Back to Journals » Journal of Inflammation Research » Volume 14
Prognostic Significance of Tumor-Associated Macrophages in Chondroblastoma and Their Association with Response to Adjuvant Radiotherapy
Authors Zheng BW, Yang ML, Huang W, Zheng BY, Zhang TL, Li J, Lv GH, Yan YG, Zou MX
Received 27 February 2021
Accepted for publication 22 April 2021
Published 17 May 2021 Volume 2021:14 Pages 1991—2005
DOI https://doi.org/10.2147/JIR.S308707
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Monika Sharma
Bo-Wen Zheng,1,2 Min-Liang Yang,2 Wei Huang,3 Bo-Yv Zheng,4 Tao-Lan Zhang,5 Jing Li,2 Guo-Hua Lv,2 Yi-Guo Yan,1,* Ming-Xiang Zou1,*
1Department of Spine Surgery, The First Affiliated Hospital, University of South China, Hengyang, 421001, People’s Republic of China; 2Department of Spine Surgery, The Second Xiangya Hospital, Central South University, Changsha, 410011, People’s Republic of China; 3Health and Management Centre, The First Affiliated Hospital, University of South China, Hengyang, 421001, People’s Republic of China; 4Department of Orthopedics Surgery, General Hospital of the Central Theater Command, Wuhan, 430061, People’s Republic of China; 5Department of Radiation Oncology, Indiana University School of Medicine, IU Simon Comprehensive Cancer Center, Indianapolis, IN, 46202, USA
*These authors contributed equally to this work
Correspondence: Ming-Xiang Zou
Department of Spine Surgery, The First Affiliated Hospital, University of South China, 69 Chuanshan Road, Hengyang, Hunan, 421001, People’s Republic of China
Tel +86 73485295624
Fax +86 73482654334
Email [email protected]
Yi-Guo Yan
Department of Spine Surgery, The First Affiliated Hospital, University of South China, 69 Chuanshan Road, Hengyang, Hunan, 421001, People’s Republic of China
, +86 73485295624
, +86 73482654334
Email [email protected]
Objective: Chondroblastoma (CB) is a rare and locally growing cartilage-derived tumor. Currently, clinical implications of tumor-associated macrophages (TAMs) in CB remain unclear. In this study, we sought to analyze the relationship between TAM parameters (including densities of CD68+ and CD163+ cells as well as the CD163+/CD68+ ratio) and clinicopathological characteristics and survival of patients.
Methods: Immunohistochemistry was used to assess TAM subtypes for CD68 and CD163, as well as the expression levels of p53, CD34, and Ki-67 on tumor cells in 132 tissue specimens retrieved between July 2002 and April 2020. Then, TAM parameters were retrospectively analyzed for their associations with patient outcomes (local recurrence-free survival [LRFS] and overall survival [OS]) and clinicopathological features.
Results: TAM densities were significantly higher in axial chondroblastoma tissue than in extra-axial chondroblastoma tissue. Moreover, the number of CD163+ TAMs was positively correlated with tumor invasion of surrounding tissues and high expression of CD34 and Ki-67 on tumor cells, whereas CD163+ cell density and the CD163/CD68 ratio were negatively associated with patient response to adjuvant radiotherapy. Univariate Kaplan–Meier analysis revealed that the number of CD68+ and CD163+ lymphocytes was significantly associated with both LRFS and OS. Multivariate Cox regression analysis showed that CD163+ and CD68+ cell levels were independent prognostic factors of LRFS, while TAM data independently predicted OS. More importantly, in subgroup analysis based on three significant factors in univariate survival analysis (including tumor location, adjuvant radiotherapy, and surrounding tissue invasion by tumors), the TAM parameters still displayed good prognostic performance.
Conclusion: These data suggest that TAM may significantly affect the biological behavior of CB. We hypothesize that modulating the TAM level or polarization status in the microenvironment may be an effective approach for CB treatment.
Keywords: chondroblastoma, tumor-associated macrophages, tumor immune microenvironment, prognostic factors, survival analysis
Introduction
Chondroblastoma (CB) is a rare cartilage-derived tumor with locally aggressive growth characteristics. CB commonly involves the long bone epiphysis and accounts for less than 1% of all bone tumors.1 Due to resistance to traditional chemotherapy, the current treatment of choice for CB mainly includes surgery and radiotherapy.2 Although surgery is indicated for patients with neurological deficits, spinal instability and/or worsening symptoms caused by tumors, complete resection of CB lesion may be difficult due to its locally aggressive nature and proximity to important neurovascular structures. It has also been reported that radiotherapy can be administered for patients with postoperative recurrence and those who cannot be treated surgically.3 However, radiotherapy may even cause malignant changes in this disease.4 Therefore, the therapeutic options for CB patients are limited at present and the recurrence risk of this disease is high after surgery (approximately 10–35% of patients experience relapse),5 which exerts a significant adverse effect on the long-term quality of life and survival of patients.
Tumor-associated macrophages (TAMs) constitute an important component of the tumor microenvironment. Studies have shown that TAMs can interact with cancer cells to promote neovascularization and increase radiotherapy resistance, thus accelerating the invasion and metastasis of tumors.6 Moreover, it has been suggested that TAM infiltration in the microenvironment is closely associated with the survival of patients with various human malignancies.7 Specifically, the number of CD163+ TAMs was shown to be negatively correlated with better survival in patients with gastric cancer, breast cancer, and glioma,8–10 while the density of CD68+ cells was related to poor prognosis in non-small cell lung cancer and renal clear cell carcinoma.11,12 Furthermore, for patients with colon cancer and non-small cell lung cancer, the inclusion of the CD163+/CD68+ ratio was found to significantly improve the accuracy of traditional tumor-node metastasis staging in predicting patient outcomes.13,14
Previous studies have demonstrated that many factors can affect CB prognosis. For example, it has been reported that CB lesions located in the pelvis and proximal humerus have a poor prognosis.15,16 Similarly, studies have found that patient age and secondary cyst formation within the lesion are significantly associated with postoperative CB recurrence.16–18 However, there is currently a lack of studies analyzing the clinical implications of TAMs in CB. Considering the locally aggressive feature and potentially malignant transformation of CB, we consider similarities in the functional role of TAM among CB and cancers. Moreover, assessing the effect of microenvironmental TAMs on the biological behavior of CB may help optimize preoperative risk stratification and provide direction for the development of new therapeutic approaches. In this study, we aimed to analyze the relationship between TAM parameters (including the densities of CD68+ and CD163+ cells as well as the CD163+/CD68+ ratio) and the clinicopathological characteristics and survival of patients.
Methods and Materials
Patients and Tissue Samples
A total of 132 CB patients who underwent surgical resection at the Second Xiangya Hospital from July 1, 2002, to April 30, 2020, were retrospectively included in this study. Patients’ characteristics are detailed in Table 1. Demographic information (age and sex), clinical features (including tumor location, tumor size, duration of symptoms, preoperative and postoperative neurological status) and treatment (including type of surgery, radiotherapy and chemotherapy) were directly obtained from medical records. Surrounding tissue invasion by tumors was recorded when the tumor lesion had an extraosseous invasion into surrounding tissues, which was evaluated by preoperative magnetic resonance imaging (MRI).19 Tumors were recorded as axial chondroblastoma (ACB) or extra-axial chondroblastoma (EACB), according to the site involved. The pathological diagnosis was independently confirmed by two neuropathologists based on hematoxylin and eosin (HE)-stained sections, and the presence of aneurysmal bone cyst (ABC) and chicken-wire calcification in the tumor tissue was also assessed. Analyzing the expression levels of several key proteins (including CD34, Ki-67 and p53) in CB tissues was also performed, which was detailed below. The type of surgical resection was classified as Enneking appropriate (EA) or Enneking inappropriate (EI) based on the pathological analysis of the postoperative specimens.20 The revised response evaluation criteria for solid tumors were used to evaluate the efficacy of radiotherapy21 and patients were divided into ineffective group (tumor had progression in images) or effective group (tumor had complete remission, partial remission, or was stable) according to this method. In this study, we included patients harboring histopathologically confirmed CB, irrespective of whether tumors invaded the axial or extra-axial skeleton. In addition, we restricted our cohort to patients who had only received surgery with or without adjuvant radiotherapy. Patients who had previously received other tumor-specific treatments (such as chemotherapy) and those who had any other comorbidities (such as immunocompromised state) were excluded from this study, considering that both situations could likely distort the true TAM profile within the CB microenvironment.19
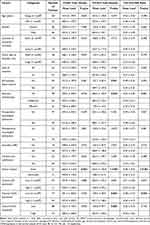 |
Table 1 Association Between TAMs Parameters and Clinicopathological Features of Chondroblastoma Patients |
Formalin-fixed paraffin-embedded blocks from 132 CB patients were retrieved from the Department of Pathology and sectioned into 4-μm-thick tissue sections for subsequent assays. This study was approved by our hospital ethics committee, and informed consent was obtained from all patients before commencing of the study.
Follow-Up of Patients
All patients received regular clinical and radiological follow-ups after surgery, and the last follow-up was performed in September 2020. As 24 patients died before the final follow-up date and 2 patients were lost during the follow-up period, only 106 patients were visited in hospital for the final follow-up. The primary outcome parameters of interest were local recurrence-free survival (LRFS) and overall survival (OS). LRFS was defined as the time interval between tumor resection and the first local recurrence, which was assessed by MRI findings and/or pathological results of surgically resected specimens,22 and OS was determined as the time interval between surgical resection of the tumors and patient death from all causes. The event was recorded as censored if no tumor relapse was observed (for LRFS analysis) or the patient was still alive (for OS analysis).
Immunohistochemistry
Immunohistochemical staining was performed as we previously described.23 Briefly, paraffin-embedded sections (4 μm) of 132 CB specimens from our institute were deparaffinized in xylene, rehydrated with a graded series of ethanol solutions and then rinsed in distilled water. The tissue sections were then treated with primary antibodies (Supplemental material 1) at 4°C overnight following antigen retrieval and blocking. After incubation with secondary biotinylated goat anti-rabbit or anti-mouse immunoglobulin, immunodetection was conducted with a streptavidin–peroxidase conjugate (Auragene, Changsha, Hunan, China), followed by visualization with the 3,3ʹ-diaminobenzidine solution and counterstaining with hematoxylin. A negative control was produced by replacing the primary antibody with a phosphate buffer solution, and a positive control was generated using human testis tissues according to a previous report.14
Evaluation of Immunohistochemistry
Semiquantitative Analysis
Immunohistochemical results were evaluated as we have previously documented.24 Briefly, using a light microscope, immunostained tissue sections were independently scored by two experienced neuropathologists who were blinded to patient data and had profound expertise in neuro-oncology. All discrepancies were resolved by consensus. Brown cytoplasmic or nuclear staining of tumor cells was recorded as positive for protein 53 (p53) and CD34 expression. Tissue sections were first scanned at low power, and five high-power fields were then randomly selected, with at least 1000 cells counted for each field. After this, the staining intensity of tumor cells in tissue sections was graded by neuropathologists as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong) according to a previously described method.19,25 The staining percentage was counted as the ratio of positively stained cells to the total number of cells assessed. For each case, the staining intensity (0–3) and staining percentage (0–100%) scores were multiplied to generate the final data. Noticeably, the Ki-67-staining index score was determined as the percentage of cells with nuclear staining, and these data were separated into low (< 10%) or high (≥ 10%) Ki-67 expression as previously suggested.26
Automated Image Analysis
Automated quantitative analysis of CD68+ and CD163+ cells was performed as previously described.19,27 Briefly, sections were viewed in regions where TAM was focally rich using Nikon’s inverted Eclipse Ti microscope, and images representative of regions of interest were taken by a Nikon DS-Ri 1-U3 digital camera (Tokyo, Japan) with NIS-Elements image analysis software AR 3.0. The number of TAMs was then counted in 5 areas (20x) using a computer-assisted image analysis method (Image-Pro Plus 6.0, Media Cybernetics Inc., Rockville, Maryland). Finally, measurements were determined as the mean number of positively stained cells per tissue surface unit in square millimeters (mm2).
Statistical Analysis
All statistical analyses were performed using SPSS 17.0 (IBM Inc., Armonk, New York). Categorical data are presented as frequencies and were analyzed by the chi-square test. Quantitative data are presented as the mean ± standard deviation and were analyzed by Student’s t-test or one-way ANOVA. The “surv_cutpoint” function in the “survminer” package of R software (version 3.6.2, R Foundation for Statistical Computing, Vienna, Austria) was used to determine cutoff values for continuous variables in survival analysis, with OS as the outcome parameter (Supplemental material 2 and Supplemental material 3).28 Patients were divided into two subgroups (≤ cutoff or > cutoff) according to these cutoff points. Specifically, this threshold value was defined as the point with the lowest P value from the Log rank test, which was corrected accordingly.29 Univariate analysis using the Kaplan–Meier method was applied to analyze the survival difference between groups. Multivariate Cox proportional hazard models were used to identify independent predictors for LRFS and OS after controlling for other covariates that were significant in univariate analysis. All tests were two-sided, and a P ≤ 0.05 was considered statistically significant.
Results
Patient and Tumor Characteristics
A total of 132 patients with CB were included in this study. Among them, 61 patients had ACB, and 71 patients had EACB (Figure 1). Sixty-five patients underwent EA resection, and 67 patients underwent EI resection. The average values for age, duration of symptoms and tumor size are 29.2 ± 13.4 years, 8.3 ± 7.4 months, and 3.9 ± 1.8 cm, respectively. Thirty-four patients received adjuvant photon radiotherapy after surgery. The average dose was 39.6 Gy/22 F, and the number of radiations ranged from 5 to 20. The mean levels of CD68+ and CD163+ cells and the CD163/CD68 ratio were 543.4 ± 201.2 (cells/mm2), 261.2 ± 101.2 (cells/mm2) and 0.51 ± 0.19, respectively. Similarly, the average immunostaining scores for p53, CD34 and Ki-67 in tumor tissues were 165.2 ± 48.5, 144.1 ± 62.9, and 10.1 ± 11.2, respectively. The mean follow-up time was 76.0 months. The median LRFS was 192.0 months, and the 1-, 3- and 5-year LRFS rates were 86%, 78%, and 75%, respectively. The median OS was 180.0 months, and the OS rates at 3 years, 5 years, and 10 years were 90%, 84%, and 70%, respectively. Based on the cutoff values obtained by R software, we divided TAM data as well as p53 and CD34 data into two groups (low or high expression) for subsequent survival analysis. Representative images of TAMs and immunohistochemical markers are shown in Figure 2.
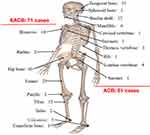 |
Figure 1 Distribution of the tumor site for 132 chondroblastoma patients. |
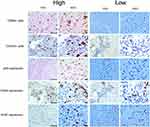 |
Figure 2 Representative images of tumor-associated macrophages parameters and immunohistochemical markers in chondroblastoma tissues. |
Association Between TAM Levels and Clinicopathological Features
The results of the association between TAM parameters and patient features are shown in Table 1. We found that the number of macrophages was significantly higher in ACB tissue than in EACB tissue. The density of CD163+ TAMs was positively correlated with tumor invasion of surrounding tissues and high Ki-67 and CD34 expression on tumor cells. In contrast, CD163+ cell density and the CD163/CD68 ratio were negatively associated with the patient response to radiotherapy (Figure 3).
 |
Table 2 Univariate Analysis of the Prognostic Factors of Local Recurrence-Free Survival in Patients with Chondroblastoma |
 |
Figure 3 The relationship between tumor-associated macrophages parameters and response to radiotherapy in patients with chondroblastoma. |
Influence of TAM on Survival of CB Patients
LRFS
Univariate Kaplan-Meier analysis showed that the number of CD68+ and CD163+ lymphocytes significantly affected patient survival (Table 2 and Figure 4). In addition, our analysis also revealed that type of resection, presence of surrounding tissue invasion by tumors and adjuvant radiotherapy were significantly associated with LRFS (Table 2). A multivariate Cox regression model showed that the resection modality and microenvironmental CD163+ and CD68+ cell levels could independently predict LRFS (Figure 5).
 |
Figure 4 Kaplan–Meier curves of local recurrence-free survival of chondroblastoma patients stratified by CD68+ cell density and CD163+ cell density. |
OS
Univariate Kaplan-Meier analysis showed that CD68+ and CD163+ cell counts and the CD163/CD68 ratio significantly influenced OS (Table 3 and Figure 6). Moreover, our results also indicated that type of surgery, presence of tumor infiltration into surrounding tissues, adjuvant radiotherapy, and postoperative neurological status were closely related to survival (Table 3). Further, multivariate Cox regression analysis identified TAM data and modality of tumor resection as independent predictors of patient OS (Figure 7).
 |
Table 3 Univariate Analysis of the Prognostic Factors of Overall Survival in Patients with Chondroblastoma |
 |
Figure 6 Kaplan–Meier curves of overall survival of chondroblastoma patients stratified by CD68+ cell density, CD163+ cell density and CD163/CD68 ratio. |
Subgroup Analysis for the Prognostic Performance of TAMs Stratified by Significant Clinical Predictors in Univariate Analysis
Subgroup analysis based on tumor location revealed that the number of CD163+ TAMs was significantly associated with LRFS and OS in ACB patients (Supplemental material 4). In the EACB group, however, the results showed that TAM parameters significantly affected patient outcomes (Supplemental material 5).
Similarly, in patients who did not receive radiotherapy, we found that the number of CD163+ and CD68+ cells was associated with LRFS, while CD163+ and CD68+ cell densities as well as the CD163/CD68 ratio were correlated with OS (Supplemental material 6). In contrast, regarding patients who had ineffective adjuvant radiotherapy, this analysis showed that CD68+ cell density significantly affected OS (Supplemental material 7). However, due to the small sample size (n = 8), subgroup analysis was not performed in patients with good response to radiotherapy.
In patients without surrounding tissue invasion of tumors, we found that the level of CD68+ cells and the CD163/CD68 ratio was associated with OS (Supplemental material 8). However, for patients whose tumors invaded into surrounding tissues, our results revealed that CD68+ and CD163+ cell densities were closely related to patient survival (Supplemental material 9).
Discussion
In this study, we analyzed the TAM profile in the CB microenvironment and their association with the clinicopathological characteristics and survival of patients. We found that TAM parameters had a significant positive correlation with preoperative neurological deficits, surrounding tissue infiltration by tumors and high expression levels of Ki-67 and CD34 on tumor cells. Moreover, our results revealed a significant increase in TAM density for tumors located in the axial skeleton and among patients who received ineffective radiotherapy. More importantly, a multivariate Cox analysis identified CD68+ and CD163+ cell levels as independent predictors of LRFS, while CD163+ cell density and the CD163/CD68 ratio independently affected OS. These data suggest that TAMs may significantly influence the biological behavior of CB. We hypothesize that modulating TAM densities or polarization status in the microenvironment may open up a new opportunity for CB therapy.
Due to the different forms of activation and polarization,30 the prognostic value of TAMs in several cancer types is still controversial. However, a large body of evidence suggests that the levels of TAM could independently predict poor patient survival in various human malignancies.31 In line with these findings, our study showed that the number of CD68- and CD163-positive cells as well as the CD163/CD68 ratio were significantly associated with poor LRFS and/or OS of CB patients. Additionally, this study also found that TAM density was positively correlated with preoperative neurological dysfunction, tumor invasion into surrounding tissues, and a high Ki-67 index in tumor cells (the large variation in our Ki-67 data possibly indicates a different biological behavior between tumors), consistent with previous reports indicating that high TAM levels were associated with an aggressive phenotype of many cancers.32,33 Moreover, recent results from preclinical studies and clinical trials suggest that cancer patients may benefit from the use of drugs targeting the TAM.33 These data strongly imply that TAMs may influence CB development.
Currently, the specific mechanism underlying TAM affecting the clinical outcome of CB patients remains unknown. Previous studies have shown that TAMs may promote tumor initiation through the induction of angiogenesis and thus exert an effect on prognosis.33,34 Supporting this idea, we found that CD163+ cell density significantly correlated with CD34 expression in tumor tissues. These results suggest that TAMs may affect patient outcome in part by inducing CD34 expression in CB tissues. Moreover, it should be noted that CD163+ cell levels and/or the CD163/CD68 ratio were significantly associated with an aggressive CB phenotype and patient survival. It is well known that CD163 and CD163/CD68 ratios are commonly used to assess the degree of M2-type polarization of macrophages.35 Prior data found that the PI3Kγ/NF-κB pathway could regulate the polarization status of TAMs in cancer tissues.36 Further, it has been demonstrated that RANKL (as the ligand of NF-κB) expression is linked to CB progression.37 These data suggest the possibility that TAMs may influence CB outcome by regulating macrophage polarization status through the PI3Kγ/RANKL/NF-κB signaling pathway. To corroborate this speculation, our future studies will analyze the expression levels of RANKL and key proteins in PI3Kγ pathways and their association with TAM parameters. In addition, recent reports have indicated that CD47 is highly expressed in many sarcoma tissues,38 and SIRPα on TAMs can mediate immune escape by binding to CD47 to promote tumor development.39,40 Whether TAMs affect tumor progression through similar mechanisms deserves further exploration in CB.
Another major finding of this study was that the number of CD163+ macrophages and the CD163/CD68 ratio had a significantly negative association with the effectiveness of radiotherapy in CB patients. Further subgroup analysis showed that tumors with high TAM levels portended the worst survival for patients who were not treated with adjuvant radiotherapy. In agreement with this finding, it has been reported that TAMs (especially CD163+ macrophages) can increase the resistance of tumor cells to radiotherapy.41,42 In addition, studies have found that TAM parameters are correlated with the effectiveness of radiotherapy in patients with colon and head and neck cancers.43–45 Apart from this, researchers have proven that a high CD163+ cell count could predict poor response to radiotherapy and affect cause-specific survival in patients with cervical cancer.43 Altogether, these data indicate that TAMs may influence patient outcomes by increasing the resistance of CB cells to radiotherapy.
Interestingly, we also found that TAM parameters (including the number of CD68+ and CD163+ cells as well as the CD163/CD68 ratio) were significantly higher in ACB tissues than in EACB tissues. Furthermore, this analysis revealed that ACB harbored higher tumoral Ki-67 expression and an increased risk of surrounding tissue infiltration by tumors than EACB. This was also the case for patient age, tumor size, and postoperative neurological status in ACB compared to EACB, whereas other variables, including the type of surgical resection, were not significantly different between the two groups. Further subgroup analysis showed that prognostic factors were not consistent between ACB and EACB (data not shown). Taken together, these results suggest that ACB displays more aggressive behavior than EACB, similar to preceding observations showing that ACB progresses more rapidly and that patients are more likely to experience disease recurrence after surgery.3,46,47 One possible explanation could be that TAMs in the microenvironment induce an aggressive phenotype for ACB through the production of various factors or proteases,48 thereby leading to poor patient survival. This information may be useful in improving prognostic risk stratification and guiding treatment optimization for patients.
Limitations
Our study had a retrospective design. Further prospective studies with a large sample size are still needed to confirm our findings. Additionally, this study is exploratory in nature. Future studies are required to uncover the precise molecular mechanisms of how TAM affects the clinical outcome of CB patients. For example, whether TAM influences CB progression via PI3Kγ/RANKL/NF-κB or SIRPα/CD47 signaling pathways deserves further exploration. Further studies involving TAM quantification and transcriptome sequencing using pre- and postradiotherapy CB tissue samples are also needed to help confirm the exact molecular mechanisms by which TAMs promote resistance to radiotherapy. Nevertheless, despite lack of experimental data, our findings may still provide useful information for prognostic risk stratification and therapeutic optimization of patients.
Conclusion
Our data suggest that TAM may significantly affect the biological behavior of CB. We hypothesize that modulating the TAM level or polarization status in the microenvironment may be an effective therapeutic approach for patients.
Ethics Approval and Consent to Participate
The study protocol was approved by the Institutional Review Board at the First Affiliated Hospital, University of South China, Hunan, P.R. China. Written informed consent was obtained from each patient for publication of this study. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
We thank Dr. Yi Jiang and Dr. Xiao-Ling She from the Department of Pathology, the Second Xiangya Hospital, Central South University for pathological analysis of the study. We also thank American Journal Experts for assistance in the preparation of this manuscript. This work was supported by the National Natural Science Foundation of China (82003802 to TLZ, 81871821 to JL and 82002364 MXZ), the Natural Science Foundation of Hunan Province (2019JJ50542 to TLZ), the Project for Clinical Research of Hunan Provincial Health Commission (20201978 to TLZ and 20201956 to MXZ) and China Scholarship Council (CSC201808430085 to TLZ).
Authorship
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Tathe SP, Parate SN, Jaiswal KN, Randale AA. Intraoperative crush smear cytology of vertebral chondroblastoma: a diagnostic challenge. Diagn Cytopathol. 2018;46(1):79–82. doi:10.1002/dc.23799
2. Zheng BW, Huang W, Liu FS, et al. Clinicopathological and prognostic characteristics in spinal chondroblastomas: a pooled analysis of individual patient data from a single institute and 27 studies. Global Spine J. 2021:219256822110057. doi:10.1177/21925682211005732.
3. Chung OM, Yip SF, Ngan KC, Ng WF. Chondroblastoma of the lumbar spine with cauda equina syndrome. Spinal Cord. 2003;41(6):359–364. doi:10.1038/sj.sc.3101458
4. Angelini A, Hassani M, Mavrogenis AF, et al. Chondroblastoma in adult age. Eur J Orthop Surg Traumatol. 2017;27(6):843–849. doi:10.1007/s00590-017-1996-7
5. Herget GW, Maier D, Südkamp NP, et al. Anatomical reconstruction of the acromion using an autologous iliac crest graft for treatment of recurrent chondroblastoma: a case report. JBJS Case Connect. 2019;9(3):e0086. doi:10.2106/JBJS.CC.19.00086
6. Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33(3):119–126. doi:10.1016/j.it.2011.12.001
7. Salmaninejad A, Valilou SF, Soltani A, et al. Tumor-associated macrophages: role in cancer development and therapeutic implications. Cell Oncol (Dordr). 2019;42(5):591–608. doi:10.1007/s13402-019-00453-z
8. Huang X, Pan Y, Ma J, et al. Prognostic significance of the infiltration of CD163(+) macrophages combined with CD66b(+) neutrophils in gastric cancer. Cancer Med. 2018;7(5):1731–1741. doi:10.1002/cam4.1420
9. Jeong H, Hwang I, Kang SH, Shin HC, Kwon SY. Tumor-associated macrophages as potential prognostic biomarkers of invasive breast cancer. J Breast Cancer. 2019;22(1):38–51. doi:10.4048/jbc.2019.22.e5
10. Hu WM, Yang YZ, Zhang TZ, Qin CF, Li XN. LGALS3 is a poor prognostic factor in diffusely infiltrating gliomas and is closely correlated with cd163+ tumor-associated macrophages. Front Med (Lausanne). 2020;7:182. doi:10.3389/fmed.2020.00182
11. Rakaee M, Busund LR, Jamaly S, et al. Prognostic value of macrophage phenotypes in resectable non-small cell lung cancer assessed by multiplex immunohistochemistry. Neoplasia. 2019;21(3):282–293. doi:10.1016/j.neo.2019.01.005
12. Nakanishi H, Miyata Y, Mochizuki Y, et al. Pathological significance and prognostic roles of densities of CD57+ cells, CD68+ cells, and mast cells, and their ratios in clear cell renal cell carcinoma. Hum Pathol. 2018;79:102–108. doi:10.1016/j.humpath.2018.05.007
13. Yang C, Wei C, Wang S, et al. Elevated CD163(+)/CD68(+) ratio at tumor invasive front is closely associated with aggressive phenotype and poor prognosis in colorectal cancer. Int J Biol Sci. 2019;15(5):984–998. doi:10.7150/ijbs.29836
14. Hwang I, Kim JW, Ylaya K, et al. Tumor-associated macrophage, angiogenesis and lymphangiogenesis markers predict prognosis of non-small cell lung cancer patients. J Transl Med. 2020;18(1):443. doi:10.1186/s12967-020-02618-z
15. Lin PP, Thenappan A, Deavers MT, Lewis VO, Yasko AW. Treatment and prognosis of chondroblastoma. Clin Orthop Relat Res. 2005;NA(438):103–109. doi:10.1097/01.blo.0000179591.72844.c3
16. Xu H, Nugent D, Monforte HL, et al. Chondroblastoma of bone in the extremities: a multicenter retrospective study. J Bone Joint Surg Am. 2015;97(11):925–931. doi:10.2106/JBJS.N.00992
17. Konishi E, Nakashima Y, Mano M, et al. Chondroblastoma of extra-craniofacial bones: clinicopathological analyses of 103 cases. Pathol Int. 2017;67(10):495–502. doi:10.1111/pin.12586
18. Laitinen MK, Stevenson JD, Evans S, et al. Chondroblastoma in pelvis and extremities- a single centre study of 177 cases. J Bone Oncol. 2019;17:100248. doi:10.1016/j.jbo.2019.100248
19. Zou MX, Lv GH, Wang XB, et al. Clinical impact of the immune microenvironment in spinal chordoma: immunoscore as an independent favorable prognostic factor. Neurosurgery. 2019;84(6):E318–E333. doi:10.1093/neuros/nyy274
20. Fisher CG, Saravanja DD, Dvorak MF, et al. Surgical management of primary bone tumors of the spine: validation of an approach to enhance cure and reduce local recurrence. Spine (Phila Pa 1976). 2011;36(10):830–836. doi:10.1097/BRS.0b013e3181e502e5
21. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
22. Zou MX, Zheng BW, Liu FS, et al. The relationship between tumor-stroma ratio, the immune microenvironment, and survival in patients with spinal chordoma. Neurosurgery. 2019;85(6):E1095–e1110. doi:10.1093/neuros/nyz333
23. Zou MX, Peng AB, Lv GH, et al. Expression of programmed death-1 ligand (PD-L1) in tumor-infiltrating lymphocytes is associated with favorable spinal chordoma prognosis. Am J Transl Res. 2016;8(7):3274–3287.
24. Zou MX, Lv GH, Li J, et al. Upregulated human telomerase reverse transcriptase (hTERT) expression is associated with spinal chordoma growth, invasion and poor prognosis. Am J Transl Res. 2016;8(2):516–529.
25. Bellmunt J, Mullane SA, Werner L, et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol. 2015;26(4):812–817. doi:10.1093/annonc/mdv009
26. Zhou M, Chen K, Yang H, et al. Expression of insulin-like growth factor II mRNA-binding protein 3 (IMP3) in sacral chordoma. J Neurooncol. 2014;116(1):77–82. doi:10.1007/s11060-013-1274-4
27. Gabrielson A, Wu Y, Wang H, et al. Intratumoral CD3 and CD8 T-cell densities associated with relapse-free survival in HCC. Cancer Immunol Res. 2016;4(5):419–430. doi:10.1158/2326-6066.CIR-15-0110
28. Zou MX, Pan Y, Huang W, et al. A four-factor immune risk score signature predicts the clinical outcome of patients with spinal chordoma. Clin Transl Med. 2020;10(1):224–237. doi:10.1002/ctm2.4
29. Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst. 1994;86(11):829–835. doi:10.1093/jnci/86.11.829
30. Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66(2):605–612. doi:10.1158/0008-5472.CAN-05-4005
31. Chen X, Chen J, Zhang W, et al. Prognostic value of diametrically polarized tumor-associated macrophages in multiple myeloma. Oncotarget. 2017;8(68):112685–112696. doi:10.18632/oncotarget.22340
32. Minami K, Hiwatashi K, Ueno S, et al. Prognostic significance of CD68, CD163 and folate receptor-β positive macrophages in hepatocellular carcinoma. Exp Ther Med. 2018;15(5):4465–4476. doi:10.3892/etm.2018.5959
33. Yang Q, Guo N, Zhou Y, Chen J, Wei Q, Han M. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm Sin B. 2020;10(11):2156–2170. doi:10.1016/j.apsb.2020.04.004
34. Shu Y, Cheng P. Targeting tumor-associated macrophages for cancer immunotherapy. Biochim Biophys Acta Rev Cancer. 2020;1874(2):188434. doi:10.1016/j.bbcan.2020.188434
35. Shabo I, Svanvik J. Expression of macrophage antigens by tumor cells. Adv Exp Med Biol. 2011;714:141–150. doi:10.1007/978-94-007-0782-5_7
36. Kaneda MM, Messer KS, Ralainirina N, et al. PI3Kγ is a molecular switch that controls immune suppression. Nature. 2016;539(7629):437–442. doi:10.1038/nature19834
37. Huang L, Cheng YY, Chow LT, Zheng MH, Kumta SM. Receptor activator of NF-kappaB ligand (RANKL) is expressed in chondroblastoma: possible involvement in osteoclastic giant cell recruitment. Mol Pathol. 2003;56(2):116–120. doi:10.1136/mp.56.2.116
38. Dancsok AR, Gao D, Lee AF, et al. Tumor-associated macrophages and macrophage-related immune checkpoint expression in sarcomas. Oncoimmunology. 2020;9(1):1747340. doi:10.1080/2162402X.2020.1747340
39. Chao MP, Weissman IL, Majeti R. The CD47-SIRPα pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol. 2012;24(2):225–232. doi:10.1016/j.coi.2012.01.010
40. Zhang W, Huang Q, Xiao W, et al. Advances in anti-tumor treatments targeting the CD47/SIRPα axis. Front Immunol. 2020;11:18. doi:10.3389/fimmu.2020.00018
41. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416. doi:10.1038/nrclinonc.2016.217
42. Anfray C, Ummarino A, Andón FT, Allavena P. Current strategies to target tumor-associated-macrophages to improve anti-tumor immune responses. Cells. 2019;9(1):46. doi:10.3390/cells9010046
43. Lippens L, Van Bockstal M, De Jaeghere EA, et al. Immunologic impact of chemoradiation in cervical cancer and how immune cell infiltration could lead toward personalized treatment. Int J Cancer. 2020;147(2):554–564. doi:10.1002/ijc.32893
44. Shaikh S, Noshirwani A, West N, Perry S, Jayne D. Can macrophages within the microenvironment of locally invasive rectal cancers predict response to radiotherapy? Lancet. 2015;385 Suppl 1:S87. doi:10.1016/S0140-6736(15)60402-0
45. Ou D, Adam J, Garberis I, et al. Influence of tumor-associated macrophages and HLA class I expression according to HPV status in head and neck cancer patients receiving chemo/bioradiotherapy. Radiother Oncol. 2019;130:89–96. doi:10.1016/j.radonc.2018.08.013
46. Ilaslan H, Sundaram M, Unni KK. Vertebral chondroblastoma. Skeletal Radiol. 2003;32(2):66–71. doi:10.1007/s00256-002-0599-4
47. Kim SA, Cho KJ, Park YK, et al. Chondroblastoma of the lumbar spine - A case Report and Review of the literature. Korean J Pathol. 2011;45(5):532. doi:10.4132/KoreanJPathol.2011.45.5.532
48. Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12(1):76. doi:10.1186/s13045-019-0760-3
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


