Back to Journals » International Journal of General Medicine » Volume 15
Prognostic Value of Albumin-to-Alkaline Phosphatase Ratio for EGFR-Mutated Advanced Non-Small-Cell Lung Cancer Patients Treated with First-Line EGFR-TKIs: A Large Population-Based Study and Literature Review
Authors Gan Y , Ren J, Xian J, Yu H, Jin J, Li D, Li W
Received 11 November 2021
Accepted for publication 25 February 2022
Published 29 March 2022 Volume 2022:15 Pages 3405—3416
DOI https://doi.org/10.2147/IJGM.S348912
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yuncui Gan,1,* Jing Ren,2,* Jinghong Xian,3,* He Yu,1 Jing Jin,1 Dan Li,1 Weimin Li1
1Department of Respiratory and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, People’s Republic of China; 2Integrated Care Management Center, West China Hospital, Sichuan University, Chengdu, People’s Republic of China; 3Department of Clinical Research, West China Hospital, Sichuan University, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Weimin Li, Department of Respiratory and Critical Care Medicine, West China Hospital, Sichuan University, No. 37 Guoxue Alley, Chengdu, 610041, Sichuan Province, People’s Republic of China, Tel/Fax +86 288 558 2944, Email [email protected]
Background: Resistance inevitably develops in epidermal growth factor receptor (EGFR)-mutated advanced non-small-cell lung cancer (NSCLC) patients after treatment of EGFR tyrosine kinase inhibitors (EGFR-TKIs). The albumin-to-alkaline phosphatase ratio (AAPR), a novel index, has been reported to be associated with survival in various cancers. In this study, we explored the prognostic value of AAPR in EGFR-mutated advanced NSCLC patients treated with first-line EGFR-TKIs.
Methods: The clinical and pretreatment laboratory data were retrospectively extracted from hospital medical system. The Log-rank and Kaplan–Meier analyses were adopted to detect differences in survival between groups. Univariate and multivariate Cox’s proportional hazard regression models were applied to assess the prognostic value of AAPR for progression-free survival (PFS) and overall survival (OS).
Results: Totally, 598 EGFR-mutated NSCLC patients with stage IIIB-IV were enrolled into this study. The median age of all patients was 60 years, and 56.9% were women. About 97% patients had common EGFR gene mutations of deletions in exon 19 (19 del) or a point mutation in exon 21 (L858R). Using receiver operating characteristic (ROC) curve analysis and the Youden index, the optimal cut-off value of pretreatment AAPR was 0.47. Patients with high AAPR achieved longer median PFS and OS than patients with low AAPR (14.0 months vs 10.4 months, P< 0.01; 58.2 months vs 36.7 months, P< 0.001, respectively). The multivariate analysis by Cox’s proportional hazards regression model demonstrated that AAPR was an independent prognostic factor for both PFS (HR: 0.813, 95% CI: 0.673– 0.984, P=0.033) and OS (HR: 0.629, 95% CI: 0.476– 0.830, P=0.001).
Conclusion: Pretreatment AAPR, measured as part of routine blood biochemical test, may be a reliable prognostic indicator in EGFR-mutated advanced NSCLC patients treated with first-line first-generation EGFR-TKIs.
Keywords: AAPR, NSCLC, EGFR-TKI, prognosis
Introduction
Lung cancer is the leading cause of cancer-related deaths all over the world, with a five-year survival rate of approximately 20%.1–3 Non-small-cell lung cancer (NSCLC) accounts for 83% of all primary lung cancer, and the majority are diagnosed with stage III or IV due to lack of typical symptoms in early disease.4 Approximately 50% of Asian NSCLC patients could have an actionable targeted driver gene mutation.5 The application of molecular targeted therapy has improved survival in patients with NSCLC, especially advanced patients with epidermal growth factor receptor (EGFR) sensitizing mutation treated with EGFR tyrosine kinase inhibitors (EGFR-TKIs).6 However, resistance eventually develops after treatment and the period of progression varies from patients to patients.7–10 Therefore, it is important for clinicians to identify patients with high risk of progression and death.
Albumin (ALB), synthesized by hepatocytes and commonly found in serum, is considered as an index reflecting nutritional and inflammatory conditions. Under inflammatory conditions, the production of ALB is suppressed by the activation of inflammatory cytokines, such as IL-1 and TNF-α.11 Low serum albumin levels have been shown to correlate with poor survival in various cancers.12–14 Alkaline phosphatase (ALP), a group of isoenzymes, is widely distributed in human liver, bone, kidney and other tissues. The main function of ALP is catalyzing the removal of the phosphate groups from nucleic acid molecules. Several studies have reported that elevated serum ALP was related to poor prognosis in pancreatic cancer,15 prostate cancer16 and spinal metastatic disease.17
Recently, some researchers have combined two hematological indicators into one for better reflection of systemic inflammation and metabolism status. AAPR, the albumin-to-alkaline phosphatase ratio and an easily acquisitive marker, has been revealed to be a favorable prognostic indicator in hepatocellular carcinoma,18 breast cancer19 and nasopharyngeal carcinoma.20 A few studies focus on the clinical significance of AAPR in NSCLC patients. Our previous study has found that AAPR may serve as a prognostic factor for OS in metastatic NSCLC. However, the association between AAPR and EGFR mutations was not analyzed in this study.21 Thus, in the present study, we mainly explore the possibility of AAPR as a prognostic indicator for progression-free survival (PFS) and overall survival (OS) in EGFR-mutated advanced NSCLC patients treated with first-line first-generation EGFR-TKIs.
Materials and Methods
Study Population
In this retrospective cohort study, we reviewed medical records of patients initially diagnosed NSCLC with TNM stage IIIB to IV between 2008 and 2018 in West China Hospital of Sichuan University. Patients who met the following criteria were subsequently enrolled in this study: 1) pathologically confirmed NSCLC; 2) with EGFR sensitizing mutation (common mutations: in-frame deletions in exon 19 (19 del) and Leu858Arg (L858R), uncommon mutations: G719X, L861Q and S768I); 3) receiving first-line first-generation EGFR-TKI targeted therapy; 4) serum ALB and ALP were measured before treatment; and 5) follow-up data were available. All patients were restaged according to the eighth edition of the TNM classification. Patients with a history of other primary cancers, active infection and known liver or renal or bone diseases were excluded because these conditions might affect the values of data. Our study was approved by West China Hospital Research Ethics Committee and abided by the Declaration of Helsinki and other ethical guidelines at present. Informed consent from patients was waived because of the retrospective nature of the study. All related data were analyzed anonymously and treated with confidentiality.
Follow Up and Data Collection
The exact date of progression was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. PFS was assessed through medical records, and OS was acquired through telephone follow-up or checking medical records. PFS was calculated from the date of initial treatment of TKI to the date of disease progression or last follow-up. Time from the date of initial treatment of TKI to the date of death from any cause or last follow-up was defined as OS. The last follow-up was in January 2021.
Baseline clinical pathological parameters, including age, gender, smoking status, family history of malignancy, histology, distant metastases, EGFR mutation status, tumor TNM stage and medications were collected from the hospital medical system. Furthermore, pretreatment laboratory indices covering ALB and ALP were retrieved from the hospital database. AAPR was calculated as the ratio of the serum ALB to the serum ALP.
Statistical Analysis
By setting OS as the state variable, receiver operating characteristic (ROC) curve analysis and the Youden index were used to determine the optimal cut-off value for AAPR. The cut was done by pROC package in R software. All patients were divided into two groups according to the cut-off value of AAPR. Continuous variables were presented as medians and ranges. Comparison of variables between divided groups was performed using Chi-square tests for dichotomous variables and Mann-Whitney U-test for continuous variables. The Kaplan–Meier method and Log rank test were also used to analyze prognosis based on PFS and OS for different groups. Subsequently, univariate and multivariate Cox proportional hazard regression models were performed to adjust potential confounding factors. Candidate variables with a P<0.1 in univariate analysis were included in the multivariate model. All statistical analyses were performed in R software (version 3.6.3). A two-sided P<0.05 was considered statistically significant.
Results
Clinicopathological Characteristics of Patients
We screened 1757 patients treated with EGFR-TKIs in West China hospital during the study period. After excluding 438 patients without PFS data, 52 patients with TNM stage I–IIIA, 368 patients without available serum ALB and ALP data, 233 patients who received non-first-line EGFR-TKIs and 68 patients without EGFR-sensitizing mutation, a total of 598 EGFR-mutated advanced NSCLC patients who received first-line first-generation EGFR-TKIs were included in this study (Figure 1). The clinicopathological characteristics are summarized in Table 1. Among these patients, 340 (56.9%) were females and 151 (26.7%) had a current/past history of smoking. The median age of all NSCLC patients was 60 years (range: 22–95 years) and 283 (47.3%) were aged older than 60 years. Of the 598 cases, 77 (13.6%) had a family history of malignancy and 348 (58.4%) had a tumor in the right lung. According to the histology, most of the patients (580; 97.0%) had an adenocarcinoma, while 18 (3.0%) patients had other types. Based on the eighth edition of the TNM classification system, 570 (95.3%) patients were stage IV. At the initial diagnosis, 472 out of 598 patients (78.9%) had developed two or fewer metastasis sites, 263 (44.0%) patients had bone metastasis and 59 (9.8%) patients had liver metastasis. In addition, most of the patients (97.0%) had common EGFR mutations of 19 del or L858R, while others had uncommon EGFR mutations of G719X, L861Q or S768I. All patients were given first-line first-generation EGFR-TKIs (73.1% gefitinib and 26.9% Icotinib).
 |
Table 1 Baseline Clinicopathological Characteristics of 598 Advanced NSCLC Patients According to Pretreatment AAPR Level |
 |
Figure 1 Flowchart of patient selection. |
Correlation Analysis Between Pretreatment AAPR and Clinicopathological Characteristics or Laboratory Parameters
According to the analysis, an AAPR value of 0.47 corresponded to the maximum Youden index value. Thus, we identified that 0.47 was the optimal cut-off value for the pretreatment AAPR. A total of 271 (45.3%) patients with AAPR>0.47 were categorized into the high AAPR group and the other 327 (54.7%) patients with AAPR≤0.47 were defined as low AAPR group. The area under the curve (AUC) for the pretreatment AAPR was 0.603 (P<0.001) (Figure 2).
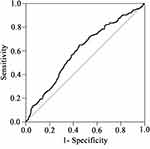 |
Figure 2 ROC analysis of the pretreatment AAPR by setting overall survival as the endpoint. AUC = 0.603, 95% CI = 0.557–0.65. |
The relationships between clinicopathological characteristics and pretreatment AAPR are shown in Table 1. Significant differences between the low and high AAPR groups were identified in the number of metastasis sites, liver and bone metastasis status (all P<0.001). However, no significant differences were found in age, gender, smoking status, tumor location, tumor histology type, TNM stage, EGFR mutation status and brain metastasis status between the two groups (all P>0.05). Further analysis revealed that patients with higher AAPR had higher ALB concentration and lower ALP concentration than those with lower AAPR (42.1 vs 39.7 g/L, P<0.001; 72.0 vs 116.0 U/L, P<0.001, respectively).
Kaplan–Meier Survival Analysis of AAPR for PFS and OS
The median OS and PFS of all patients were 44.8 months (range: 1.4–108.6 months) and 12.0 months (range: 0.4–65.7 months), respectively. 523 patients developed disease progression and 237 patients died during the follow-up period. As for the analysis of AAPR, patients in the high AAPR group had a longer median PFS and OS than patients in the low APPR group (14.0 months vs 10.4 months, P<0.01; 58.2 months vs 36.7 months, P<0.001, respectively) (Figure 3A and B). Then, we conducted stratified analyses. In patients without smoking history, those with high AAPR had a favorable PFS and OS than those with low AAPR (P<0.05 for PFS and P<0.001 for OS, respectively) (Figure 3C and D), but no significant difference was found in smokers (all P>0.05). Similar trends were also observed in patients with adenocarcinomas (P<0.01 and P<0.001, respectively) (Figure 3E and F).
Furthermore, when the analyses were stratified by the number of metastasis sites and liver/bone metastasis status, our results demonstrated that prognosis was better in the high AAPR group than in the low AAPR group in patients with two or fewer metastasis sites and those with liver/bone metastasis (all P<0.05) (Figure S1A, B, E and F). In the subgroup of patients with more than two metastasis sites, those with higher AAPR had a longer OS than those with lower APPR (P<0.01), but not PFS (P>0.05) (Figure S1C and S1D). No significant differences were found in patients without liver/bone metastasis divided by AAPR level (P>0.05) (Figure S1G and S1H).
Univariate and Multivariate Analyses for PFS and OS
As shown in Table 2, the univariate Cox proportional hazards model showed that APPR level, liver metastasis and the number of metastasis sites at initial diagnosis were found to be significantly correlated with both PFS and OS (P=0.002, P=0.047 and P<0.001 for PFS, respectively; P<0.001, P=0.006 and P=0.004 for OS, respectively). Furthermore, significant correlations were found between age, smoking status, tumor histology type and PFS (P=0.005, P=0.026 and P<0.001, respectively), but not with OS (all P>0.05). However, bone metastasis was only associated with a risk of death, with a HR of 1.354 (95% CI=1.049–1.749). There was no significant correlation between different EGFR-TKIs and survival (all P>0.05).
 |
Table 2 Univariate Cox Regression Analysis of PFS and OS for All NSCLC Patients |
Then, multivariate analysis of Cox proportional hazards model was conducted to assess independent prognostic factors (Table 3). The result demonstrated that AAPR was an independent prognostic factor for both PFS (HR: 0.813, 95% CI: 0.673–0.984, P=0.033) and OS (HR: 0.629, 95% CI: 0.476–0.830, P=0.001). The number of metastatic sites was significantly correlated with PFS (HR=1.409, 95% CI=1.106–1.795, P=0.005) but not with OS (P>0.05) in multivariate analysis (Table 3). Furthermore, we also noted that patients more than 60 years old at initial treatment and patients with adenocarcinomas seemed to be favorable prognostic factors for PFS (HR=0.821, 95% CI: 0.686–0.983, P=0.032; HR=0.283, 95% CI: 0.174–0.462, P<0.001, respectively). Kaplan–Meier survival curves for subgroup analysis are shown in Figure 4.
 |
Table 3 Multivariate Cox Regression Analysis of PFS and OS for All NSCLC Patients |
Discussion
EGFR mutation is the most common type of gene mutations in Asians with lung cancer.22 First-generation EGFR-TKIs, including gefitinib, icotinib and erlotinib, are still the dominant medications among Chinese patients due to its lower cost. However, many patients inevitably develop resistance, with average PFS ranging from 9 to 15 months.23 In this study, we explored the potential prognostic value of AAPR, a novel and easily acquisitive index, in 598 EGFR-mutated advanced NSCLC patients treated with first-line first-generation EGFR-TKIs for the first time. Our results demonstrated that AAPR was an independent prognostic factor for PFS and OS, especially in nonsmokers and adenocarcinoma patients.
ALB level reflects liver function, nutritional status and human defense capabilities. A decrease in ALB concentration is a sign of malnutrition and weakened immunity. In addition, the alteration in protein binding has a relevant impact on drug half-life. Therefore, hypoalbuminemia may lead to a poor response to anti-tumor therapeutics.24 ALP is a hydrolase enzyme which widely exists in the liver, kidneys and bones. Serum ALP is often used as a biomarker for liver and bone health. Additionally, ALP has been reported to play an important role in protection against inflammation and immune response.25 Many studies have found that decreased ALB and elevated ALP are associated with poor survival in lung cancer patients.26,27 AAPR, the ratio of ALB to ALP, has more powerful prognostic value than ALB or ALP alone. Chan et al firstly described its use in hepatocellular carcinoma and found that decreased AAPR was associated with poor overall and disease-free survival regardless of treatment options.18 Hereafter, this index has been validated in various cancers.15–17 Consistently, our data showed that low AAPR correlated with poor PFS and OS. In the stratification analysis of liver/bone metastasis status, there was a tendency that patients with low AAPR had a poor survival. Low AAPR usually means lower ALB level or higher ALP level, which may be related to liver or bone metastasis, decreased nutritional status and poor liver function and may result in shorter exposure to EGFR-TKIs. To some extent, AAPR may reflect the body condition of patients. Performance score is often used to assess treatment response and tolerance to chemotherapy. Zhou et al found that AAPR<0.35 is associated with 55% higher risk of death after adjusting for performance score,28 which indicated that AAPR might be an independent prognostic factor for OS in lung cancer patients. In our study, performance score was not included into Cox regression model due to missing data. Further studies including potential confounding factors, such as ECOG-PS, are needed to validate our results.
The most common mechanism of acquired resistance to EGFR-TKIs is the development of EGFR T790M mutation. Other mechanisms include activation of alternative pathways and transformation of histology and phenotype. Osimertinib, the first third-generation EGFR-TKI, is used for both EGFR sensitizing and T790M resistance mutation. Other second line therapies after drug resistance include platinum-containing chemotherapy and targeted therapies for MET or HER2 amplification or BRAF mutation.29 Osimertinib had greater efficacy than platinum-containing chemotherapy in patients with T790M mutation (median PFS: 10.1 vs 4.4 months, P<0.001).30 PFS mainly depend on the time when tumor develops the corresponding mechanisms of acquired resistance. OS is not only affected by the drug resistance, but also by many other factors, such as nutritional status, performance score and posterior line therapy. In our study, we found that the difference in median OS between groups was more significant than that in PFS (58.2 months vs 36.7 months, P<0.001; 14.0 months vs 10.4 months, P<0.01; respectively). AAPR is associated with liver or bone metastasis, nutritional status and liver function, so it is more likely correlated with OS. We also revealed that icotinib had comparable efficacy with gefitinib, which was consistent with the ICOGEN trial. In our study, posterior treatment was not included into survival analysis due to missing data.
In the past two years, some researchers investigated the prognostic value of AAPR in lung cancer,21,28,31–36 the detailed information of these studies is summarized in Table 4. Different cut-offs were used among these researches for different cancer types. Li et al explored the prognostic impact of AAPR in metastatic NSCLC patients for the first time and found that elevated AAPR was an independent prognostic indicator for favorable OS.21 Furthermore, this novel index was applied to the prognosis of limited or extensive stage small-cell lung cancer (SCLC) patients.28,33,34 In our study, we also revealed that pretreatment AAPR could be served as a favorable prognostic indicator for both PFS and OS. However, we did not analyze the monitoring role of dynamic changes in AAPR on tumor progression. Further researches are needed to detect the optimal cut-off points of AAPR in specific cancer types and to explore the monitoring role of dynamic changes of AAPR on tumor progression during medication.
 |
Table 4 Basic Information About Published Articles of AAPR and Lung Cancer |
Recently, Zhou et al analyzed 808 advanced NSCLC patients and found that medium and high AAPR were associated with a decreased risk of death.35 In Liu’s study, AAPR was found to be a biomarker for prognosis and treatment choice in driver mutation-negative advanced NSCLC.36 We also confirmed that low AAPR correlated with shorter PFS and OS in EGFR-mutated advanced NSCLC patients in multivariate analysis, which is consistent with other reports about AAPR in advanced NSCLC.21,35 Further stratified analysis of smoking status and tumor histology type demonstrated that the prognostic impact of AAPR on PFS and OS was strong in non-smokers and patients with adenocarcinomas, while this trend was not apparent in smokers and patients with non-adenocarcinomas. The phenomenon should be further validated because EGFR mutations mainly present in adenocarcinoma and non-smokers37 and the number of smokers and non-adenocarcinomas was small after stratification in our study. Moreover, in multivariate analysis, we found that having more than two metastasis sites was significantly correlated with poor PFS and OS, while old age at initial treatment and adenocarcinoma were also related to more favorable PFS.
There were several limitations in our study. First, this retrospective study collected the data from a single center, which may cause selection bias. A prospective study including more patients from multicenter is needed to validate our findings. Second, the high censoring rate at the end of the follow-up may influence the accurate estimation of OS. Third, patients with undetected liver, renal or bone disease may be enrolled into our study. Moreover, confounding factors, such as tumor size, performance status and posterior line therapy, that may also influence OS, were not included into conjunction analysis due to unavailable or missing data. Finally, ROC curve analysis was performed to get the optimal cut-off value of AAPR in our study. Whether our cut-off value can be used in other cohorts needs further validation.
Conclusions
Pretreatment AAPR, a quick and low-cost index, may be a favorable prognostic factor for both PFS and OS in EGFR-mutated advanced NSCLC treated with first-line EGFR-TKIs. Future studies are warranted to unravel its underlying role in survival prediction and to overcome the limitations of our study.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81871890 and 91859203 to WM Li); Sichuan International/Hong Kong, Macao and Taiwan Science and Technology Innovation Cooperation Project: molecular imaging research on targeted treatment of lung cancer (2018HH0161). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that no competing interests exist in this work.
References
1. Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2016, Bethesda, MD: National Cancer Institute; 2019. Available from: https://seer.cancer.gov/csr/1975_2016/. based on November 2018 SEER data submission, posted to the SEER web site.
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
4. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. doi:10.3322/caac.21565
5. Shi Y, Au SK, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154–162. doi:10.1097/JTO.0000000000000033
6. Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311. doi:10.1016/S0140-6736(16)30958-8
7. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first line treatment for European patients with advanced EGFR mutation positive non small cell lung cancer (EURTAC): a multicentre, open label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi:10.1016/S1470-2045(11)70393-X
8. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19:2240–2247. doi:10.1158/1078-0432.CCR-12-2246
9. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi:10.1056/NEJMoa0909530
10. Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–222. doi:10.1016/S1470-2045(13)70604-1
11. Mcmillan DC, Watson WS, O’Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. 2001;39(2):210–213. doi:10.1207/S15327914nc392_8
12. Takaaki F, Toshinaga S, Hiroki M, et al. Serum albumin is superior to prealbumin for predicting short-term recurrence in patients with operable colorectal cancer. Nutr Cancer. 2012;64(8):1169–1173. doi:10.1080/01635581.2012.718034
13. Oñate-Ocaña LF, Aiello-Crocifoglio V, Gallardo-Rincón D, et al. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol. 2007;14(2):381–389. doi:10.1245/s10434-006-9093-x
14. Miura K, Hamanaka K, Koizumi T, et al. Clinical significance of preoperative serum albumin level for prognosis in surgically resected patients with non-small cell lung cancer: comparative study of normal lung, emphysema, and pulmonary fibrosis. Lung Cancer. 2017;111:S0169500217303823.
15. Xiao Y, Lu J, Chang W, et al. Dynamic serum alkaline phosphatase is an indicator of overall survival in pancreatic cancer. BMC Cancer. 2019;19(1):785. doi:10.1186/s12885-019-6004-7
16. Sonpavde G, Pond GR, Berry WR, et al. Serum alkaline phosphatase changes predict survival independent of PSA changes in men with castration-resistant prostate cancer and bone metastasis receiving chemotherapy. Urol Oncol. 2012;30(5):607–613. doi:10.1016/j.urolonc.2010.07.002
17. Karhade AV, Thio QC, Kuverji M, Ogink PT, Ferrone ML, Schwab JH. Prognostic value of serum alkaline phosphatase in spinal metastatic disease. Brit J Cancer. 2019;120(6):640–646. doi:10.1038/s41416-019-0407-8
18. Chan AW, Chan SL, Mo FK, et al. Albumin-to-alkaline phosphatase ratio: a novel prognostic index for hepatocellular carcinoma. Dis Markers. 2015;2015:564057. doi:10.1155/2015/564057
19. Long ZQ, Hua X, Zhang WW, et al. Prognostic impact of the pretreatment albumin to alkaline phosphatase ratio for nonmetastatic breast cancer patients. Cancer Manag Res. 2019;11:4809–4814. doi:10.2147/CMAR.S200759
20. Nie M, Peng S, Chen C, et al. Albumin-to-alkaline phosphatase ratio: a novel prognostic index of overall survival in cisplatin-based chemotherapy-treated patients with metastatic nasopharyngeal carcinoma. J Cancer. 2017;8(5):809–815. doi:10.7150/jca.17536
21. Li D, Yu H, Li W. Albumin-to-alkaline phosphatase ratio at diagnosis predicts survival in patients with metastatic non-small-cell lung cancer. Onco Targets Ther. 2019;12:5241–5249. doi:10.2147/OTT.S203321
22. Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–181. doi:10.1038/nrc2088
23. Recondo G, Facchinetti F, Olaussen KA, Besse B, Friboulet L. Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? Nat Rev Clin Oncol. 2018;15(11):694–708. doi:10.1038/s41571-018-0081-4
24. Rabbani A, Ahn SN. Structure, enzymatic activities, glycation and therapeutic potential of human serum albumin: a natural cargo. Int J Biol Macromol. 2019;123:979–990. doi:10.1016/j.ijbiomac.2018.11.053
25. Rader BA. Alkaline phosphatase, an unconventional immune protein. Front Immunol. 2017;3(8):897. doi:10.3389/fimmu.2017.00897
26. Yang JR, Xu JY, Chen GC, et al. Post-diagnostic C-reactive protein and albumin predict survival in Chinese patients with non-small cell lung cancer: a prospective cohort study. Sci Rep. 2019;9(1):8143. doi:10.1038/s41598-019-44653-x
27. Zhou Y, Yu QF, Peng AF, Tong WL, Liu JM, Liu ZL. The risk factors of bone metastases in patients with lung cancer. Sci Rep. 2017;7(1):8970. doi:10.1038/s41598-017-09650-y
28. Zhou S, Wang H, Jiang W, Yu Q, Zeng A. Prognostic value of pretreatment albumin-to-alkaline phosphatase ratio in extensive-disease small-cell lung cancer: a retrospective cohort study. Cancer Manag Res. 2020;12:2015–2024. doi:10.2147/CMAR.S247967
29. Wu SG, Shi JY. Management of acquired resistance to EGFR-TKI targeted therapy in advanced non-small cell lung cancer. Mol cancer. 2018;17(1):38. doi:10.1186/s12943-018-0777-1
30. Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640. doi:10.1056/NEJMoa1612674
31. Zhang L, Zhang H, Yue D, et al. The prognostic value of the preoperative albumin to alkaline phosphatase ratio in patients with non-small cell lung cancer after surgery. Thorac Cancer. 2019;10(7):1581–1589. doi:10.1111/1759-7714.13107
32. Li SJ, Lv WY, Du H, et al. Albumin-to-alkaline phosphatase ratio as a novel prognostic indicator for patients undergoing minimally invasive lung cancer surgery: propensity score matching analysis using a prospective database. Int J Surg. 2019;69:32–42. doi:10.1016/j.ijsu.2019.07.008
33. Li X, Li B, Zeng H, et al. Prognostic value of dynamic albumin-to-alkaline phosphatase ratio in limited stage small-cell lung cancer. Future Oncol. 2019;15(9):995–1006. doi:10.2217/fon-2018-0818
34. Li B, Jiang C, Wang R, et al. Prognostic value of a nomogram based on the dynamic albumin-to-alkaline phosphatase ratio for patients with extensive-stage small-cell lung cancer. Onco Targets Ther. 2020;13:9043–9057. doi:10.2147/OTT.S262084
35. Zhou S, Jiang W, Wang H, Wei N, Yu Q. Predictive value of pretreatment albumin-to-alkaline phosphatase ratio for overall survival for patients with advanced non-small cell lung cancer. Cancer Med. 2020;9(17):6268–6280. doi:10.1002/cam4.3244
36. Liu X, Li Y, Zhao Q, Jiang H, Ni J, Cai H. Albumin-to-alkaline phosphatase ratio: a novel prognostic index for patients with driver mutation-negative advanced non-small cell lung cancer. Clin Respir J. 2021;15(5):540–549. doi:10.1111/crj.13339
37. Toyooka S, Soh J, Shigematsu H, Aoe M, Date H. The impact and role of EGFR gene mutation on non-small cell lung cancer. Cancer Chemoth Pharm. 2006;58(1 Supplement):25–31. doi:10.1007/s00280-006-0312-8
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


