77:131–137. Polish. | ||
Lee HW, Choi YW, Han JH, et al. Expression of excision repair cross-complementation group 1 protein predicts poor outcome in advanced non-small cell lung cancer patients treated with platinum-based doublet chemotherapy. Lung Cancer. 2009;65:377–382. | ||
Renouf DJ, Wood-Baker R, Ionescu DN, et al. BCL-2 expression is prognostic for improved survival in non-small cell lung cancer. J Thorac Oncol. 2009;4:486–491. | ||
Zhao ZH, Wang SF, Cong DG, Yu L, Wang J. Correlation of the expression of pokemon with p14 and bc1-2 and their effects on prognosis of non small cell lung cancer. Tumor. 2008;4:121–124. | ||
Yoo J, Jung JH, Lee MA, et al. Immunohistochemical analysis of non-small cell lung cancer: correlation with clinical parameters and prognosis. J Korean Med Sci. 2007;22:318–325. | ||
Hu X, Song N, Liu Y, et al. Prognostic value of Bcl-2 and cyclooxygenase expressions in non-small cell lung cancer. Chin J Cancer Prev Treat. 2006;4:1704–1708. | ||
Wang L, Sun L, Dong C, et al. Prognosis significance of expression of apoptosis, P53 and Bcl-2 in non-small cell lung cancer. Chin J Histochem Cytochem. 2006;4:156–162. | ||
Yaren A, Oztop I, Kargi A, et al. Bcl-2 and c-kit expression in non-small-cell lung cancer and their effects on prognosis. Int J Clin Pract. 2006;60:675–682. | ||
Yilmaz A, Savaş I, Dizbay Sak S, et al. Distribution of Bcl-2 gene expression and its prognostic value in non-small cell lung cancer. Tuberk Toraks. 2005;53:323–329. | ||
Groeger AM, Esposito V, De Luca A, et al. Prognostic value of immunohistochemical expression of p53, bax, Bcl-2 and Bcl-xL in resected non-small-cell lung cancers. Histopathology. 2004;44:54–63. | ||
Shibata Y, Hidaka S, Tagawa Y, Nagayasu T. Bcl-2 protein expression correlates with better prognosis in patients with advanced non-small cell lung cancer. Anticancer Res. 2004;24:1925–1928. | ||
Kren L, Brazdil J, Hermanova M, et al. Prognostic significance of anti-apoptosis proteins survivin and bcl-2 in non-small cell lung carcinomas: a clinicopathologic study of 102 cases. Appl Immunohistochem Mol Morphol. 2004;12:44–49. | ||
Ludovini V, Gregorc V, Pistola L, et al. Vascular endothelial growth factor, p53, Rb, Bcl-2 expression and response to chemotherapy in advanced non-small cell lung cancer. Lung Cancer. 2004;46:77–85. | ||
Grossi F, Loprevite M, Chiaramondia M, et al. Prognostic significance of K-ras, p53, bcl-2, PCNA, CD34 in radically resected non-small cell lung cancers. Eur J Cancer. 2003;39:1242–1250. | ||
Huang CI, Neuberg D, Johnson BE, Wei JY, Christiani DC. Expression of bcl-2 protein is associated with shorter survival in nonsmall cell lung carcinoma. Cancer. 2003;98:135–143. | ||
Gregorc V, Ludovini V, Pistola L, et al. Relevance of p53, bcl-2 and Rb expression on resistance to cisplatin-based chemotherapy in advanced non-small cell lung cancer. Lung Cancer. 2003;39:41–48. | ||
Han JY, Hong EK, Choi BG, et al. Death receptor 5 and Bcl-2 protein expression as predictors of tumor response to gemcitabine and cisplatin in patients with advanced non-small-cell lung cancer. Med Oncol. 2003;20:355–362. | ||
Krug LM, Miller VA, Filippa DA, Venkatraman E, Ng KK, Kris MG. Bcl-2 and bax expression in advanced non-small cell lung cancer: lack of correlation with chemotherapy response or survival in patients treated with docetaxel plus vinorelbine. Lung Cancer. 2003;39:139–143. | ||
Poleri C, Morero JL, Nieva B, et al. Risk of recurrence in patients with surgically resected stage I non-small cell lung carcinoma: histopathologic and immunohistochemical analysis. Chest. 2003;123:1858–1867. | ||
Tomita M, Matsuzaki Y, Edagawa M, Shimizu T, Hara M, Onitsuka T. Prognostic significance of bcl-2 expression in resected pN2 non-small cell lung cancer. Eur J Surg Oncol. 2003;29:654–657. | ||
Lai RS, Wang JS, Hsu HK, Chang HC, Lin CH, Lin MH. Prognostic evaluation of the expression of p53 and bcl-2 oncoproteins in patients with surgically resected non-small cell lung cancer. Jpn J Clin Oncol. 2002;32:393–397. | ||
Hanaoka T, Nakayama J, Haniuda M, Sato TA. Immunohistochemical demonstration of apoptosis-regulated proteins, Bcl-2 and Bax, in resected non-small-cell lung cancers. Int J Clin Oncol. 2002;7:152–158. | ||
Han H, Landreneau RJ, Santucci TS, et al. Prognostic value of immunohistochemical expressions of p53, HER-2/neu, and bcl-2 in stage I non-small-cell lung cancer. Hum Pathol. 2002;33:105–110. | ||
Hwang JH, Lim SC, Kim YC, Park KO, Ahn SJ, Chung WK. Apoptosis and bcl-2 expression as predictors of survival in radiation-treated non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2001;50:13–18. | ||
Laudanski J, Niklinska W, Burzykowski T, Chyczewski L, Niklinski J. Prognostic significance of p53 and bcl-2 abnormalities in operable nonsmall cell lung cancer. Eur Respir J. 2001;17:660–666. | ||
Tanaka F, Otake Y, Yanagihara K, et al. Apoptosis and p53 status predict the efficacy of postoperative administration of UFT in non-small cell lung cancer. Br J Cancer. 2001;84:263–269. | ||
Cox G, Walker RA, Muller S, Abrams KR, Steward WP, O’Byrne KJ. Does immunointensity account for the differences in prognostic significance of Bcl-2 expression in non-small cell lung cancer? Pathol Oncol Res. 2000;6:87–92. | ||
Moldvay J, Scheid P, Wild P, et al. Predictive survival markers in patients with surgically resected non-small cell lung carcinoma. Clin Cancer Res. 2000;6:1125–1134. | ||
van de Vaart PJ, Belderbos J, de Jong D, et al. DNA-adduct levels as a predictor of outcome for NSCLC patients receiving daily cisplatin and radiotherapy. Int J Cancer. 2000;89:160–166. | ||
Chen Y, Sato M, Fujimura S, et al. Expression of Bcl-2, Bax, and p53 proteins in carcinogenesis of squamous cell lung cancer. Anticancer Res. 1999;19:1351–1356. | ||
D’Amico TA, Massey M, Herndon JE 2nd, Moore MB, Harpole DH Jr. A biologic risk model for stage I lung cancer: immunohistochemical analysis of 408 patients with the use of ten molecular markers. J Thorac Cardiovasc Surg. 1999;117:736–743. | ||
Huang C, Kohno N, Inufusa H, Kodama K, Taki T, Miyake M. Overexpression of bax associated with mutations in the loop-sheet-helix motif of p53. Am J Pathol. 1999;155:955–965. | ||
Mehdi SA, Tatum AH, Newman NB, et al. Prognostic markers in resected stage I and II non small-cell lung cancer: an analysis of 260 patients with 5 year follow-up. Clin Lung Cancer. 1999;1:59–67; discussion 68–59. | ||
Silvestrini R, Costa A, Lequaglie C, et al. Bcl-2 protein and prognosis in patients with potentially curable non-small-cell lung cancer. Virchows Arch. 1998;432:441–444. | ||
Anton RC, Brown RW, Younes M, Gondo MM, Stephenson MA, Cagle PT. Absence of prognostic significance of bcl-2 immunopositivity in non-small cell lung cancer: analysis of 427 cases. Hum Pathol. 1997;28:1079–1082. | ||
Apolinario RM, van der Valk P, de Jong JS, et al. Prognostic value of the expression of p53, bcl-2, and bax oncoproteins, and neovascularization in patients with radically resected non-small-cell lung cancer. J Clin Oncol. 1997;15:2456–2466. | ||
Higashiyama M, Doi O, Kodama K, Yokouchi H, Nakamori S, Tateishi R. bcl-2 oncoprotein in surgically resected non-small cell lung cancer: possibly favorable prognostic factor in association with low incidence of distant metastasis. J Surg Oncol. 1997;64:48–54. | ||
Ishida H, Irie K, Itoh T, Furukawa T, Tokunaga O. The prognostic significance of p53 and bcl-2 expression in lung adenocarcinoma and its correlation with Ki-67 growth fraction. Cancer. 1997;80:1034–1045. | ||
Koukourakis MI, Giatromanolaki A, O’Byrne KJ, et al. Potential role of bcl-2 as a suppressor of tumour angiogenesis in non-small-cell lung cancer. Int J Cancer. 1997;74:65–570. | ||
Pastorino U, Andreola S, Tagliabue E, et al. Immunocytochemical markers in stage I lung cancer: relevance to prognosis. J Clin Oncol. 1997;15:2858–2865. | ||
O’Neill AJ, Staunton MJ, Gaffney EF. Apoptosis occurs independently of bcl-2 and p53 over-expression in non-small cell lung carcinoma. Histopathology. 1996;29:45–50. | ||
Ohsaki Y, Toyoshima E, Fujiuchi S, et al. bcl-2 and p53 protein expression in non-small cell lung cancers: correlation with survival time. Clin Cancer Res. 1996;2:915–920. | ||
Fontanini G, Vignati S, Bigini D, et al. Bcl-2 protein: a prognostic factor inversely correlated to p53 in non-small-cell lung cancer. Br J Cancer. 1995;71:1003–1007. | ||
Ritter JH, Dresler CM, Wick MR. Expression of bcl-2 protein in stage T1N0M0 non-small cell lung carcinoma. Hum Pathol. 1995;4:1227–1232. | ||
Walker C, Robertson L, Myskow M, Dixon G. Expression of the BCL-2 protein in normal and dysplastic bronchial epithelium and in lung carcinomas. Br J Cancer. 1995;72:164–169. | ||
Pezzella F, Turley H, Kuzu I, et al. Bcl-2 protein in non-small-cell lung carcinoma. N Engl J Med. 1993;329:690–694. | ||
Martin B, Paesmans M, Berghmans T, et al. Role of Bcl-2 as a prognostic factor for survival in lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2003;89:55–64. | ||
Martinez-Arribas F, Alvarez T, Del Val G, et al. Bcl-2 expression in breast cancer: a comparative study at the mRNA and protein level. Anticancer Res. 2007;27:219–222. | ||
Maohua X, Yun Y, Taofeek KO, et al. Bcl-2 induces DNA replication stress by inhibiting rib nucleotide reductase. Cancer Res. 2014;74(1):212–223. | ||
Maohua X, Dongkyoo P, Shuo Y, et al. Bcl-2 inhibits recruitment of Mre11 complex to DNA double-strand breaks in response to high-linear energy transfer radiation. Nucleic Acids Res. 2015;43(2):960–972. | ||
Schmidt LH, Görlich D, Spieker T, et al. Prognostic impact of Bcl-2 depends on tumor histology and expression of MALAT-1 lncRNA in non-small-cell lung cancer. J Thorac Oncol. 2014;9(9):1294–1304. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

