Back to Journals » Cancer Management and Research » Volume 14
Prognostic Value of a Serum Panel of Inflammatory Factors in Non-Metastatic Nasopharyngeal Carcinoma Patients Undergoing Radical Radiotherapy with Adjuvant Chemotherapy
Authors Liang T, Xiao D, Lu S, Ye X, Xiao Z
Received 6 May 2022
Accepted for publication 17 August 2022
Published 16 September 2022 Volume 2022:14 Pages 2763—2772
DOI https://doi.org/10.2147/CMAR.S371922
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Ting Liang,1– 6 Ding Xiao,2,3,7 Shanshan Lu,2,3 Xu Ye,8 Zhiqiang Xiao1– 3
1National Clinical Research Center of Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, 410008, People’s Republic of China; 2Research Center of Carcinogenesis and Targeted Therapy, Xiangya Hospital, Central South University, Changsha, 410008, People’s Republic of China; 3Higher Educational Key Laboratory for Cancer Proteomics and Translational Medicine of Hunan Province, Xiangya Hospital, Central South University, Changsha, 410008, People’s Republic of China; 4Department of Hematology, Xiangya Hospital, Central South University, Changsha, 410008, People’s Republic of China; 5Hunan Hematology Oncology Clinical Medical Research Center, Changsha, 410008, People’s Republic of China; 6National Clinical Research Center for Hematologic Diseases, The First Affiliated Hospital of Soochow University, Suzhou, 215006, People’s Republic of China; 7Department of Pathology, Xiangya Hospital, Central South University, Changsha, 410008, People’s Republic of China; 8Department of Radiation Oncology, Hunan Cancer Hospital, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, 410008, People’s Republic of China
Correspondence: Zhiqiang Xiao, National Clinical Research Center of Geriatric Disorders, Xiangya Hospital, Central South University, #87 Xiangya Road, Changsha, 410008, People’s Republic of China, Tel +86-13873146236, Email [email protected]
Purpose: To evaluate the prognostic value of interleukin (IL)-6, IL-8, granulocyte-macrophage colony-stimulating factor (GM-CSF), leukemia inhibitory factor (LIF), and macrophage migration inhibitory factor (MIF) in non-metastatic nasopharyngeal carcinoma (NPC) patients undergoing radical radiotherapy.
Patients and Methods: A serum panel compromising the inflammatory factors was analyzed in 372 NPC patients before and after radiotherapy. Independent prognostic factors were screened out using multivariate Cox regression analysis. A prediction model was built based on the training set data and validated using the test set data. The prognostic value of these factors was evaluated using the time-dependent receiver operating characteristic (ROC) curve and an integrated time-averaged area under the curve (AUC).
Results: The baseline levels of IL-6, GM-CSF, and MIF were independent factors associated with poor OS and DMFS. A predictive model base established combining the baseline levels of these factors. The AUC values for the test set were 0.9828, 0.9968, and 0.9571 at 1, 3, and 5 years, respectively, compared to 0.9978, 0.9981, and 0.9222 for the training set, respectively. The AUC values for DMFS at 1, 3, and 5-years for the training set were 0.8744, 0.8951, and 0.9358, respectively, compared to 0.9525, 0.9663, and 0.9625 for the test set, respectively. The combination of post-treatment levels of IL-6, GM-CSF, and LIF also had good predictive value for OS with an AUC value > 0.85 during follow-up.
Conclusion: IL-6, GM-CSF, and MIF baseline levels are powerful prognostic factors for non-metastatic NPC patients. The combination of these factors effectively predicts OS and DMFS in non-metastatic NPC patients.
Keywords: nasopharyngeal carcinoma, inflammatory factor, radical radiotherapy, prognostic value, overall survival
Introduction
Nasopharyngeal carcinoma (NPC) is the most common malignant tumor in the head and neck with a high incidence in China.1 NPC is highly sensitive to radiotherapy, which is the backbone of treatment for NPC. Neoadjuvant chemotherapy is also indispensable for highly differentiated cases, late-stage cases, and recurrent cases.2 The local-regional control of NPC has made great progress through intensity-modulated radiotherapy (IMRT) or chemoradiation, while distant metastasis has been the main cause of treatment failure.3,4 Patients with metastasis have a significantly higher risk of death than those without metastasis.5 Thus, identifying appropriate prognostic markers and evaluating the survival and metastasis of patients in advance may help to adjust the treatment plan to obtain better treatment outcomes.
Recent studies have shown that the inflammatory microenvironment is an important participant in tumor progression. The contradictory process of immune killing and immune escape exists in all stages of tumor development.6,7 NPC has a close relationship with Epstein-Barr virus (EBV) infection, and studies have shown that inflammation-based indices are associated with the prognosis of NPC patients.8–12 However, most of these studies did not perform an analysis of receiver operating characteristic curves (ROCs), the area under which (or AUC) shows the exact prognostic value. Few studies have conducted ROC analysis, but the prognostic value of the indices used was not high, with AUC ranging from 0.6–0.8.10,11,13–15 Therefore, it is worth searching for inflammatory factors with higher prognostic value. In addition, these studies selected baseline indicators as the predictors. Even if the baseline data are not obtained for various reasons, it is worth investigating whether the factor levels after treatment still have prognostic value.
Interleukin (IL)-6, IL-8, granulocyte-macrophage colony-stimulating factor (GM-CSF), leukemia inhibitory factor (LIF), and macrophage migration inhibitory factor (MIF) are related to the progress of NPC.16–21 The objective of the present study was to evaluate the prognostic value of these factors for NPC patients before and after radical radiotherapy with adjuvant chemotherapy and to identify the best prediction combination.
Materials and Methods
Study Cohort
This prospective study was approved by the Ethics Committee of Xiangya Hospital of Central South University (Approval number 20140161) and the Ethics Committee of Hunan Cancer Hospital (Approval number 20140586). Our study complied with the Declaration of Helsinki. The patients were enrolled between March 2014 and July 2017 with 161 patients from Xiangya Hospital of Central South University and 211 patients from Hunan Cancer Hospital. All patients were pathologically diagnosed with M0 stage NPC without distant metastasis, and had never received radiotherapy and/or chemotherapy. Signed informed consent was obtained from all participants.
All patients received radical radiotherapy. The total dose of radiotherapy was 60–70 Gy (2 Gy/time, 5 times per week for 6–7 weeks) with 60 Gy to the negative neck lymph nodes and 70 Gy to the positive neck lymph nodes. Adjuvant chemotherapy with platinum-based regimens was performed 1 month after radiotherapy. Patients were administrated one or two of cisplatin, 5-fluorouracil, and taxane every 3 weeks for 2–3 cycles.
Follow-Up and Clinical Endpoints
All patients underwent outpatient follow-up, once every 3 months in the first year, once every 6 months in the next two years, and once a year thereafter. The median follow-up length was 49.5 months. The last follow-up time was May 2021. Clinical outcomes, including overall survival (OS), progression-free survival (PFS), local recurrence-free survival (LRFS), and distant metastasis-free survival (DMFS), were observed. OS was defined as the interval between the end of radiotherapy and death from any cause. PFS was defined as the interval between the end of radiotherapy and the first progression of the disease (local recurrent or distant metastasis) or death from any cause. LRFS was defined as the interval between the end of radiotherapy and local recurrence or death from any cause. DMFS was defined as the interval between the end of radiotherapy and distant metastasis or death from any cause.
Cytokine Measurement
Peripheral blood samples of the patients were taken before, immediately after, and 3 months after radiotherapy. The serum levels of IL-6, IL-8, GM-CSF, MIF, and LIF were measured using commercial ELISA kits (Genetimes Technology Inc, Shanghai, China).
Statistical Analysis
All data were analyzed using R 3.6.3 software (R Foundation, Vienna, Austria). P<0.05 was considered statistically significant. The samples were randomly divided into a training set and a test set at a ratio of 2:1 for two-stage analysis. The data of the training set were analyzed using univariate and multivariate Cox regression models to screen out possible prognostic factors. The Cox regression analysis was performed using the function coxph() from the survival package for R language (https://CRAN.R-project.org/package=survival). In Cox regression analysis, the grouping cut-off value of each cytokine was the median value of all samples. The hazard ratio (HR) and 95% confidence interval (CI) were calculated to indicate the impact of each factor on treatment outcome. According to the results of Cox regression, each cytokine had a corresponding coefficient. A prediction model of risk score was established based on the expression level of dependent factors and their corresponding coefficient. A risk score was generated for each patient using the following formula: 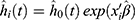 (where, x is the expression level of cytokine i and β is its corresponding coefficient). The median risk score was taken as the grouping point and the patients of the training set were divided into a high-risk group and a low-risk group. The Kaplan-Meier method was used to plot the cumulative curves of OS, PFS, LRFS, and DMFS. The difference between the high-risk group and the low-risk group was compared by Log rank test. The data of the test set were then used to verify the effectiveness of the prediction model. The predictive value of the studied cytokines was further evaluated by time-dependent ROC.22
(where, x is the expression level of cytokine i and β is its corresponding coefficient). The median risk score was taken as the grouping point and the patients of the training set were divided into a high-risk group and a low-risk group. The Kaplan-Meier method was used to plot the cumulative curves of OS, PFS, LRFS, and DMFS. The difference between the high-risk group and the low-risk group was compared by Log rank test. The data of the test set were then used to verify the effectiveness of the prediction model. The predictive value of the studied cytokines was further evaluated by time-dependent ROC.22
Results
Baseline Characteristics
In total, 374 NPC patients were enrolled in this study. The median follow-up time was 49.5 months, ranging from 4–74 months. A summary of the baseline characteristics is shown in Table 1. The ratio of males to females was approximately 1.65:1. Most of the patients were nonsmokers and had no family history. Pathological results showed that the patients were mainly diagnosed with stage III to IV keratinized squamous cell carcinoma or non-keratinized undifferentiated carcinoma. The median values of IL-6, IL-8, GM-CSF, LIF, and MIF in the total cohort were 4.10 pg/mL (1.04–38.29 pg/mL), 58.21 pg/mL (2.39–1343.94 pg/mL), 8.26 pg/mL (2.88–674.21 pg/mL), 5.05 pg/mL (1.08–32.77 pg/mL), and 350.98 pg/mL (137.10–3090.80 pg/mL), respectively.
 |
Table 1 The Baseline Characteristics (N=374) |
Survival Analysis
We analyzed the effect of baseline characteristics on survival endpoints by univariate and multivariate Cox regression analyses. The grouping of each cytokine was based on the median value of the total samples. The results of univariate analysis are shown in the forest plot (Supplementary Figure 1). Multivariate analysis determined that IL-6 > 4.1 pg/mL, GM-CSF ≤ 8.26 pg/mL, and MIF ≤ 350.98 pg/mL were independent factors associated with poor OS and DMFS. Because no influencing factors were found to be statistically associated with PFS and LRFS, we focused on the prognostic factors of OS and DMFS in the following analysis. The results of multivariate analysis are shown in Table 2. A reference prediction model was established based on the Cox regression analysis results of the training set, and each patient was assigned a risk value. The training set was divided into a high-risk group and a low-risk group based on the median risk value of the training set. There was a significant difference in OS, PFS, LRFS, and DMFS between the two groups (Figure 1). The test set data were analyzed with the prediction model. Figure 2 shows that OS, PFS, LRFS, and DMFS were significantly different between the high-risk group and the low-risk group. These results suggested that the prediction model combining the baseline levels of IL-6, GM-CSF, and MIF effectively predicts the prognosis of NPC patients undergoing radiotherapy with adjuvant chemotherapy.
 |
Table 2 Multivariate Cox Regression Analysis on Factors for OS and DMFS in the Training Set |
ROC Analysis
We next evaluated the prognostic value of IL-6, GM-CSF, and MIF for OS by time-dependent ROC (Figure 3). The results showed that the prediction model had good predictive value for OS and DMFS with an AUC value > 0.85 during the follow-up. The AUC values for OS at 1, 3, and 5 years for the training set were 0.9828 (95% CI: 0.9564, 1.0000), 0.9968 (95% CI: 0.9901, 1.0000), and 0.9571 (95% CI: 0.9263, 0.9879), respectively. When the prediction model was applied in the test set, the AUC values at 1, 3, and 5 years was 0.9978 (95% CI: 0.9922, 1.0000), 0.9981 (95% CI: 0.9951, 1.0000), and 0.9222 (95% CI: 0.8898, 0.9546), respectively. The AUC values for DMFS at 1, 3, and 5 years for the training set was 0.8744 (95% CI: 0.8214, 0.9213), 0.8951 (95% CI: 0.8346, 0.9564), and 0.9358 (95% CI: 0.9049, 0.9646), respectively, and those for the test set were 0.9525 (95% CI: 0.9034, 0.9759), 0.9 0.9663 (95% CI: 0.9358, 0.9967), and 0.9625 (95% CI: 0.8885, 0.9728), respectively.
Comparison of the Prognostic Value of Cytokine Combinations at Different Time Points
The above prediction model was established using the baseline cytokine levels. We further investigated the prognostic value of post-treatment cytokine levels for OS. Univariate and multivariate Cox regression analyses determined that the potential risk factors for OS were IL-6, GM-CSF, and LIF after treatment, as well as IL-6, IL-8, GM-CSF, and LIF at 3 months after treatment (Table 3). The predictive value of cytokine combinations at different time points for OS was then evaluated by time-dependent ROC. The results are shown in Figure 4 and Table 4. The combinations of baseline levels and post-treatment levels had better diagnostic values, which remained > 0.85 during the follow-up.
 |
Table 3 Multivariate Cox Regression Analysis of Post-Treatment Cytokines for OS in the Training Set |
 |
Table 4 AUC Values of Cytokine Combinations for OS at Different Time Points |
Discussion
In the present study, we found that inflammatory cytokines may be good prognostic factors for non-metastatic NPC patients undergoing radiotherapy with adjuvant chemotherapy. The baseline combination of IL-6, GM-CSF, and MIF resulted in an AUC value > 0.85 for both OS and DMFS. Moreover, the combination of post-treatment levels of IL-6, GM-CSF, and LIF also had good predictive value for OS, with an AUC value > 0.85 during follow-up.
Our analysis showed that baseline levels of IL-6, GM-CSF, and MIF were independent prognostic factors of NPC. The important effects of these cytokines on NPC have been reported in previous studies. In general, IL-6 expression is a risk factor for poor prognosis in cancer patients, which is consistent with the results of the present study. The expression of IL-6 in NPC tissues is a risk factor for poor prognosis of NPC and is associated with lymph node metastasis.23 The serum IL-6 level is related to treatment. A significant decrease in serum IL-6 has been observed in treated NPC patients compared to untreated NPC patients.24,25 In terms of the molecular mechanism, IL-6 promotes the proliferation, invasion, and migration of NPC cells by activating the JAK2/STAT3 signaling pathway.23 The role of GM-CSF in different types of tumors is contradictory. Although most studies have indicated that GM-CSF stimulates tumor progression, GM-CSF also has anti-proliferative effects in certain types of tumor cell lines and stages of development.26 In a mouse model of NPC, adenovirus-mediated expression of the GM-CSF gene effectively inhibits the growth of tumors, suggesting the therapeutic potential of GM-CSF.27 The present study also indicated that high expression of serum GM-CSF was favorable for better prognosis of NPC patients. These data suggested that GM-CSF has an anti-cancer effect on NPC. However, the relationship between the MIF expression level and the prognosis of patients is controversial. Several studies have reported that MIF is strongly expressed in NPC tissues, and high expression of MIF is positively correlated with angiogenesis, lymph node metastasis, and locoregional failure of NPC patients.28,29 Interfering with MIF expression reduces downstream IL-8 expression and the growth of NPC spheres.30 In contrast, Li et al reported that increased expression of MIF in tumor-infiltrating lymphocytes is an independent factor for better OS and disease-free survival of NPC patients.31 Our results showed that a high serum MIF level was a favorable factor for OS and DMFS, which agreed with the study reported by Li et al. Thus, the expression of MIF in the tumor environment is related to the intratumoral immune response, which is inconsistent with the role of MIF in tumor entities.31
An increasing number of studies have investigated the feasibility of using inflammation-based indices as prognostic factors of NPC. The Glasgow Prognostic Score (GPS), a scoring system based on serum C-reactive protein level and albumin concentration, is an independent prognostic factor of OS with AUC values at 6 months, 1 year, and 2 years of 0.805, 0.705, and 0.705, respectively.8,13 The systemic immune-inflammation index (SII), another integrated index based on platelet count and NLR, has been shown to be associated with OS and PFS in NPC patients with an approximate AUC value of 0.6 for OS.10,11,15,32 An elevated neutrophil to lymphocyte ratio (NLR) is associated with a lower survival rate and worse treatment response to chemotherapy with an AUC value of 0.619 for OS.11,12,14,33,34 Li et al proposed an index combining the C-reactive protein to albumin ratio and platelet to lymphocyte ratio, resulting in an AUC value of 0.670 for OS prediction.35 One advantage of these studies is that indicators of routine clinical detection can be used. However, regardless of the combination is used, the diagnostic value of these integrated indices is poor. In contrast, the combination of the baseline levels of IL-6, GM-CSF, and MIF had powerful predictive value with AUC values > 0.85 for both OS and DMFS. In addition, we found that the combination of post-treatment levels of IL-6, GM-CSF, and LIF had the same prognostic value as the pretreatment levels. Therefore, if the pretreatment blood sample is not collected for various reasons, the blood sample can still be collected for prognosis analysis after radiotherapy to provide guidance for subsequent adjuvant treatment.
The present study had several limitations. We only selected patients at the M0 stage in this study to more accurately observe the prognostic effect of research factors on metastasis. Such inclusion criteria led to insufficient sample representation in predicting OS. Moreover, the levels of inflammatory factors evaluated in this study are likely to change in different M stages, suggesting that the predictive value of these factors in all NPC patients may vary from the conclusion of this study. In addition, IL-6, GM-CSF, and MIF are not routinely tested indicators in clinical practice, indicating that additional detection work is needed, which may limit the application of our research conclusions. Moreover, external independent studies are needed to validate the conclusion of this study.
Conclusion
In summary, our study indicated that inflammatory factors are powerful prognostic factors. The baseline levels of IL-6, GM-CSF, and MIF effectively predict the survival and metastasis of NPC patients undergoing radical radiotherapy with adjuvant chemotherapy.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
This work was supported by the National Natural Science Foundation of China (82170552, 82103638, 81874132), National Natural Sciences Foundation of Hunan province (2022JJ40805), and the Research Foundation of Education Bureau of Hunan Province, China (19K101, 20K137, 21A0008).
Disclosure
The authors report no conflicts of interests in this work.
References
1. Chen W, Zheng R, Baade PD., et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi:10.3322/caac.21338
2. Pastor M, Lopez Pousa A, Del Barco E, et al. SEOM clinical guideline in nasopharynx cancer (2017). Clin Transl Oncol. 2018;20:84–88. doi:10.1007/s12094-017-1777-0
3. Zhang MX, Li J, Shen GP, et al. Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: a 10-year experience with a large cohort and long follow-up. Eur J Cancer. 2015;51:2587–2595. doi:10.1016/j.ejca.2015.08.006
4. Tian YM, Liu MZ, Zeng L, et al. Long-term outcome and pattern of failure for patients with nasopharyngeal carcinoma treated with intensity-modulated radiotherapy. Head Neck. 2019;41:1246–1252. doi:10.1002/hed.25545
5. Siti-Azrin AH, Norsa’adah B, Naing NN. Prognostic factors of nasopharyngeal carcinoma patients in a tertiary referral hospital: a retrospective cohort study. BMC Res Notes. 2017;10:705. doi:10.1186/s13104-017-2990-1
6. Lin Q, Jin S, Han M, Zheng W, Liu J, Wei X. Inflammation in the tumor microenvironment. J Immunol Res. 2018;2018:1965847. doi:10.1155/2018/1965847
7. Jang JH, Kim DH, Surh YJ. Dynamic roles of inflammasomes in inflammatory tumor microenvironment. NPJ Precis Oncol. 2021;5:18. doi:10.1038/s41698-021-00154-7
8. Proctor MJ, Morrison DS, Talwar D, et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. Br J Cancer. 2011;104:726–734. doi:10.1038/sj.bjc.6606087
9. Lu J, Chen XM, Huang HR, et al. Detailed analysis of inflammatory cell infiltration and the prognostic impact on nasopharyngeal carcinoma. Head Neck. 2018;40:1245–1253. doi:10.1002/hed.25104
10. Oei RW, Ye L, Kong F, et al. Prognostic value of inflammation-based prognostic index in patients with nasopharyngeal carcinoma: a propensity score matching study. Cancer Manag Res. 2018;10:2785–2797. doi:10.2147/CMAR.S171239
11. Li Q, Yu L, Yang P, Hu Q. Prognostic value of inflammatory markers in nasopharyngeal carcinoma patients in the intensity-modulated radiotherapy era. Cancer Manag Res. 2021;13:6799–6810. doi:10.2147/CMAR.S311094
12. Li XH, Chang H, Xu BQ, et al. An inflammatory biomarker-based nomogram to predict prognosis of patients with nasopharyngeal carcinoma: an analysis of a prospective study. Cancer Med. 2017;6:310–319. doi:10.1002/cam4.947
13. Chen C, Sun P, Dai QS, Weng HW, Li HP, Ye S. The Glasgow Prognostic Score predicts poor survival in cisplatin-based treated patients with metastatic nasopharyngeal carcinoma. PLoS One. 2014;9:e112581. doi:10.1371/journal.pone.0112581
14. Ye L, Oei RW, Kong F, et al. Prognostic values of hematological biomarkers in nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. Eur Arch Otorhinolaryngol. 2018;275:1309–1317. doi:10.1007/s00405-018-4956-x
15. Jiang W, Chen Y, Huang J, et al. Systemic immune-inflammation index predicts the clinical outcome in patients with nasopharyngeal carcinoma: a propensity score-matched analysis. Oncotarget. 2017;8:66075–66086. doi:10.18632/oncotarget.19796
16. Chang KP, Chang YT, Wu CC, et al. Multiplexed immunobead-based profiling of cytokine markers for detection of nasopharyngeal carcinoma and prognosis of patient survival. Head Neck. 2011;33:886–897. doi:10.1002/hed.21557
17. Ke L, Xiang Y, Xia W, et al. A prognostic model predicts the risk of distant metastasis and death for patients with nasopharyngeal carcinoma based on pre-treatment interleukin 6 and clinical stage. Clin Immunol. 2016;164:45–51. doi:10.1016/j.clim.2016.01.004
18. Li XJ, Peng LX, Shao JY, et al. As an independent unfavorable prognostic factor, IL-8 promotes metastasis of nasopharyngeal carcinoma through induction of epithelial-mesenchymal transition and activation of AKT signaling. Carcinogenesis. 2012;33:1302–1309. doi:10.1093/carcin/bgs181
19. Zhao W, Xiang Y, Zhang Z, et al. Pharmacological inhibition of GSK3 promotes TNFα-induced GM-CSF via up-regulation of ERK signaling in nasopharyngeal carcinoma (NPC). Int Immunopharmacol. 2020;83:106447. doi:10.1016/j.intimp.2020.106447
20. Liu SC, Chang YS. Role of leukemia inhibitory factor in nasopharyngeal carcinogenesis. Mol Cell Oncol. 2014;1:e29900. doi:10.4161/mco.29900
21. Xue N, Xing S, Ma W, Sheng J, Huang Z, Xu Q. Combination of plasma MIF and VCA-IgA improves the diagnostic specificity for patients with nasopharyngeal carcinoma. Technol Cancer Res Treat. 2020;19:1533033820935773. doi:10.1177/1533033820935773
22. Kamarudin AN, Cox T, Kolamunnage-Dona R. Time-dependent ROC curve analysis in medical research: current methods and applications. BMC Med Res Methodol. 2017;17(1):53. doi:10.1186/s12874-017-0332-6
23. Zhuang M, Ding X, Song W, et al. Correlation of IL-6 and JAK2/STAT3 signaling pathway with prognosis of nasopharyngeal carcinoma patients. Aging. 2021;13:16667–16683. doi:10.18632/aging.203186
24. Tan EL, Selvaratnam G, Kananathan R, Sam CK. Quantification of Epstein-Barr virus DNA load, interleukin-6, interleukin-10, transforming growth factor-β1 and stem cell factor in plasma of patients with nasopharyngeal carcinoma. BMC Cancer. 2006;6(1):227. doi:10.1186/1471-2407-6-227
25. Jin YB, Zhang GY, Lin KR, et al. Changes of plasma cytokines and chemokines expression level in nasopharyngeal carcinoma patients after treatment with definitive intensity-modulated radiotherapy (IMRT). PLoS One. 2017;12:e0172264. doi:10.1371/journal.pone.0172264
26. Hong IS. Stimulatory versus suppressive effects of GM-CSF on tumor progression in multiple cancer types. Exp Mol Med. 2016;48:e242. doi:10.1038/emm.2016.64
27. Ren SP, Wang L, Wang H, et al. Gene therapy for human nasopharyngeal carcinoma by adenovirus-mediated transfer of human p53, GM-CSF, and B7-1 genes in a mouse xenograft tumor model. Cancer Biother Radiopharm. 2008;23:591–602. doi:10.1089/cbr.2007.0447
28. Liao B, Zhong BL, Li Z, Tian XY, Li Y, Li B. Macrophage migration inhibitory factor contributes angiogenesis by up-regulating IL-8 and correlates with poor prognosis of patients with primary nasopharyngeal carcinoma. J Surg Oncol. 2010;102:844–851. doi:10.1002/jso.21728
29. Pei XJ, Wu TT, Li B, Tian XY, Li Z, Yang QX. Increased expression of macrophage migration inhibitory factor and DJ-1 contribute to cell invasion and metastasis of nasopharyngeal carcinoma. Int J Med Sci. 2013;11:106–115. doi:10.7150/ijms.7264
30. Lo MC, Yip TC, Ngan KC, et al. Role of MIF/CXCL8/CXCR2 signaling in the growth of nasopharyngeal carcinoma tumor spheres. Cancer Lett. 2013;335:81–92. doi:10.1016/j.canlet.2013.01.052
31. Li J, Mo HY, Xiong G, et al. Tumor microenvironment macrophage inhibitory factor directs the accumulation of interleukin-17-producing tumor-infiltrating lymphocytes and predicts favorable survival in nasopharyngeal carcinoma patients. J Biol Chem. 2012;287:35484–35495. doi:10.1074/jbc.M112.367532
32. Zhou F, Liu L, Huang X, et al. Pretreatment systemic immune-inflammation index predicts survival for non-metastatic nasopharyngeal carcinoma: two independent institutional studies. J Natl Cancer Cent. 2021;2:60–67. [In press].
33. An X, Ding PR, Wang FH, Jiang WQ, Li YH. Elevated neutrophil to lymphocyte ratio predicts poor prognosis in nasopharyngeal carcinoma. Tumor Biol. 2011;32:317–324. doi:10.1007/s13277-010-0124-7
34. He JR, Shen GP, Ren ZF, et al. Pretreatment levels of peripheral neutrophils and lymphocytes as independent prognostic factors in patients with nasopharyngeal carcinoma. Head Neck. 2012;34:1769–1776. doi:10.1002/hed.22008
35. Li JP, Chen SL, Liu XM, et al. A novel inflammation-based stage (I stage) predicts overall survival of patients with nasopharyngeal carcinoma. Int J Mol Sci. 2016;17:1900. doi:10.3390/ijms17111900
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




