Back to Journals » OncoTargets and Therapy » Volume 13
Prognostic Value of a Nomogram Based on the Dynamic Albumin-to-Alkaline Phosphatase Ratio for Patients with Extensive-Stage Small-Cell Lung Cancer
Authors Li B, Jiang C, Wang R, Zou B, Xie P , Li W, Sun X, Yu J, Wang L
Received 10 May 2020
Accepted for publication 7 August 2020
Published 11 September 2020 Volume 2020:13 Pages 9043—9057
DOI https://doi.org/10.2147/OTT.S262084
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yao Dai
Butuo Li,1 Chao Jiang,2 Ruiqing Wang,3 Bing Zou,1 Peng Xie,1 Wanlong Li,1 Xindong Sun,1 Jinming Yu,1 Linlin Wang1
1Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan 250017, Shandong Province, People’s Republic of China; 2Department of Otorhinolaryngology, Head and Neck Surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong Province 250021, People’s Republic of China; 3Department of Breast Surgery, Linyi People’s Hospital, Linyi 276000, Shandong Province, People’s Republic of China
Correspondence: Linlin Wang Tel +86-531-67626142
Fax +86-531-67626141
Email [email protected]
Purpose: Small-cell lung cancer (SCLC) is known as the characteristics of high invasion, rapid progression, and poor prognosis. Therefore, identification of patients with high risk of progression and death is critical to improve the survival of patients with extensive-stage SCLC (ES-SCLC). This study was designed to determine the prognostic importance of the albumin-to-alkaline phosphatase ratio (AAPR) in the survival of patients with ES-SCLC and to develop a nomogram based on AAPR dynamics for ES-SCLC prognosis.
Patients and Methods: Characteristics were reviewed from 300 patients with ES-SCLC. Training and validation cohorts included 200 and 100 patients, respectively. We applied univariate and multivariate Cox models to assess the prognostic value of AAPR for ES-SCLC. The nomogram for progression-free survival (PFS) and overall survival (OS) of ES-SCLC patients was developed based on the multivariate survival analysis of the training cohort. External validation of the established nomogram was performed using the validation cohort.
Results: N3 stage, thoracic radiotherapy, and post-AAPR were the independent factors identified for PFS. T stage, thoracic radiotherapy, and high post-AAPR were the independent risk factors identified for death. The prognostic nomogram was established by integrating the independent significant factors for PFS and OS in the training cohort with the c-indices of 0.675 and 0.662, respectively, and validated in the validation cohort. The nomogram had superior prognosis prediction ability than did TNM stage. Decision curve analysis (DCA) also indicated clinical net benefits from the nomogram.
Conclusion: AAPR was valuable for prognosis prediction in patients with ES-SCLC and was recommended to be dynamically evaluated to guide patient treatment. Additionally, the nomogram covering post-AAPR accurately predicted individual survival probability.
Keywords: extensive-stage small-cell lung cancer; ES-SCLC, albumin-to-alkaline phosphatase ratio; AAPR, dynamic, nomogram, prognosis prediction
Introduction
Small-cell lung cancer (SCLC) accounts for 10–15% of all lung cancer cases,1 and about 70% of patients with SCLC are in the extensive stage (ES).2 The application of targeted therapy and immunotherapy have greatly improved survival in patients with NSCLC.3,4 However, it is disappointing that the search for SCLC target drugs has been unsuccessful.5 Improvements in progression-free survival (PFS) and overall survival (OS) of 1.1 and 2 months, respectively, have been achieved by the additional application of atezolizumab in ES-SCLC patients,6 yet platinum-based chemotherapy (platinum/etoposide) for 4–6 cycles is regarded as the first-line standard treatment for ES-SCLC.7 Despite high initial response rates to first-line chemotherapy of 67% to 80%,8 patients with ES-SCLC usually experience disease relapse and distant metastasis within a short timeframe, because of the extensive invasiveness property of the disease. Disease progression also occurs in patients who have not finished the planned treatment.8 Therefore, the identification of patients with a high risk of progression and death is crucial for the individualized management and improvement of patient survival in patients with ES-SCLC.
Nutritional status and inflammation of patients play vital roles in carcinogenesis and prognosis of SCLC.9,10 A decreased albumin (ALB) level is regarded as a surrogate parameter for malnutrition and systemic inflammation, which are negative indicators for cancer patients.10 Serum ALB is a reliable factor for survival of patients with tumors including those arising from colorectal cancer,11 lung cancer,12 breast cancer,13 and gastric cancer.14 Previous studies have found that pathological elevation of alkaline phosphatase (ALP) is usually associated with liver and kidney disease or bone metastasis.15–17 Interestingly, ALP plays a role in tumor growth, is closely associated with inflammation,18,19 and is regarded as a prognostic factor for SCLC patients.
Increasing amounts of evidence show the superiority of combined indices over simple markers for survival prediction, likely because of tumor heterogeneity and complexity.20 The incorporated index of albumin-to-alkaline phosphatase ratio (AAPR) has prognostic value in hepatocellular carcinoma and nasopharyngeal carcinoma, and was more powerful than simple ALB or ALP.21,22 However, the systemic and tumor status is dynamic during tumor development and treatment. Previous studies have mostly focused on baseline parameters as prognostic markers and have ignored the value of dynamic AAPR, which might be more informative and precise.21,22 The prognostic ability and complementary value of dynamic AAPR to TNM stage for the survival of patients with ES-SCLC remains unclear.
TNM stage, the traditional prognostic factor for patients with malignant tumors, only represents tumor burden.23 Recently, integration of AAPR with the TNM staging system was shown to have superior predictive accuracy to TNM stage alone.21 Nomograms, graphic illustrations of a statistical model, have been developed in lung,24 breast,25 and other cancers26 for prognosis estimation. Importantly, the nomogram based on clinicopathological characteristics could predict the survival of cancer patients more accurately than could the TNM staging system. Thus, nomograms based on dynamic AAPR might be able to provide individualized prognostic estimations in ES-SCLC patients to optimize treatment approaches.
This study was designed to analyze the prognostic value of dynamic AAPR in ES-SCLC patients, and to establish and validate nomogram based on dynamic AAPR for prognosis of ES-SCLC.
Patients and Methods
Study Population
We reviewed medical record of patients diagnosed with ES-SCLC according to the Veterans Administration Lung Study Group (VALSG) staging system in Shandong Cancer Hospital from January 2013 to May 2018. Patients who met the following criteria were enrolled in the study: 1) pathological diagnosis of SCLC; 2) imaging diagnosis of ES-SCLC; 3) cisplatin/etoposide as first-line treatment was tolerated well; and 4) available medical records. Exclusion criteria were: 1) patients who were initially diagnosed as limited-stage SCLC (LS-SCLC) and progressed to ES-SCLC; and 2) other primary cancers diagnosed before or after SCLC. Finally, 300 patients with ES-SCLC were included, 200 of which were used as the training cohort, and the remaining 100 were used as the validation cohort. Our study was performed in accordance with the principles of the 1975 Declaration of Helsinki and its later amendments or comparable ethical standards, and was approved by the Ethics Committee of Shandong Cancer Hospital (No. SDTHEC201808005). Due to the retrospective nature of the study and the fact that no case details were shown, informed consent was waived by the Ethics Committee of Shandong Cancer Hospital. All data was kept confidential.
Data Collection
All data were collected using uniform database templates to ensure consistency. The medical records of all patients were reviewed with respect to laboratory serum ALB and ALP, and the clinical factors, including age, gender, body mass index (BMI) at diagnosis (calculated by weight and height), marital status (married and unmarried [which included single, divorced, and widowed]), smoking history and index, Karnofsky performance score (KPS), TNM stage, brain, liver and bone metastasis, and thoracic radiotherapy. Pre-ALB and pre-ALP were defined as the serum ALB and ALP levels before treatment, respectively. Post-ALB and post-ALP were defined as the serum ALB and ALP levels, respectively, after planned therapy for patients without progression during systemic treatment, and after progression for patients with progression before the end of planned systemic therapy. AAPR was calculated as the ratio of absolute ALB to absolute ALP.
Tumor response was assessed based on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. PFS and OS were defined from the date of initial diagnosis to the date of progression and the date of death or the last follow-up, respectively. PFS data were collected from medical records, and OS data were obtained through telephone follow-up.
Statistical Analysis
BMI was used to categorize patients into clinical groupings of less than 18.5 kg/m2 (underweight), 18.5–25 kg/m2 (normal weight), greater than 25 kg/m2 (overweight). The ALB and ALP cut-off values were set as 40 g/L and 100 U/L, respectively, which represent the lower normal limit of ALB and upper normal limit of ALP. Receiver operating characteristic curve (ROC) was used to determine the optimal AAPR cut-off value of 0.52, by setting OS as the state variable. Patients in training and validation cohorts were dichotomized into low and high groups according to the cut-off values. The difference in variables between the training and validation cohorts was evaluated using the chi-square test. The chi-square test was also used to assess the association between AAPR and patient characteristics. We applied univariate and multivariate Cox models to assess the independent prognostic values for PFS and OS in ES-SCLC. Variables with p < 0.1 in univariate analyses were included in multivariate Cox analyses. Results are reported as hazard ratios (HR) with 95% confidence intervals (95% CIs). A two-sided p value < 0.05 was regarded as statistically significant.
The nomogram for PFS and OS of ES-SCLC was performed based on the results of multivariate survival analysis in the training cohort. The established nomogram was validated externally in the validation cohort using the “rms” package in R version 3.4.4. The nomogram performance was assessed using the Harrell’s concordance index (c-index) and calibration curve. The c-index was calculated by bootstrapping with 100 samples to rank the discrimination, and calibration was achieved by generating a plot of the predicted survival probabilities against the actuarial outcome. Then, we used the likelihood ratio (LR) and Akaike information criterion (AIC) to evaluate the discriminatory ability and veracity of the nomogram models for prognostic factors. Decision curve analyses (DCA) were performed to calculate the clinical net benefit across a range of threshold probabilities for the models using the package of “stdca” in R. All statistical analyses were accomplished using SPSS (version 24.0; IBM Corp., Armonk, NY, USA) and R version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria) and figures were generated using Graphpad Prism 7 (GraphPad Software Inc., La Jolla, CA, USA).
Results
Patient Characteristics
With a median follow-up of 11.8 months, 148 and 66 patients in the training and validation cohorts, respectively, had died. Patient characteristic are shown in Table 1. None of the examined characteristics in validation cohort significantly differed from those of the training cohort (p > 0.05). The majority of patients in our study were male with smoking history, and 31.5% of patients in the training cohort and 36% of patients in the validation cohort received additional thoracic radiotherapy.
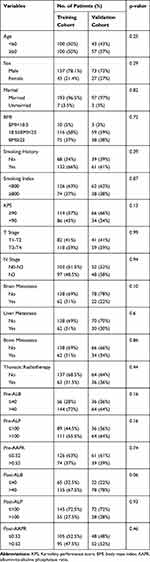 |
Table 1 Patients Characteristics in Training Cohort and Validation Cohort |
Survival-Related Prognostic Factors and Multivariate Analyses in Training Cohort
The median PFS of patients in the training cohort was 6.9 months (95% CI 6.4–7.4 months), and the PFS rates at 6 and 12 months were 59% and 13%, respectively. The median OS of patients in the training cohort was 13.6 months (95% CI 12.4–14.9 months), and the OS rates at 12 and 24 months were 48% and 17.5%, respectively. In terms of correlation of AAPR and patient characteristic (Table 2), significant positive association was found between high level of pre-AAPR and liver metastasis in training cohort.
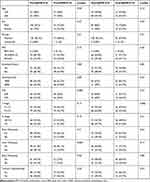 |
Table 2 Correlation Between AAPR and Patient Characteristics in Training Cohort |
Univariate analyses revealed that T3–T4 stage, N3 stage, liver metastasis, lack of thoracic radiotherapy, high post-ALP level, and low pre-AAPR and post-AAPR levels were significant indices of disease progression. Multivariate analyses, identified three of these factors as independent negative predictors: N3 stage (HR = 1.41, 95% CI 1.03–1.93, p = 0.034), without thoracic radiotherapy (HR = 0.4, 95% CI 0.29–0.56, p < 0.001), and low post-AAPR levels (HR = 0.67, 95% CI 0.49–0.92, p = 0.013) (Table 3).
 |
Table 3 Univariate and Multivariate Cox Models for PFS in Training Cohort |
Assessment of OS in the training cohort revealed that T3–T4 stage, N3 stage, liver metastasis, without thoracic radiotherapy, high pre-ALP and post-ALP levels, and low pre-ALB, pre-AAPR, and post-AAPR levels were predictive of inferior OS. T stage (HR = 1.6, 95% CI 1.13–2.28, p = 0.008), thoracic radiotherapy (HR = 0.45, 95% CI 0.32–0.66, p < 0.001), and high post-AAPR (HR = 0.62, 95% CI 0.44–0.86, p = 0.005) were identified as independent risk factors for death (Table 4).
 |
Table 4 Univariate and Multivariate Cox Models for OS in Training Cohort |
The independent PFS and OS risk factors were confirmed further using Kaplan–Meier analyses and were shown to have good prognostic capacity (Figures 1 and 2).
Prognostic Nomogram for PFS and OS
The prognostic nomogram for PFS and OS was established by the integration of independent significant factors in the training cohort (Figures 3A and 4A), with c-indices of 0.675 and 0.662, respectively. N-stage, thoracic radiotherapy, and post-AAPR were incorporated in the nomogram for PFS, and T-stage, thoracic radiotherapy and post-AAPR were incorporated in the nomogram for OS. The calibration plot revealed optimal consistency between the actual observation and the nomogram-predicted PFS and OS, for both the probability of PFS at 6 or 12 months (Figure 3B and C), and survival at 12 or 24 months (Figure 4B and C).
In the validation cohort, the median PFS was 6.9 months (95% CI 5.9–7.9 months) and median OS was 16 months (95% CI 13.8–18.1 months). The predictive accuracy of the established nomogram was further confirmed through external validation, with the c-indices of 0.697 and 0.664 for PFS and OS, respectively, in the validation cohort. PFS rates at 6 and 12 months were 64% and 12%, respectively, and survival rates at 12 and 24 months were 49% and 13%, respectively. The calibration plot illustrated the predictive probability of the established nomogram for PFS (Figure 3D and E) and OS (Figure 4D and E), with good agreement between observed and predicted values.
Comparison of Predictive Ability of the Nomogram and TNM Stage
To investigate the complementary role of dynamic AAPR in prognostic prediction, the prognostic abilities of TNM stage and the AAPR-TNM based nomogram were compared. DCA was performed to compare the potential clinical net benefit of the nomogram and TNM stage. Decision curves revealed that the nomogram had a greater net benefit for PFS and OS than did TNM stage (Figure 5). As shown in Table 5, the nomogram had significantly higher LRT χ2 and c-index values and significantly lower AIC values than the TNM staging system for PFS and OS, in both the training and validation cohorts (p < 0.05).
 |
Table 5 The Comparison of LRTχ2 and AIC for PFS and OS Between Nomogram and Prognostic Factors |
TNM stage was also shown to have an outstanding capacity for both PFS and OS prognosis in the training and validation cohorts. The nomogram c-index value was significantly higher than that of the TNM stage (0.539 for PFS and 0.567 for OS, p < 0.05) in the training cohort. The superiority of the prognostic ability of the nomogram was also demonstrated in the validation cohort with c-indices of 0.697 and 0.664 for PFS and OS, respectively, compared with 0.602 for PFS and 0.605 for OS for the TNM stage (p < 0.05) (Figure 6). The nomogram has significantly higher LRT χ2 and lower AIC values for PFS and OS than did the TNM stage in both the training and validation cohorts (Table 5).
Discussion
SCLC is known as a lung cancer subtype with characteristics of invasiveness, rapid progression, and poor prognosis. The application of targeted therapy and immunotherapy has limited advantages for patients with SCLC,27 and platinum-based chemotherapy for 4–6 cycles remains the first-line standard treatment. Therefore, it is essential to explore prognostic factors to identify high-risk patients with SCLC. AAPR, a novel accessible and non-invasive hematological prognostic factor, is already used as a prognostic predictor in patients with hepatocellular, nasopharyngeal, and pancreatic carcinoma. Here, for the first time, we demonstrate that dynamic AAPR can be used for prognosis prediction in patients with ES-SCLC. Moreover, we developed and validated dynamic AAPR-based nomograms to quantitatively estimate survival in patients with ES-SCLC.
Although we have demonstrated the prognostic value of AAPR in patients with SCLC, the mechanisms underlying the prognostic value of AAPR remains unknown. Low ALB concentration is a marker of malnutrition in patients with cancer.28 Additionally, during inflammation, ALB is a negative acute phase protein. ALB levels decrease dramatically in response to acute phase protein demands through a combination of reduced production, increased degradation, and increased loss because of the high permeability of vessels.29,30 Therefore, low ALB levels also reflect the presence of inflammation in patients with cancer. Malnutrition and systemic inflammation are associated with inferior prognosis in patients with cancer.31 Moreover, ALB is also involved in balancing cell proliferation and metabolism.32 ALP is a hydrolytic enzyme which can dephosphorylate multiple molecules including nucleotides.33 ALP functions to adjust purinergic signaling, which plays a role in the induction of inflammatory responses through ATP and ADP-mediated binding of nucleotide receptors.34 Additionally, inhibition of ALP activity reduces cancer cell viability and migration, and induces apoptosis.18 Therefore, AAPR is proposed to reflect systemic inflammation status and cell metabolism, and is associated with outcomes in patients with cancer. We also found that low pre-AAPR was associated with liver metastasis, because of the relationship between ALB, ALP, and liver function.
Low pre-AAPR could predict inferior PFS and OS in patients with ES-SCLC. This is consistent with results previously reported for patients with hepatocellular, nasopharyngeal, and pancreatic carcinoma.21,22,35 However, after multivariate analyses pre-AAPR lost the significance, and post-AAPR was an independent prognostic factor for PFS and OS. Tumor temporal heterogeneity means that either systemic or tumor status constantly changes during development and treatment. Our results show that low levels of post-AAPR, the indicator of inflammation, decreased nutritional status, and liver function during treatment, are valuable and informative in prognostic prediction. Therefore, a nomogram based on post-AAPR and incorporating other independent prognostic factors for PFS and OS was developed and compared with traditional TNM staging system.
Multivariate analyses revealed that patients with ES-SCLC receiving additional thoracic radiotherapy had superior PFS and OS with independent statistical significance. This is broadly in line with the results from randomized controlled trials36 and retrospective studies37 of thoracic radiotherapy in patients with ES-SCLC. Our results also show the important role of TNM stage for prognosis and both T and N stage were found to be associated with PFS and OS. Multivariate survival analyses revealed that N stage remained an independent prognostic factor for PFS and that T stage remained an independent prognostic factor for OS.
Here, for the first time, we included post-AAPR, rather than baseline AAPR, and established a dynamic AAPR-based nomogram for prognosis prediction in patients with ES-SCLC. We then confirmed that dynamic AAPR is both superior and complementary to the TNM staging system in prognosis prediction in these patients. The TNM staging system is based on the level of tumor burden and involves knowledge of the size or direct extent of the primary tumor, the spread to regional lymph nodes, and the presence of distant metastasis. Given the heterogeneity of the tumor micro-environment, patient characteristics, and treatments, there is a great deal of variation in outcomes among patients with same TNM stage. Increasing numbers of studies have demonstrated that information from distinct sources contribute to a more accurate prediction model.38 In our study, the post-AAPR-based nomogram integrated information about dynamic tumor and systemic status changes, treatment heterogeneity, and tumor burden. The superior performance of the nomogram compared to TNM stage was validated in the validation cohort. Therefore, we constructed a comprehensive and dynamic nomogram with the characteristic of non-invasion, and the potential role of the nomogram on clinical individual therapy was indicated by DCA results.
However, there are some limitations in our study. Firstly, this is a retrospective study from a single center which may cause selection bias, which we tried to minimize by establishing training and validation cohorts. Even if our study included information from distinct sources, other potential prognostic markers, which may have additional value, were not available for our nomogram. Therefore, this AAPR-based nomogram requires further validation and extension in a random, prospective study with a larger number of patients. Furthermore, the mechanisms underlying the prognostic role of dynamic AAPR remain to be further elucidated.
Conclusion
We have identified post-AAPR as a prognostic factor for patients with ES-SCLC, and recommend AAPR to be dynamically evaluated to guide patient treatment. Our post-AAPR nomogram accurately predicts individual survival probability and could be used as a tool for clinical decision-making.
Abbreviations
SCLC, small-cell lung cancer; PFS, progression-free survival; OS, overall survival; ALB, albumin; ALP, alkaline phosphatase; AAPR, albumin-to-alkaline phosphatase ratio; VALSG, Veterans Administration Lung Study Group; LS-SCLC, limited-stage SCLC; ES-SCLC, extensive-stage SCLC; BMI, body mass index; KPS, Karnofsky performance score; RECIST, Response Evaluation Criteria in Solid Tumors; ROC, receiver operating characteristic curve; HR, hazard ratios; CI, confidence intervals; C-index, concordance index; LR, likelihood ratio; AIC, Akaike information criterion; DCA, decision curve analyses.
Data Sharing Statement
The data in this study are available from the corresponding author upon reasonable request.
Ethical Approval and Informed Consent
This study was approved by the Ethics Committee of Shandong Cancer Hospital and Institute (No. SDTHEC201808005).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359(13):1367–1380. doi:10.1056/NEJMra0802714
2. van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet (London, England). 2011;378(9804):1741–1755. doi:10.1016/S0140-6736(11)60165-7
3. Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet (London, England). 2017;389(10066):299–311. doi:10.1016/S0140-6736(16)30958-8
4. Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018;52(Pt 1):103–109. doi:10.1016/j.semcancer.2017.11.019
5. Saito M, Shiraishi K, Goto A, Suzuki H, Kohno T, Kono K. Development of targeted therapy and immunotherapy for treatment of small cell lung cancer. Jpn J Clin Oncol. 2018;48(7):603–608. doi:10.1093/jjco/hyy068
6. Horn L, Mansfield AS, Szczesna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi:10.1056/NEJMoa1809064
7. Lara PN
8. Chan BA, Coward JI. Chemotherapy advances in small-cell lung cancer. J Thorac Dis. 2013;5(Suppl 5):S565–S578. doi:10.3978/j.issn.2072-1439.2013.07.43
9. Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4(4):221–233. doi:10.1158/1541-7786.MCR-05-0261
10. Don BR, Kaysen G. Poor nutritional status and inflammation: serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17(6):432–437. doi:10.1111/j.0894-0959.2004.17603.x
11. Boonpipattanapong T, Chewatanakornkul S. Preoperative carcinoembryonic antigen and albumin in predicting survival in patients with colon and rectal carcinomas. J Clin Gastroenterol. 2006;40(7):592–595. doi:10.1097/00004836-200608000-00006
12. Hsu S-N, Hsu Y-J, Lin C, Su S-L, Lin S-H. Proteinuria: associated with poor outcome in patients with small cell lung cancer. J Cancer Res Ther. 2018;14(10):688–693. doi:10.4103/0973-1482.191037
13. Lis CG, Grutsch JF, Vashi PG, Lammersfeld CA. Is serum albumin an independent predictor of survival in patients with breast cancer? JPEN J Parenter Enteral Nutr. 2003;27(1):10–15. doi:10.1177/014860710302700110
14. Onate-Ocana LF, Aiello-Crocifoglio V, Gallardo-Rincon D, et al. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol. 2007;14(2):381–389. doi:10.1245/s10434-006-9093-x
15. Rupp C, Stiehl A, Trauner M, Gotthardt DN. Editorial: further evidence for the role of serum alkaline phosphatase as a useful surrogate marker of prognosis in PSC - authors’ reply. Aliment Pharmacol Ther. 2015;41(1):151–152. doi:10.1111/apt.13020
16. Damera S, Raphael KL, Baird BC, Cheung AK, Greene T, Beddhu S. Serum alkaline phosphatase levels associate with elevated serum C-reactive protein in chronic kidney disease. Kidney Int. 2011;79(2):228–233. doi:10.1038/ki.2010.356
17. Lim SM, Kim YN, Park KH, et al. Bone alkaline phosphatase as a surrogate marker of bone metastasis in gastric cancer patients. BMC Cancer. 2016;16:385. doi:10.1186/s12885-016-2415-x
18. Rao SR, Snaith AE, Marino D, et al. Tumour-derived alkaline phosphatase regulates tumour growth, epithelial plasticity and disease-free survival in metastatic prostate cancer. Br J Cancer. 2017;116(2):227–236. doi:10.1038/bjc.2016.402
19. Rader BA. Alkaline phosphatase, an unconventional immune protein. Front Immunol. 2017;8:897. doi:10.3389/fimmu.2017.00897
20. Kuroda D, Sawayama H, Kurashige J, et al. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. 2018;21(2):204–212. doi:10.1007/s10120-017-0744-3
21. Cai X, Chen Z, Chen J, et al. Albumin-to-alkaline phosphatase ratio as an independent prognostic factor for overall survival of advanced hepatocellular carcinoma patients without receiving standard anti-cancer therapies. J Cancer. 2018;9(1):189–197. doi:10.7150/jca.21799
22. Nie M, Sun P, Chen C, et al. Albumin-to-alkaline phosphatase ratio: a novel prognostic index of overall survival in cisplatin-based chemotherapy-treated patients with metastatic nasopharyngeal carcinoma. J Cancer. 2017;8(5):809–815. doi:10.7150/jca.17536
23. Jhun BW, Lee KJ, Jeon K, et al. Clinical applicability of staging small cell lung cancer according to the seventh edition of the TNM staging system. Lung Cancer. 2013;81(1):65–70. doi:10.1016/j.lungcan.2013.03.005
24. Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. 2015;33(8):861–869. doi:10.1200/JCO.2014.56.6661
25. Li S, Zhao J, Zhu L, Su F, Chen K. Development and validation of a nomogram predicting the overall survival of stage IV breast cancer patients. Cancer Med. 2017;6(11):2586–2594. doi:10.1002/cam4.1224
26. Necchi A, Sonpavde G, Lo Vullo S, et al. Nomogram-based prediction of overall survival in patients with metastatic urothelial carcinoma receiving first-line platinum-based chemotherapy: retrospective international study of invasive/advanced cancer of the urothelium (RISC). Eur Urol. 2017;71(2):281–289. doi:10.1016/j.eururo.2016.09.042
27. Ferrara R, Mezquita L, Besse B. Progress in the management of advanced thoracic malignancies in 2017. J Thorac Dis. 2018;13(3):301–322. doi:10.1016/j.jtho.2018.01.002
28. McIntosh EN, Laurent LL. Nutritional assessment of the hospitalized patient. Am Fam Physician. 1983;27(1):169–175.
29. Ishizuka M, Nagata H, Takagi K, Horie T, Kubota K. Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. Ann Surg. 2007;246(6):1047–1051. doi:10.1097/SLA.0b013e3181454171
30. de Mutsert R, Grootendorst DC, Indemans F, Boeschoten EW, Krediet RT, Dekker FW. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J Renal Nutr. 2009;19(2):127–135. doi:10.1053/j.jrn.2008.08.003
31. Lamb GWA, Aitchison M, Ramsey S, Housley SL, Mcmillan DC. Clinical utility of the Glasgow Prognostic Score in patients undergoing curative nephrectomy for renal clear cell cancer: basis of new prognostic scoring systems. Br J Cancer. 2012;106(2):279–283. doi:10.1038/bjc.2011.556
32. Nojiri S, Joh T. Albumin suppresses human hepatocellular carcinoma proliferation and the cell cycle. Int J Mol Sci. 2014;15(3):5163–5174. doi:10.3390/ijms15035163
33. Cauwels A, Rogge E, Vandendriessche B, Shiva S, Brouckaert P. Extracellular ATP drives systemic inflammation, tissue damage and mortality. Cell Death Dis. 2014;5:e1102. doi:10.1038/cddis.2014.70
34. Peters E, Geraci S, Heemskerk S, et al. Alkaline phosphatase protects against renal inflammation through dephosphorylation of lipopolysaccharide and adenosine triphosphate. Br J Pharmacol. 2015;172(20):4932–4945. doi:10.1111/bph.13261
35. Pu N, Gao S, Xu Y, et al. Alkaline phosphatase-to-albumin ratio as a prognostic indicator in pancreatic ductal adenocarcinoma after curative resection. J Cancer. 2017;8(16):3362–3370. doi:10.7150/jca.20917
36. Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a Phase 3 randomised controlled trial. Lancet. 2015;385(9962):36–42. doi:10.1016/S0140-6736(14)61085-0
37. Li-Ming X, Zhao LJ, Simone CB
38. Wang S, Yang L, Ci B, et al. Development and validation of a nomogram prognostic model for SCLC patients. J Thorac Dis. 2018;13(9):1338–1348. doi:10.1016/j.jtho.2018.05.037
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






