Back to Journals » OncoTargets and Therapy » Volume 13
Prognostic Value and Molecular Regulatory Mechanism of MSTO2P in Hepatocellular Carcinoma: A Comprehensive Study Based on Bioinformatics, Clinical Analysis and in vitro Validation
Authors Yan L, Yue C , Xu Y, Jiang X, Zhang L, Wu J
Received 12 January 2020
Accepted for publication 10 March 2020
Published 27 March 2020 Volume 2020:13 Pages 2583—2598
DOI https://doi.org/10.2147/OTT.S245741
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Lijun Yan,1,* Chaosen Yue,2,* Yingchen Xu,1 Xinceng Jiang,1 Lijun Zhang,1 Jixiang Wu1
1Department of General Surgery, Beijing Tongren Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Breast Center, Beijing Tongren Hospital, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jixiang Wu; Lijun Zhang
Department of General Surgery, Beijing Tongren Hospital, Capital Medical University, No. 1 Dongjiaominxiang, Dongcheng District, Beijing 100730, People’s Republic of China
Email [email protected]; [email protected]
Background: Accumulating studies have shown that pseudogenes could become key regulators in human cancers. Misato family member 2, pseudogene (MSTO2P) is overexpressed in lung and gastric cancer and affects the biological functions of tumor cells. However, the role of MSTO2P in hepatocellular carcinoma (HCC) is unreported.
Purpose: This study aimed to examine the diagnostic and prognostic value of MSTO2P in HCC, to investigate the effects of MSTO2P on the biological functions of HCC cells, and to explore the potential mechanisms of MSTO2P in HCC.
Methods: Relevant data on HCC were downloaded from the Gene Expression Omnibus database and the Cancer Genome Atlas database and used to analyze MSTO2P expression and the role of MSTO2P in HCC prognosis. MSTO2P in HCC cell lines was knocked down by shRNA to study the effects of MSTO2P on cell proliferation, apoptosis, metastasis and invasion in HCC. Expressions of the main proteins involved in epithelial–mesenchymal transition and the PI3K/AKT/mTOR signaling pathway in HCC were examined via Western blot analysis.
Results: MSTO2P had significant diagnostic and prognostic value in HCC. MSTO2P was highly expressed in HCC tissues and cells, and MSTO2P increased HCC cell proliferation, invasion and metastasis. MSTO2P knockdown also increased E-cadherin expression and decreased N-cadherin and Vimentin expression. Additionally, MSTO2P increased the expressions of proteins in the PI3K/AKT/mTOR pathway, including PI3K, p-AKT and p-mTOR.
Conclusion: MSTO2P might be used as a potential target for diagnosing and curing HCC. MSTO2P may affect HCC cell proliferation, apoptosis, metastasis and invasion through the PI3K/AKT/mTOR pathway.
Keywords: MSTO2P, hepatocellular carcinoma, epithelial–mesenchymal transition
Introduction
Hepatocellular carcinoma (HCC) has high morbidity and mortality. HCC is reported to be the fifth most common cancer in men and the ninth most common cancer in women.1 The incidence of liver cancer is related to many factors, such as hepatitis B, hepatitis C, alcoholic hepatitis, and steatohepatitis.2–4 According to the Barcelona Clinic Liver Cancer (BCLC)5 staging, liver cancer treatments include surgical resection, radiofrequency ablation, arterial chemoembolization, and liver transplantation. Although great progress has been made in diagnosing and treating liver cancer, its recurrence remains high; thus, tumor markers are still needed for early diagnosis and prognosis of liver cancer.
Pseudogenes were first reported in 19776 and were not initially considered to have transcriptional activity or protein coding capacity. In 2012, Kalyana-Sundaram et al detected 2082 pseudogene transcripts in 293 tissue samples, of which, 218 were expressed in cancer samples.7 In 2014, Han et al suggested that pseudogenes could be used as biomarkers for cancer and to determine disease prognosis.8 In addition to their traditional roles as antisense RNA,9 endo-siRNA or esiRNA,10–13 pseudogenes can serve as endogenous competitors for miRNA, RNA-binding proteins19 and translation machinery.14–18
As a pseudogene, misato family member 2, pseudogene (MSTO2P) is highly expressed in gastric cancer20 and lung cancer21 and affects the biological characteristics of these cancers. However, whether MSTO2P influences HCC occurrence and development remains unclear. In our study, we first determined the prognostic value and the molecular regulatory mechanism of pseudogene MSTO2P in HCC based on bioinformatics, clinical analysis and in vitro validation. We found that MSTO2P was related to the biological functions of liver cancer and affected the prognosis of HCC patients.
Materials and Methods
Analysis of MSTO2P Expression in HCC Based on the Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) Database
To verify whether MSTO2P is overexpressed in HCC patients, 424 samples containing MSTO2P gene expression data were studied based on the TCGA database (https://cancergenome.nih.gov/), of which, 374 were HCC patients, and 50 were non-HCC patients. Next, 10 microarrays were searched in the GEO database (https://www.ncbi.nlm.nih.gov/geo/) based on the GPL570 platform, and each microarray included MSTO2P gene expression data from normal liver tissue and HCC tissue. All data were analyzed using SPSS 22.0 software (IBM, USA); p<0.05 was considered significant. Stata 15.0 software (StataCorp LLC., USA) was used for the meta-analysis of the TCGA and GEO data. The diagnostic value of MSTO2P in HCC was evaluated via a receiver operating characteristic (ROC) curve.
Prognostic Value of MSTO2P in HCC Based on Clinical Data
Clinical information on HCC patients was downloaded from the TCGA database and used to investigate the effect of MSTO2P on the prognosis of HCC patients. Each sample included survival time, survival status, age, gender, pathological grade, clinical stage and MSTO2P gene expression data. RMS, foreign and survival packages in R software were used to establish a nomogram prediction model based on the multivariate Cox regression analysis, and the model was evaluated via the calibration curve.
Gene Ontology Function and Kyoto Encyclopedia of Genes and Genomes Pathway Analysis
To understand how MSTO2P affects tumorigenesis and HCC development, we selected MSTO2P-associated genes in humans via the Multi Experiment Matrix (MEM) database (https://biit.cs.ut.ee/mem/) and performed Gene Ontology (GO) functional annotation and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment through DAVID 6.8 (https://david-d.ncifcrf.gov/). The results were visualized using the ggplot2 and GOplot packages in R software.
Tissue Samples and Cell Cultures
This study was approved by the Ethics Committee of Beijing Tongren Hospital. All HCC and adjacent normal liver tissues used in the study were collected at Beijing Tongren Hospital affiliated to Capital Medical University. No patients underwent other treatments prior to surgery. Written informed consent, which was in accordance with the Declaration of Helsinki, was obtained from each patient. The normal liver cell line, L02, and the HCC cell lines, HepG2, Huh-7, Hep3B, SMMC7721, QSG7701, and BEL7402, were purchased from the Cancer Hospital Chinese Academy of Medical Sciences. Dulbecco’s modified Eagle’s medium, RPMI-1640 cell culture media, fetal bovine serum, trypsin, and 100X penicillin-streptomycin solution were purchased from Gibco (Waltham, MA, USA). The cells were cultured in an incubator at 37°C with 5% CO2.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from the tissues and the L02, HepG2, Huh-7, Hep3B, SMMC7721, QSG7701, and BEL7402 cells using TRIzol reagent (Vazyme, Nanjing, China). The GoScript™ cDNA Synthesis Kit (Promega, WI, USA) was used to synthesize the cDNA. All steps were performed per the manufacturer’s protocol. The primers were as follows: GAPDH forward 5′-TCATTGACCTCAACTACATGG-3′ and reverse 5′-TCGCTCCTGGAAGATGGTG-3′ and MSTO2P forward 5′-CAGGGCGGGGAATAATAACT-3′ and reverse 5′-GGAACACAGGCGATAAGGAA-3′. We used the 2−ΔΔCT method to determine the relative expressions of MSTO2P in the tissues and cell lines.
Cell Transfection
To investigate the knockdown effect of MSTO2P in vitro, the MSTO2PshRNA (#1, #2, #3) and MSTO2PshRNANC plasmids were introduced into HepG2 and BEL7402 cells, respectively. The transfected cells were cultured at 37°C in a 5% CO2 incubator. qPCR was used to detect MSTO2P and the internal reference, GAPDH, in transfected cells 48 hrs after transfection. All experiments were performed in triplicate per the experimental procedures.
Effects of Silencing MSTO2P on Cell Function
The MSTO2PshRNA plasmid was introduced into 293T cells to generate a high titer of the lentivirus containing MSTO2P shRNA. Twenty microliters of virus and polybrene (Sigma, St. Louis, MO, USA) at a final concentration of 4 µg/mL was added to a cell culture dish. Cells were incubated for 48 hrs at 37°C. The complete medium of 1 µg/mL puromycin (Sigma) was used for resistance screening. After 3–4 generations, the collected cells were used to detect the levels of apoptosis, cell cycles, cell clone formation, migration ability and cell invasion ability. Each experiment was performed in triplicate.
Effects of Silencing MSTO2P on Apoptosis (Apoptosis Analysis)
After centrifugation, the cell culture medium was mixed with propidium iodide (PI; Cwbiotech, Beijing, China) and Annexin V-FITC staining solution (Cwbiotech), then incubated at room temperature for 20 mins. Aluminum foil (Miaojie, Shanghai, China) was used to prevent exposure to light. Flow cytometry (BD Biosciences, San Jose, CA, USA) analysis showed Annexin V-FITC staining as green fluorescence and PI staining as red fluorescence.
Effects of MSTO2P Silencing on the Cell Cycle (Cell Cycle Analysis)
PI staining solution (0.5 mL) was added to each tube, the cell precipitates were slowly and fully suspended, and the cell suspensions were incubated at 37°C for 30 mins in a dark, warm bath. Flow cytometry was used to detect red fluorescence at the excitation wavelength of 488 nm; light scattering was detected simultaneously. Flow cytometry analysis was completed within 24 hrs after staining.
Effect of MSTO2P Silencing on Cell Proliferation Ability (Colony-Formation Assay)
The cells were blown into a single-cell suspension with the corresponding medium, then inoculated into a 6-well plate. The cells were cultured for 7 days in an incubator at 37°C. The medium was removed, and 1 mL of crystal violet staining solution (Solarbio, Beijing, China) was added to each hole. The crystal violet was then removed, and images were captured.
Effects of MSTO2P Silencing on Cell Migration (Wound-Healing Assay)
A straight line was drawn along the center of the medium with a 200-µL pipette tip. Phosphate-buffered saline (Solarbio) was used to remove loose cells, which were added to the culture medium. Photos were taken to assess differences in cell migration between the shRNA and scramble groups.
Effect of MSTO2P Silencing on Cell Invasion Ability (Transwell Assay)
Matrigel (25 μL; BD Biosciences) and 200 μL of the cell suspensions were added into the upper chambers of Transwell plates (Corning, NY, USA). Complete culture medium (700 μL; Gibco) was added into the subchambers of the Transwell plates. The cells were incubated for 24 hrs, then stained with crystal violet solution for 30 mins. Images were captured to evaluate the cell invasion ability.
Western Blotting
RIPA buffer cell lysate (100 μL; Cwbiotech) and an ultrasonic fragmentation apparatus (Scientz, Ningbo, China) were used to lyse cells. A bicinchoninic acid (BCA) kit (Cwbiotech) was used to determine the protein concentration. A 10% SDS-PAGE gel (Cwbiotech) and 100-V stable electrophoresis (Biorad, CA, USA) were configured based on the molecular weight of the detected target protein. The electrophoresis stopped when the loading-buffer dye reached the bottom of the gel, then the membrane was wet-transferred. The cells were sealed with 5% skim milk powder (TBST) for 1 hr. When the primary antibody incubation was complete, the membrane was washed with TBST. The secondary antibody was diluted with TBST (Cwbiotech) sealant and incubated for 1 hr.
Statistical Analysis
All experiments were repeated three times, and the experimental data are expressed as the mean ± SD. SPSS 22.0 software (IBM, USA), Stata 15.0 software (StataCorp LLC., USA) and GraphPad Prism 8 software (GraphPad Software Inc., USA) were used for statistical analysis. One-way analysis of variance or an unpaired Student’s t-test were used for between-group comparisons. Differences with P<0.05 were considered statistically significant.
Results
MSTO2P Was Highly Expressed in HCC Tissues Based on the TCGA and GEO Databases
Ten GEO datasets (GSE29721, GSE45267, GSE45436, GSE55092, GSE62232, GSE6764, GSE84402, GSE102097, GSE107170, and GSE121248) with MSTO2P expression data in the GEO database were collected. Table 1 lists the details for the 10 GEO datasets and TCGA data. MSTO2P was highly expressed in GSE29721, GSE45267, GSE45436, GSE55092, GSE62232, GSE84402, GSE102097, GSE107170, and GSE121248 and the TCGA database (Figure 1A–E, G–K). There was no significant difference in GSE6764 (Figure 1F). A meta-analysis was used to integrate the data downloaded from the GEO and TCGA databases. The standard mean difference (SMD) of MSTO2P expression was 1.80 (95% CI: 1.26–2.33; I2=92.7%; P<0.001) by the random-effects model (Figure 2A and B). MSTO2P was overexpressed in HCC tissues compared with normal liver tissues. The ROC curve suggested that MSTO2P had good diagnostic value for HCC (Figure 3A–K).
 |
Table 1 GEO and TCGA Data |
MSTO2P Was Related to HCC Patient Prognoses Based on Clinical Data Downloaded from TCGA
We analyzed the clinical data of 344 HCC patients from TCGA. In the nomogram prognostic model based on multifactor analysis, patients with high MSTO2P expression had higher scores and worse 3-year and 5-year survival rates compared with patients with low MSTO2P expression (Figure 4A). Calibration curves were used to verify the model; no deviations from the reference line indicated that the model was highly credible (Figure 4B and C). Our results suggested that MSTO2P had significant prognostic value for HCC patients.
GO and KEGG Analysis
GO items were classified into three major groups: biological processes (BP), cellular components (CC), and molecular functions (MF). Significantly enriched GO terms, including cell cycle, M phase, cellular response to stress, DNA replication, DNA repair, damaged DNA binding, RNA splicing, RNA modification, RNA polymerase activity, methylation, and 3ʹ–5ʹ-exoribonuclease activity, were discovered and indicated that MSTO2P might be related to HCC oncogenesis (Figure 5A–C). Table 2 shows the KEGG analysis results.
 |
Table 2 KEGG Analysis of MSTO2P-Related Genes in HCC |
MSTO2P Was Upregulated in HCC Tissues and Cell Lines
RT-PCR analysis was used to detect MSTO2P expression in 20 pairs of HCC and adjacent normal liver tissues. The results indicated that MSTO2P was significantly overexpressed in HCC tissues compared with that in adjacent liver tissues (Figure 6A). PCR was used to detect MSTO2P expression in L02, HepG2, Huh-7, Hep3B, SMMC7721, QSG7701, and BEL7402 HCC cells. Compared with normal liver cells, MSTO2P expression was significantly higher in the HCC cell lines (P<0.05; Figure 6B). The HepG2 and BEL7402 cells had the highest MSTO2P expressions and were thus selected to study the influence of MSTO2P silencing on the biological functions of HCC cells.
shRNA with the Best Silencing Effect Was Selected
shRNA1, shRNA2 and shRNA3 were transfected into HepG2 and BEL7402 cells. MSTO2P expression was detected via PCR, and the shRNA2 with the best silencing effect was used in subsequent experiments (Figure 6C).
Silencing MSTO2P Inhibited HCC Cell Proliferation, Increased HCC Cell Apoptosis and Affected the HCC Cell Cycle
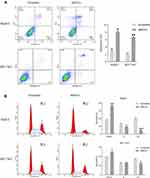
Knocking down MSTO2P in HepG2 and BEL7402 cells showed that cell proliferation was significantly reduced compared with that of the control group (Figure 6D). The results showed that MSTO2P knockdown suppressed HCC cell generation. Flow cytometry was used to detect the effects of MSTO2P knockdown on HCC cell apoptosis and cell cycles. HCC cell apoptosis was significantly increased after silencing MSTO2P compared with that of the control group (Figure 7A). HCC cells in the G0/G1 phase were increased after MSTO2P knockdown compared with those of the control group (Figure 7B).
Silencing MSTO2P Significantly Inhibited HCC Cell Invasion and Migration
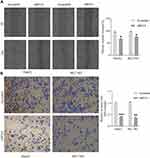
The effects of MSTO2P knockdown on HCC cell invasion and migration were detected via wound-healing and Transwell assays. HCC cell invasion and migration were significantly impeded after knocking down MSTO2P (Figure 8A and B).
MSTO2P Might Affect HCC Cell Functions Through the PI3K/AKT/mTOR Pathway
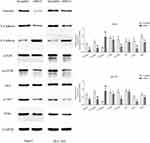
We used Western blotting to detect the epithelial–mesenchymal transition (EMT) markers, E-cadherin, N-cadherin and Vimentin. Compared with the control group, MSTO2P knockdown yielded increased E-cadherin expression and decreased N-cadherin and Vimentin expressions. To investigate how MSTO2P affected HCC cell functions, we used Western blotting to detect the expressions of major proteins in the PI3K/AKT/mTOR signaling pathway. Silencing MSTO2P decreased the PI3K, p-AKT and p-mTOR protein expressions, but the AKT and mTOR protein expressions were not significantly changed (Figure 9). This suggested that MSTO2P might affect HCC cell functions through the PI3K/AKT/mTOR pathway.
Discussion
HCC is a lethal tumor with high incidence that causes great harm in China. HCC is difficult to treat because of its insidious onset and rapid invasive growth. The etiology and exact molecular mechanisms of HCC are unclear, and the HCC pathogenesis is a complex process with multiple factors and steps. Studies have shown that HCC is caused by mutations and an abnormal accumulation of multiple genes affected by multiple environmental and genetic factors, including genomic instability, abnormal cell signaling pathways, abnormal cell cycles, apoptosis, abnormal regulation of aging, and tumor angiogenesis. Therefore, the HCC pathogenesis must be clarified, and biomarkers for diagnosing and treating HCC must be determined.
Pseudogenes have been called “junk genes” because they have lost the ability to code proteins. Over the years, many studies have been conducted on pseudogenes. Pseudogenes are indicated to play broad and multifaceted roles in human cancer. MSTO2P, as a pseudogene, is highly expressed in both lung and gastric cancer and affects the biological functions of lung cancer and gastric cancer cells. Here, we used 10 GEO datasets and TCGA data to analyze MSTO2P expression in liver cancer, then meta-analysis was used to integrate the results. MSTO2P expression in the liver cancer tissues was significantly higher than that in the normal tissues. We also performed ROC curve and nomogram prediction model analysis based on clinical data from 344 HCC patients downloaded from TCGA. The results revealed that MSTO2P had good diagnostic and prognostic benefits. Subsequently, we examined MSTO2P expression in HCC tissues and cell lines and found that MSTO2P was highly expressed in both HCC tissues and cell lines. We performed GO functional annotation analysis using DAVID 6.8 and discovered that pseudogene MSTO2P might influence the pathogenic processes of HCC, including the cell cycle, M phase, cellular response to stress, DNA replication, DNA repair, damaged DNA binding, and RNA splicing. Next, we selected two cell lines with high expression levels to explore the biological effects of MSTO2P on HCC cell functions. The regulatory mechanism of MSTO2P in HCC cells in vitro was also examined. Our results demonstrated that MSTO2P knockout significantly inhibited HCC cell proliferation, metastasis and invasion and increased HCC cell apoptosis, revealing pseudogene MSTO2P can exert a tumorigenesis-promoting effect on HCC.
EMT is associated with the development of some chronic diseases and invasion and metastasis of epithelial-derived tumors.22,23 We examined the EMT markers, E-cadherin, N-cadherin, and Vimentin. The results suggested that E-cadherin was increased, and N-cadherin and Vimentin were decreased after MSTO2P knockdown. We think that MSTO2P promotes EMT in HCC. The PI3K/AKT/mTOR pathway is triggered in several cancers and plays a critical role in regulating physiological processes such as cell proliferation, the cell cycle, apoptosis, metabolism and migration.24 Previous studies have shown that the PI3K/AKT signaling pathway affects cancer metastasis and invasion by affecting the EMT of tumor cells.25,26 The PI3K/AKT pathway also induces EMT by interacting with other signaling pathways such as TGF-b,27 NF-kB,28 and Wnt/β-catenin.29 In our study, we carried out KEGG pathway analysis on the target genes of MSTO2P but found no clear pathway associated with HCC. Previous studies have indicated that the PI3K/AKT pathway can play an important role in the cell cycle and the EMT process in cancers, and our results showed that MSTO2P affected the cell cycle and the EMT of HCC. Thus, we evaluated whether MSTO2P could affect the EMT and other physiological processes of HCC through the PI3K/AKT pathway, and we detected the proteins involved in the PI3K/AKT pathway. PI3K, p-AKT and p-mTOR expressions were significantly reduced after MSTO2P knockout, indicating that MSTO2P might affect the biological functions of HCC cells through the PI3K/AKT/mTOR pathway. Additional experiments are needed to verify this.
Our research had some limitations. Our study on the prognostic value of MSTO2P for HCC was based on a retrospective analysis, and a prospective cohort is needed to confirm our results. Further experiments including vivo animal models are also needed to elucidate the biological mechanism of MSTO2P in HCC.
Conclusions
This study was the first to elaborate the diagnostic value, prognostic value and molecular regulatory mechanism of MSTO2P in HCC cells. MSTO2P was significantly overexpressed in HCC tissues and cells and highly correlated with HCC patient prognoses. In addition, our experiment confirmed that MSTO2P affected the EMT of liver cancer cells and may affect HCC cell proliferation, apoptosis, metastasis and invasion through the PI3K/AKT/mTOR pathway. These results suggest that MSTO2P may be a therapeutic target with good diagnostic and prognostic value for HCC tissues and cells.
Acknowledgments
This research was funded by Beijing Hospitals Authority Youth Programme (code: QML20180203), the Priming Scientific Research Foundation for the Junior Researcher in Beijing Tongren Hospital, Capital Medical University (code: 2018-YJJ-ZZL-043), the Beijing Natural Science Foundation (code: 7194248) and Beijing Dongcheng District Excellent Talent Training Subsidy Project-Youth Backbone Individual Program.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi:10.1002/ijc.29210
2. Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129–1133. doi:10.1016/S0140-6736(81)90585-7
3. Koike K, Tsutsumi T, Fujie H, et al. Molecular mechanism of viral hepatocarcinogenesis. Oncology. 2002;62(Suppl. 1):29–37. doi:10.1159/000048273
4. Beasley RP. Hepatitis B virus as the etiologic agent in hepatocellular carcinoma. HEPATOLOGY. 1982;2(Suppl):21S–26S.
5. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi:10.1002/hep.24199
6. Jacq C, Miller JR, Brownlee GG. A pseudogene structure in 5S DNA of Xenopus laevis. Cell. 1977;12(1):109–120. doi:10.1016/0092-8674(77)90189-1
7. Kalyana-Sundaram S, Kumar-Sinha C, Shankar S, et al. Expressed pseudogenes in the transcriptional landscape of human cancers. Cell. 2012;149(7):7. doi:10.1016/j.cell.2012.04.041
8. Han L, Yuan Y, Zheng S, et al. The pan-cancer analysis of pseudogene expression reveals biologically and clinically relevant tumour subtypes. Nat Commun. 2014;5(1):
9. Korneev SA, Park JH, O’Shea M. Neuronal expression of neural nitric oxide synthase (nNOS) protein is suppressed by an antisense RNA transcribed from an NOS pseudogene. J Neurosci. 1999;19(18):7711. doi:10.1523/JNEUROSCI.19-18-07711.1999
10. Okamura K, Lai EC. Endogenous small interfering RNAs in animals. Nat Rev Mol Cell Biol. 2008;9(9):673–678. doi:10.1038/nrm2479
11. Watanabe T, Totoki Y, Toyoda A, et al. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. NATURE. 2008;453(7194):539–543. doi:10.1038/nature06908
12. Tam OH, Aravin AA, Stein P, et al. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. NATURE. 2008;453(7194):534–538. doi:10.1038/nature06904
13. Chan WL, Yuo CY, Yang WK, et al. Transcribed pseudogene ψPPM1K generates endogenous siRNA to suppress oncogenic cell growth in hepatocellular carcinoma. Nucleic Acids Res. 2013;41:3734–3747.
14. Tay Y, Kats L, Salmena L, et al. Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell. 2011;147(2):0–357. doi:10.1016/j.cell.2011.09.029
15. Kumar MS, Armenteros-Monterroso E, East P, et al. HMGA2 functions as a competing endogenous RNA to promote lung cancer progression. Nature. 2013;505(7482):212–217. doi:10.1038/nature12785
16. Karreth FA, Tay Y, Perna D, et al. In vivo identification of tumor- suppressive PTEN ceRNAs in an oncogenic BRAF-induced mouse model of melanoma. Cell. 2011;147(2):382–395. doi:10.1016/j.cell.2011.09.032
17. Sumazin P, Yang X, Chiu HS, et al. An extensive MicroRNA-mediated network of RNA-RNA interactions regulates established oncogenic pathways in glioblastoma. Cell. 2011;147(2):0–381. doi:10.1016/j.cell.2011.09.041
18. Wang L, Guo Z-Y, Zhang R, et al. Pseudogene OCT4-pg4 functions as a natural micro RNA sponge to regulate OCT4 expression by competing for miR-145 in hepatocellular carcinoma. Carcinogenesis. 2013;34(8):1773–1781. doi:10.1093/carcin/bgt139
19. Han YJ, Ma SF, Yourek G, et al. A transcribed pseudogene of MYLK promotes cell proliferation. FASEB J. 2011;25(7):2305–2312. doi:10.1096/fsb2.v25.7
20. Li H, Zhu H, Zhou Y, et al. Long non-coding RNA MSTO2P promotes the proliferation and colony formation in gastric cancer by indirectly regulating miR-335 expression. Tumour Biol. 2017;39(6):101042831770550.
21. Wang LJ, Sun GZ, Chen YF. LncRNA MSTO2P promotes proliferation and autophagy of lung cancer cells by up-regulating EZH2 expression. Eur Rev Med Pharmacol Sci. 2019;23(8):3375–3382. doi:10.26355/eurrev_201904_17701
22. Talbot LJ, Bhattacharya SD, Kuo PC. Epithelial-mesenchymal transition, the tumor microenvironment, and metastatic behavior of epithelial malignancies. Int J Biochem Mol Biol. 2012;3(2):117–136.
23. Lee SJ, Kim KH, Park KK. Mechanisms of fibrogenesis in liver cirrhosis: the molecular aspects of epithelial-mesenchymal transition. World J Hepatol. 2014;6(4):207–216. doi:10.4254/wjh.v6.i4.207
24. Lunardi A, Webster KA, Papa A, et al. Role of aberrant PI3K pathway activation in gallbladder tumorigenesis. Oncotarget. 2014;5(4):894–900. doi:10.18632/oncotarget.v5i4
25. Bakin AV, Tomlinson AK, Bhowmick NA, et al. Phosphatidylinositol-3 kinase function is required for TGFβ-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem. 2000. doi:10.1074/jbc.M005912200
26. Xu Q, Ma J, Lei J, et al. Mangostin suppresses the viability and epithelial-mesenchymal transition of pancreatic cancer cells by downregulating the PI3K/Akt pathway. Biomed Res Int. 2014;2014:1–12.
27. Hofmann AF. In memoriam: Dr. Herbert Falk (1924–2008). Hepatology. 2010;49(1):1–3. doi:10.1002/hep.22746
28. Julien S, Puig I, Caretti E, et al. Activation of NF-κB by Akt upregulates snail expression and induces epithelium mesenchyme transition. Oncogene. 2007;26(53):7445–7456. doi:10.1038/sj.onc.1210546
29. Savagner P. The epithelial-mesenchymal transition (EMT) phenomenon. Ann Oncol. 2010;21(Supplement7):vii89–vii92. doi:10.1093/annonc/mdq292
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.