Back to Journals » OncoTargets and Therapy » Volume 7
Prognostic significance of thymidylate synthase in postoperative non-small cell lung cancer patients
Authors Zhao H, Ma G, Zou B, Li M, Lin S, Zhao L, Guo Y, Huang Y, Tian Y, Xie D, Zhang L
Received 28 March 2014
Accepted for publication 30 April 2014
Published 16 July 2014 Volume 2014:7 Pages 1301—1310
DOI https://doi.org/10.2147/OTT.S65067
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Hong-Yun Zhao,1,* Guo-Wei Ma,1,* Ben-Yan Zou,1,* Mei Li,1 Su-Xia Lin,1 Li-Ping Zhao,2 Ying Guo,1 Yan Huang,1 Ying Tian,1 Dan Xie,1 Li Zhang1
1Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, People’s Republic of China; 2Department of Medical Oncology, Zhongshan Hospital of Sun Yat-Sen University, Zhongshan People’s City Hospital, Zhongshan, People’s Republic of China
*The first three authors contributed equally to this work
Abstract: The aim of the present study was to investigate the clinicopathologic/prognostic significance of thymidylate synthase (TS), orotate phosphoribosyltransferase (OPRT), and thymidine phosphorylase (TP) proteins in postoperative non-small cell lung cancer (NSCLC) patients. Microarray slides from a set of 178 NSCLC patients were used for the detection of TS, OPRT, and TP expression by immunohistochemistry. The correlation between clinicopathologic factors and protein expression of three proteins was analyzed. Ninety seven carcinomas (57.4%) were TS-positive, 90 carcinomas (53.9%) were OPRT-positive, and 102 carcinomas (69.4%) were TP-positive. Compared with the TS-positive patients, the overall survival (OS) was significantly lower in the TS-negative patients (hazard ratio [HR] =1.766, 95% confidence interval [CI] =1.212–2.573, P=0.003). Significant differences between TS-positive and TS-negative patients was also observed in the following stratified analyses: 1) adenocarcinoma subgroup (HR =2.079, 95% CI =1.235–3.500, P=0.006); 2) less than 60-year-old subgroup (HR =1.890, 95% CI =1.061–3.366, P=0.031); 3) stage II/III subgroup (HR =1.594, 95% CI =1.036–2.453, P=0.034); and 4) surgery plus adjuvant therapy subgroup (HR =1.976, 95% CI =1.226–3.185, P=0.005). However, the OS was not significantly correlated with OPRT or TP protein expression. This study demonstrates that the TS level in tumor tissues may be a useful marker to predict the postoperative OS in NSCLC patients.
Keywords: orotate phosphoribosyltransferase, thymidine phosphorylase
Introduction
Lung cancer is a leading cause of cancer deaths worldwide1 Non-small cell lung cancer (NSCLC), which accounts for approximately 80% of all lung cancers, is a very heterogeneous group of malignancies with a poor prognosis.2 For stages I–IIIa, surgery is still the first curative treatment for NSCLC patients. In addition, various combined-modality therapies, including chemotherapy and radiation therapy, have been assessed for improving the treatment outcome in NSCLC patients.3 Although the 5-year survival rate after complete resection has been shown to be 67%–79% for stage I patients,4 the 3-year survival rate has been only 35%–55% and 28% for stage II and stage III NSCLC patients, respectively.5 The heterogeneous biological properties of NSCLC may affect the prognosis for patients with resectable NSCLC, and it is hard for the international lung cancer tumor-node-metastasis (TNM) staging system to fully assess the prognosis of patients. Therefore, the TNM staging system needs to be improved by some molecular markers, which will help, not only to predict the recurrence as early as possible but also, to determine the indication of optimal therapeutic strategy as adjuvant therapies. However, reliable biomarkers are very limited.
Previous studies6–9 have demonstrated that the genes involved in nucleotide metabolism and DNA repair play important roles in determining the biological phenotype and prognosis of NSCLC, including Bcl-2, HER2, ribonucleotide reductase M1 (RRM1), and excision repair cross complement 1 (ERCC1). All of these are involved in DNA repair in the intracellular nucleotide excision repair system. With the development of molecular biology, other biomarkers that play important roles in nucleotide synthesis and DNA repair have attracted attention worldwide. Among them, nucleotide metabolic enzymes, such as thymidylate synthase (TS), orotate phosphoribosyltransferase (OPRT), and thymidine phosphorylase (TP) have already been investigated for their prognostic potentials in different tumors.10–14,18–21
TS is the downstream member of ribonucleotide reductase and crucial for the formation of deoxyribothymidine monophosphate (dTMP) that is required for DNA synthesis and repair. TS expression is reported to have an influence on the prognosis of tumors.10,11 OPRT is a constitutively expressed enzyme that regulates the de novo pyrimidine nucleotide biosynthetic pathway, namely the conversion of orotic acid to orotidine monophosphate, and is also involved in salvage biosynthetic pathways. Previous studies showed that OPRT could be a prognostic marker in different cancers, including colorectal cancer (CRC), pancreatic cancer, and renal cancer.12–14 TP is involved in the catalyzation of reversible phosphorylation of thymine to deoxyribose-1-phosphate and thymine, and plays important roles in angiogenesis, cancer invasiveness, metastasis, and antiapoptosis.15 TP is most frequently expressed in breast cancer, followed by lung cancer (adenocarcinoma [ADC] and squamous cell carcinoma).16 Previous study found that a high level of TP expression is associated with more extensive angiogenesis, unfavorable clinical and laboratory findings, and poor clinical outcome in CRC.17 Subsequent studies also confirmed the prognostic role of TP in other types of cancer: transitional cell carcinoma of the bladder, cervical cancer, gastric carcinoma,18–20 and lung cancer.21
Based on our previous studies of prognostic biomarkers in NSCLC patients22,23 and their roles in nucleotide metabolism and DNA repair, we assumed that TS, OPRT, and TP might be potential prognostic markers for resectable NSCLC. However, there have been very few studies of these potential biomarkers in NSCLC; further, previous results have been contradictory,21,24–26 especially for OPRT and TP, and have not confirmed which molecular marker can predict the prognosis of resectable NSCLC.
Therefore, in the present study, we performed a large cohort retrospective clinical study to identify whether TS, OPRT, and TP are useful molecular markers for the prognosis of overall survival (OS) in resected NSCLC patients. We used immunohistochemistry (IHC) to detect the intratumoral expression of these three potential markers and analyzed the correlation between the protein expression of the three potential biomarkers and clinicopathologic factors in resectable NSCLC patients.
Materials and methods
Patients and tissue specimens
The study sample included a total of 178 consecutive NSCLC patients who underwent radical surgery from February 1994 to January 1998 at the Cancer Center of Sun Yat-Sen University. These cases were selected based on the availability of resection tissue and follow-up data. Patients who had previous malignant disease or a second primary tumor were excluded. Tumor tissue samples from these patients were obtained from the surgical pathology archives of the Department of Pathology at the Cancer Center. The study was approved by the medical ethics committee of the Cancer Center of Sun Yat-Sen University.
The pathologic TNM status of NSCLC was assessed according to the criteria of TNM classification of the International Union Against Cancer, 6th edition. Data on tumor differentiation grades and smoking history were missing for the majority of patients. The median age of patients was 60 years (range, 24–94 years), and the male/female ratio was 2.8:1. The patient demographics are listed in Table 1. All patients were followed up regularly until December 2010. The median follow-up time was 64.5±58.7 months, and 117 cancer-related deaths were reported.
In the majority of cases, lobectomy was performed (160 of 178 patients), while 18 patients underwent pneumonectomy. Sixty-four percent of patients (114/178) received neoadjuvant and/or adjuvant chemotherapy/radiotherapy. Systemic chemotherapy was used in adjuvant settings, including mitomycin/vinblastine/cisplatin, etoposide/cisplatin, and tegafur (FT-207).
Construction of tissue microarrays (TMA)
The TMA was constructed according to the method as previously described.27 Briefly, individual donor tissue blocks and the corresponding histological hematoxylin and eosin (H&E)-stained slides were overlaid for tissue TMA sampling. The tissue was sampled using a tissue microarray instrument (Beecher Instruments Inc., Sun Prairie, WI, USA). A 0.6 mm diameter cylinder of tissue was removed and re-embedded into a predetermined position in a recipient paraffin block. Three core samples were selected from each primary tumor. Multiple sections (5 μm thick) were cut from the TMA block and mounted on microscope slides.
Immunohistochemistry
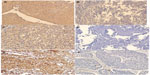
The primary antibodies used in this study were mouse anti-human TS monoclonal antibody (MAB4130, clone TS106, Merck Millipore, Billerica, MA, USA) (1:50 dilution in phosphate-buffered saline [PBS]); Rabbit anti-human OPRT polyclonal antibody (14830-1-AP, Proteintech Group, Chicago, IL, USA) (1:100 dilution in PBS); and mouse anti-human TP monoclonal antibody (ab3135, Abcam plc, Cambridge, UK) (1:50 dilution in PBS). Multiple 5 μm TMA sections were deparaffinized and rehydrated through graded alcohol. Prior to immunostaining, the TMA sections (for TS and OPRT staining) were treated by microwaving for 23 minutes in 10 mM citrate buffer (pH 6.0). For antigen retrieval, tissue slides were boiled in 10 mM citrate buffer (pH 6.0) in a pressure cooker for 10 minutes (for TS and OPRT) or in a microwave for 10 minutes (for TP). The sections were treated with 0.3% hydrogen peroxide for 15 minutes to quench the endogenous peroxidase activity, rinsed in 150 mM PBS (pH 7.6), then incubated with the primary antibodies at 4°C overnight. All incubation was performed in a moist chamber. Subsequently, the slides were incubated with appropriate biotinylated secondary antibodies (PV-6001 Two-Step IHC Detection Reagent, Merck Millipore, Billerica, MA, USA) at a concentration of 1:100 for 30 minutes at 37°C and then reacted with streptavidin-peroxidase conjugate for 30 minutes at 37°C and 3′-3′ diaminobenzidine (DAB; K5007 substrate buffer DAB+ Chromogen, Dako Denmark A/S, Glostrup, Denmark) as a chromogen substrate. The nucleus was counterstained using Meyer’s hematoxylin. A negative control was obtained by replacing the primary antibody with normal murine IgG. The labeling indices for TS, TP, and OPRT proteins were determined by calculating the percentage of immunoreactive cells in more than 500 cancer cells (Figure 1).
TS and OPRT proteins were observed in the cytoplasm, while TP protein was observed in both the cytoplasm and nucleus, by immunostaining. Two independent pathologists (DX and ML) who were blinded to clinicopathologic information, performed the scorings. All sections were scored in a semiquantitative manner according to methods described previously,28 reflecting both the intensity and percentage of stained cells. Intensity was classified as 0 (no staining), t1 (weak staining), t2 (moderate staining), or t3 (strong staining), and grades 0 and 1 were categorized as negative, and grades 2 and 3 as positive for TS, TP, and OPRT.
Statistical analysis
SPSS software (SPSS Standard version 16.0; SPSS Inc., Chicago, IL, USA) was used for the data analysis. OS was defined as the period from the date of surgery to death or last follow-up visit. The chi-square test was used to assess the possible correlation between expression of TS, TP, and OPRT and clinicopathologic parameters. Univariate analysis by the Kaplan–Meier method and log-rank test was carried out by comparing OS and three biomarkers. The relative risks (RRs) of OS associated with TS/TP/OPRT expression status were estimated from univariate Cox proportional hazards model. Multivariate survival analyses of all parameters were used in the Cox regression model. P<0.05 (two-sided) was considered statistically significant.
Results
Expression of TS, OPRT, and TP in NSCLC samples
The evaluation of TS, OPRT, and TP expression was performed blind to clinical outcomes. The noninformative samples, including unrepresentative samples, samples with too few tumor cells (<300 cells per case), and lost samples, were not used in data compilation. IHC results were obtained from the expressions of TS in 169 cases, OPRT in 167 cases, and TP in 147 cases, respectively.
TS staining showed that 97 carcinomas (57.4%) were TS-positive (Figure 1A). The level of TS expression was not correlated to sex, tumor status, nodal status, pathological stage, histology types, or treatment model in the whole NSCLC cohort (P>0.05), but the frequency of TS-positive tumors in patients <60 years was significantly higher than that in patients ≥60 years (65.1% versus 49.4%) (P=0.039) (Table 1). In addition, 90 carcinomas (53.9%) were OPRT-positive tumors (Figure 1C), and 102 carcinomas (69.4%) were TP-positive tumors (Figure 1E). The frequency of OPRT-positive tumors was obviously high in the patients with lower pathologic nodal (pN) stage and lower pathological stage (P=0.041 and P=0.044, respectively) (Table 1), but neither OPRT nor TP expression was correlated to clinicopathologic parameters, such as tumor size, histology types, treatment model, etc (Table 1).
Correlation of TS/OPRT/TP expression with clinicopathologic features and patient survival
In the whole cohort, the 5-year and 10-year survival rates were 42% and 31%, respectively. Using univariate analysis, the TS-positive patients had a significantly higher OS rate than did the TS-negative patients (P=0.003) (Figure 2A and Table 2). Stratified analysis revealed that the positive correlation between TS expression and OS was found in ADC (P=0.005) (Figure 2B) but not in squamous cell carcinoma and adenosquamous cell carcinoma. Furthermore, similar positive correlation was also found using the following parameters: patients <60 years (P=0.028) (Figure 2C), pathologic tumor size (pT)1/T2 (P<0.001) (Figure 2D), pN0/N1 (P<0.010) (Figure 2E), stage II/III (P=0.032) (Figure 2F), and surgery plus chemotherapy and/or radiation (P=0.002) (Figure 2G). Results from hazard ratios (HRs) showed that the TS-negative group had higher risk of death, in the whole group and in subgroups, such as ADC, patients <60 years, pT1/T2, pN0/N1, and stage II/III, surgery plus chemotherapy and/or radiation (Table 3). However, neither OPRT expression nor TP expression was significantly correlated to OS, in the whole cohort and subgroups. In addition, Kaplan–Meier analysis demonstrated that some clinicopathologic prognostic parameters, such as tumor pN status (P<0.001) and clinical stage (P<0.001), were related to patient survival, but no significant correlation was found between survival and other clinicopathologic parameters (data not shown).
Multivariate Cox’s proportional hazards model analyses
Multiple Cox regression was applied with backward elimination for the selection of the prognostic factors for OS and revealed that TS status (TS negative versus positive) (HR =1.604, 95% CI =1.040–2.472, P=0.032) and pN status (N2 versus N0) (HR =2.900, 95% CI =1.777–4.735, P<0.001) were independent prognostic factors for OS. The proportional hazards assumption was adequately met.
Discussion
The present study indicated that TS expression could predict the postoperative survival, especially in NSCLC patients with the following characteristics: <60 years old, stage II/III, ADC, and undergoing surgery plus chemotherapy and/or radiation as adjuvant therapy. However, the present study did not confirm the clinical prognostic biomarkers of TP and OPRT in resectable NSCLC patients. To our knowledge, this work is the first study to investigate the prognostic potential of TS, OPRT, and TP in resectable NSCLC patients, in a big cohort with long follow-up time.
TS is a key enzyme for pyrimidine nucleotide synthesis and DNA-damage repair29 thus it is reasonable to assume that TS might play an important role in regulating the malignant potential in some types of cancers. Moreover, Chu et al revealed that TS protein regulates p53 expression at the translational level.30 Since p53 plays a substantial role in the regulation of cell cycle progression, DNA synthesis, and apoptosis, the regulation of p53 expression by TS may be involved in the clinical correlation of TS expression with prognosis. Many previous studies reported that TS level can predict the overall outcome in patients at an early stage of CRC,31,32 and gastrointestinal and breast carcinomas.33,34 However, the prognostic potential of TS is still controversial, even in CRC patients, with some reports failing to demonstrate a relationship between TS expression and survival.35,36
In NSCLC patients, some studies demonstrated that a high level of TS is related to poor survival;21,24,25 however, the present study found that TS expression was positively correlated to OS. Our result is consistent with the findings from Zheng et al26 that high level of TS protein is significantly related to longer survival in resected NSCLC patients, suggesting that high expression of TS is a prognostic factor for long survival in resectable NSCLC patients. These results were similar to the findings obtained from RRM1 and ERCC1, which are also involved in nucleotide synthesis and DNA damage repair.9 Moreover, univariate analysis of the HR model revealed that TS-negative patients had higher risk of death after complete resection; TS was an independent marker of survival by the multivariate analysis. Interestingly, the expression of TS mRNA (messenger RNA) and/or protein was inversely correlated to OS in breast cancer,37 rectal cancer,38 and gastric cancer.39 The opposite results in different studies may have arisen from the following possible factors: 1) different methodologies – the most common technique used for survival analysis is IHC, but results from reverse-transcription polymerase chain reaction (RT-PCR) used in several studies21,40 reported different correlation results in NSCLC patients;26 2) patient stage and treatments – different from previous findings, this study included NSCLC patients from stage I to stage III, and only a small number of patients (34.8%) were treated with tegafur; and 3) cell proliferation – to our knowledge, although TS could be associated with cell proliferation, the significance of TS level in cancers is still uncertain.41
As we know, patients at a late stage of cancer are associated with poor survival. In the present study, the significant difference between TS expression and patient survival was observed mostly in stage II to III cases, suggesting that TS expression is a good prognostic factor for patients at stage II to III. For stage I NSCLC, TS-positive patients had slightly longer, but not significantly longer OS, which is consistent with the results from Zheng et al.26 Moreover, we found TS-positive patients had significantly longer OS than did TS-negative patients in the small tumor size (pT1/T2) and lower lymph node metastasis (pN0/N1) subgroups. This suggests that TS expression might have been shown to affect the prognosis of stage I patients if we had increased the sample number of stage I. In addition, because of compounding factors in stage III (25% of patients had only received chemotherapy, 32% of patients had only received radiotherapy, 11% of patients had received concurrent radiochemotherapy, and 32% of patients had not received any adjuvant chemotherapy or radiotherapy), whether TS expression is also related to the prognosis of stage II or stage III NSCLC patients needs further investigation.
In the present study, we found that the rate of TS-positive patients was higher in the subgroup of <60 years, indicating that younger patients are associated with longer survival. However, the OS rate in TS-negative patients <60 years was lower than that in TS-positive patients ≥60 years (Table 2), suggesting that TS expression may affect the outcome of OS, regardless of age.
The present study observed that the patients had better OS after adjuvant therapies, suggesting that TS-positive patients may be more sensitive to adjuvant therapies. However, previous studies42,43 found that low TS expression may be an important predictive factor for better treatment efficacy of pemetrexed in NSCLC patients. Another study44 found that patients with high TS expression had longer OS after receiving pacitaxel plus carboplatin chemotherapy. Since the patients in our study did not receive pemetrexed, pacitaxel, and carboplatin as adjuvant therapy, it will be interesting to investigate whether TS expression is associated with the efficacy of adjuvant therapy.
In addition, we also observed that the frequency of OPRT- positive was significantly lower in tumors with lymph node metastasis compared with tumors without lymph node metastasis, and lower in advanced stage than in early stage. These results are similar to those of previous studies.45,46 We failed to find a correlation between OPRT expression and patient survival, and this finding may suggest that OPRT expression may be a marker to predict lymphatic metastasis prior to surgery. Our results did not show a correlation between TP and survival, which is different from a previous study;21 however, in NSCLC patients, the study of the prognostic value of TP has been quite limited.
Conclusion
The present study suggests that TS expression in tumor tissue may be useful for predicting postoperative survival in resectable NSCLC patients, especially in the patients with ADC, stage II/III, age <60 years, and adjuvant therapy. However, further studies are needed to fully elucidate the importance of TS in the prognosis of lung cancer.
Acknowledgments
This project was supported by the Guangdong Province Natural Science Fund (number S2012040007455). We thank all patients and their families for their participation in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
Cox G, Jones JL, Andi A, Waller DA, O’Byrne KJ. A biological staging model for operable non-small cell lung cancer. Thorax. 2001;56(7):561–566. | |
Grondin SC, Liptay MJ. Current concepts in the staging of non-small cell lung cancer. Surg Oncol. 2002;11(4):181–190. | |
Strauss GM, Herndon JE, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26(31):5043–5051. | |
Naruke T, Tsuchiya R, Kondo H, Asamura H. Prognosis and survival after resection for bronchogenic carcinoma based on the 1997 TNM-staging classification: the Japanese experience. Ann Thorac Surg. 2001;71(6):1759–1764. | |
Martin J, Ginsberg RJ, Venkatraman ES, et al. Long-term results of combined-modality therapy in resectable non-small-cell lung cancer. J Clin Oncol. 2002;20(8):1989–1995. | |
Kim YC, Park KO, Kern JA, et al. The interactive effect of Ras, HER2, P53 and Bcl-2 expression in predicting the survival of non-small cell lung cancer patients. Lung Cancer. 1998;22(3):181–190. | |
Strauss GM, Kwiatkowski DJ, Harpole DH, Lynch TJ, Skarin AT, Sugarbaker DJ. Molecular and pathologic markers in stage I non-small-cell carcinoma of the lung. J Clin Oncol. 1995;13(5):1265–1279. | |
Olaussen KA, Dunant A, Fouret P, et al; IALT Bio Investigators. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355(10):983–991. | |
Zheng Z, Chen T, Li X, Haura E, Sharma A, Bepler G. DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. N Engl J Med. 2007;356(8):800–808. | |
Yamachika T, Nakanishi H, Inada K, et al. A new prognostic factor for colorectal carcinoma, thymidylate synthase, and its therapeutic significance. Cancer. 1998;82(1):70–77. | |
Kuniyasu T, Nakamura T, Tabuchi Y, Kuroda Y. Immunohistochemical evaluation of thymidylate synthase in gastric carcinoma using a new polyclonal antibody: the clinical role of thymidylate synthase as a prognostic indicator and its therapeutic usefulness. Cancer. 1998;83(7):1300–1306. | |
Ochiai T, Nishimura K, Noguchi H, et al. Prognostic impact of orotate phosphoribosyl transferase activity in resectable colorectal cancers treated by 5-fluorouracil-based adjuvant chemotherapy. J Surg Oncol. 2006;94(1):45–50. | |
Nio Y, Toga T, Maruyama R, Fukushima M. Expression of orotate phosphoribosyl transferase in human pancreatic cancer: implication for the efficacy of uracil and tegafur-based adjuvant chemotherapy. Oncol Rep. 2007;18(1):59–64. | |
Mizutani Y, Wada H, Yoshida O, Fukushima M, Nakanishi H, Miki T. Significance of orotate phosphoribosyltransferase activity in renal cell carcinoma. J Urol. 2004;171(2 Pt 1):605–610. | |
Toi M, Atiqur Rahman M, Bando H, Chow LW. Thymidine phosphorylase (platelet-derived endothelial-cell growth factor) in cancer biology and treatment. Lancet Oncol. 2005;6(3):158–166. | |
Kamoshida S, Shiogama K, Shimomura R, et al. Immunohistochemical demonstration of fluoropyrimidine-metabolizing enzymes in various types of cancer. Oncol Rep. 2005;14(5):1223–1230. | |
Takebayashi Y, Akiyama S, Akiba S, et al. Clinicopathologic and prognostic significance of an angiogenic factor, thymidine phosphorylase, in human colorectal carcinoma. J Natl Cancer Inst. 1996;88(16):1110–1117. | |
Arima J, Imazono Y, Takebayashi Y, et al. Expression of thymidine phosphorylase as an indicator of poor prognosis for patients with transitional cell carcinoma of the bladder. Cancer. 2000;88(5):1131–1138. | |
Hata K, Takebayashi Y, Iida K, Fujiwaki R, Fukumoto M, Miyazaki K. Expression of thymidine phosphorylase in human cervical cancer. Anticancer Res. 1999;19(1B):709–716. | |
Takebayashi Y, Miyadera K, Akiyama S, et al. Expression of thymidine phosphorylase in human gastric carcinoma. Jpn J Cancer Res. 1996; 87(3):288–295. | |
Grimminger PP, Schneider PM, Metzger R, et al. Low thymidylate synthase, thymidine phosphorylase, and dihydropyrimidine dehydrogenase mRNA expression correlate with prolonged survival in resected non-small-cell lung cancer. Clin Lung Cancer. 2010;11(5):328–334. | |
He LR, Zhao HY, Li BK, et al. Overexpression of AIB1 negatively affects survival of surgically resected non-small-cell lung cancer patients. Ann Oncol. 2010;21(8):1675–1681. | |
He LR, Zhao HY, Li BK, et al. Overexpression of eIF5A-2 is an adverse prognostic marker of survival in stage I non-small cell lung cancer patients. Int J Cancer. 2011;129(1):143–150. | |
Nakagawa T, Tanaka F, Otake Y, et al. Prognostic value of thymidylate synthase expression in patients with p-stage I adenocarcinoma of the lung. Lung Cancer. 2002;35(2):165–170. | |
Miyoshi T, Kondo K, Toba H, et al. Predictive value of thymidylate synthase and dihydropyrimidine dehydrogenase expression in tumor tissue, regarding the efficacy of postoperatively administered UFT (tegafur + uracil) in patients with non-small cell lung cancer. Anticancer Res. 2007;27(4C):2641–2648. | |
Zheng Z, Li X, Schell MJ, et al. Thymidylate synthase in situ protein expression and survival in stage I nonsmall-cell lung cancer. Cancer. 2008;112(12):2765–2773. | |
Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4(7):844–847. | |
McCarty KS, Szabo E, Flowers JL, et al. Use of a monoclonal anti-estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Cancer Res. 1986;46(Suppl 8):4244s–4248s. | |
Danenberg PV. Thymidylate synthetase – a target enzyme in cancer chemotherapy. Biochim Biophys Acta. 1977;473(2):73–92. | |
Chu E, Copur SM, Ju J, et al. Thymidylate synthase protein and p53 mRNA form an in vivo ribonucleoprotein complex. Mol Cell Biol. 1999;19(2):1582–1594. | |
Ichikawa W, Uetake H, Shirota Y, et al. Combination of dihydropyrimidine dehydrogenase and thymidylate synthase gene expressions in primary tumors as predictive parameters for the efficacy of fluoropyrimidine-based chemotherapy for metastatic colorectal cancer. Clin Cancer Res. 2003;9(2):786–791. | |
Edler D, Kressner U, Ragnhammar P, et al. Immunohistochemically detected thymidylate synthase in colorectal cancer: an independent prognostic factor of survival. Clin Cancer Res. 2000;6(2):488–492. | |
Tsujitani S, Konishi I, Suzuki K, et al. Expression of thymidylate synthase in relation to survival and chemosensitivity in gastric cancer patients. J Exp Clin Cancer Res. 2000;19(2):189–195. | |
Pestalozzi BC, Peterson HF, Gelber RD, et al. Prognostic importance of thymidylate synthase expression in early breast cancer. J Clin Oncol. 1997;15(5):1923–1931. | |
Findlay MP, Cunningham D, Morgan G, Clinton S, Hardcastle A, Aherne GW. Lack of correlation between thymidylate synthase levels in primary colorectal tumours and subsequent response to chemotherapy. Br J Cancer. 1997;75(6):903–909. | |
Yamada H, Iinuma H, Watanabe T. Prognostic value of 5-fluorouracil metabolic enzyme genes in Dukes’ stage B and C colorectal cancer patients treated with oral 5-fluorouracil-based adjuvant chemotherapy. Oncol Rep. 2008;19(3):729–735. | |
Nishimura R, Nagao K, Miyayama H, et al. Thymidylate synthase levels as a therapeutic and prognostic predictor in breast cancer. Anticancer Res. 1999;19(6C):5621–5626. | |
Johnston PG, Fisher ER, Rockette HE, et al. The role of thymidylate synthase expression in prognosis and outcome of adjuvant chemotherapy in patients with rectal cancer. J Clin Oncol. 1994;12(12):2640–2647. | |
Lenz HJ, Leichman CG, Danenberg KD, et al. Thymidylate synthase mRNA level in adenocarcinoma of the stomach: a predictor for primary tumor response and overall survival. J Clin Oncol. 1996;14(1):176–182. | |
Hashimoto H, Ozeki Y, Sato M, et al. Significance of thymidylate synthase gene expression level in patients with adenocarcinoma of the lung. Cancer. 2006;106(7):1595–1601. | |
Allegra CJ, Parr AL, Wold LE, et al. Investigation of the prognostic and predictive value of thymidylate synthase, p53, and Ki-67 in patients with locally advanced colon cancer. J Clin Oncol. 2002;20(7):1735–1743. | |
Liu Y, Yin TJ, Zhou R, Zhou S, Fan L, Zhang RG. Expression of thymidylate synthase predicts clinical outcomes of pemetrexed-containing chemotherapy for non-small-cell lung cancer: a systemic review and meta-analysis. Cancer Chemother Pharmacol. 2013;72(5):1125–1132. | |
Nicolson MC, Fennell DA, Ferry D, et al. Thymidylate synthase expression and outcome of patients receiving pemetrexed for advanced nonsquamous non-small-cell lung cancer in a prospective blinded assessment phase II clinical trial. J Thorac Oncol. 2013;8(7):930–939. | |
Nakagawa Y, Shimizu T, Takahashi N, Hashimoto S. Impact of thymidylate synthase protein expression on efficacy of chemotherapy in advanced lung cancer patients. Mol Clin Oncol. 2013;1(3):411–417. | |
Ochiai T, Sugitani M, Nishimura K, et al. [Correlation between clinical pathophysiologic factors and expression of orotate phosphoribosyl transferase (OPRT), thymidylate synthase (TS), and dihydropyrimidine dehydrogenase (DPD) in colorectal cancer]. Gan To Kagaku Ryoho. 2002;29(3):413–420. Japanese. | |
Ishikawa M, Miyauchi T, Kashiwagi Y. Clinical implications of thymidylate synthetase, dihydropyrimidine dehydrogenase and orotate phosphoribosyl transferase activity levels in colorectal carcinoma following radical resection and administration of adjuvant 5-FU chemotherapy. BMC Cancer. 2008;8:188. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.