Back to Journals » Cancer Management and Research » Volume 12
Prognostic Significance of the Preoperative Lymphocyte to Monocyte Ratio in Patients with Gallbladder Carcinoma
Authors Xu W , Wu X, Wang X, Yu S , Xu G, Xiong J , Zhang J , Sang X , Zheng Y, Liu W
Received 22 December 2019
Accepted for publication 23 April 2020
Published 11 May 2020 Volume 2020:12 Pages 3271—3283
DOI https://doi.org/10.2147/CMAR.S243326
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Weiyu Xu,1,* Xiaoqian Wu,2,* Xuezhu Wang,2,* Si Yu,2 Gang Xu,2 Jianping Xiong,3 Junwei Zhang,2 Xinting Sang,2 Yongchang Zheng,2 Wei Liu1
1Department of General Surgery, Beijing Friendship Hospital, Capital Medical University, Beijing 100050, People’s Republic of China; 2Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (CAMS & PUMC), Beijing 100730, People’s Republic of China; 3Department of Interventional Radiology, Beijing Friendship Hospital, Capital Medical University, Beijing 100050, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yongchang Zheng
Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (CAMS & PUMC), 1 Shuaifuyuan, Wangfujing, Beijing 100730, People’s Republic of China
Email [email protected]
Wei Liu
Department of Human Anatomy, Histology and Embryology, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, 9 Dongdan Santiao, Dongcheng District, Beijing 100005, People’s Republic of China
Email [email protected]
Background: This study was designed to investigate the prognostic value of the lymphocyte to monocyte ratio (LMR) in patients with gallbladder carcinoma (GBC).
Patients and Methods: We retrospectively enrolled 154 consecutive GBC patients from 2005 to 2017 in this study. The LMR of preoperative blood samples was calculated by dividing the lymphocyte count by the monocyte count. A receiver operating characteristic (ROC) curve was employed to identify the optimal cut-off value of the LMR in the determination of overall survival (OS). The Kaplan–Meier method was utilized to assess OS, and the Log rank test was employed to compare survival differences. Univariate and multivariate Cox regression analyses were conducted to detect independent prognostic indicators.
Results: The optimal cut-off point for the LMR was 4.76 according to the ROC curve. Patients ≤ 60 years old with an LMR ≤ 4.76 experienced significantly worse OS than those with an LMR > 4.76 (hazard ratio (HR): 0.399, 95% confidence interval (CI): 0.265– 0.602, P< 0.001); however, the prognostic value of the LMR was not determined in patients > 60 years old or among the entire study cohort (both P> 0.05). Significantly poorer OS was observed in patients > 60 years with an LMR ≤ 4.21 compared to those with an LMR > 4.21 (HR: 1.830, 95% CI: 1.129– 2.967, P=0.014). Multivariate Cox regression analysis indicated that both the high and low LMR cut-off values were independent risk factors for OS (HR: 0.272, 95% CI: 0.105– 0.704, P=0.007; HR: 0.544, 95% CI: 0.330– 0.895, P=0.017).
Conclusion: The LMR is an independent prognostic indicator for GBC patients, the cut-off value of which is age dependent.
Keywords: gallbladder cancer, lymphocyte to monocyte ratio, LMR, survival, prognosis, biomarker
Introduction
Gallbladder cancer (GBC), originating from the bile duct epithelium, ranks first in the biliary tract and sixth in the gastrointestinal tract in terms of malignancy incidence, accounting for 80–95% of all biliary tract malignancies.1,2 Due to its profound aggressiveness and the absence of specific symptoms during the early stage of disease, the poor prognosis of GBC remains a challenge despite great progress in surgical intervention as well as adjuvant chemotherapy. The majority of GBCs are unresectable at initial presentation. The 5-year survival rate of GBC patients is only approximately 5%, and their overall median survival time is less than 1 year.3,4 At present, the UICC tumor node metastasis (TNM) staging classification (7th edition) is the most reliable approach for prognostic progression and is widely applied in clinical practice. Nevertheless, the prognosis varies greatly even among patients with the same TNM stage. To this end, it is necessary to detect more reliable indicators to accurately predict tumor recurrence and patient survival.
The systemic inflammatory response is critically involved in the pathogenesis and progression of multiple malignancies.5,6 On the one hand, cells involved in the inflammatory process can generate proangiogenic molecules as well as inflammatory mediators, thereby creating a procancer microenvironment that enhances vascular invasion but suppresses the host immune response.5–7 On the other hand, tumors are capable of releasing cytokines to affect the activity of blood cells related to inflammation and immunity, such as monocytes, lymphocytes, neutrophils and platelets.8,9 As a marker of the systemic inflammatory response, the correlation between the lymphocyte to monocyte ratio (LMR), which is calculated as a simple ratio of total lymphocytes and total monocytes, and patient survival has recently been investigated in various types of cancer.10–17 As a result, most reports have suggested that the LMR is an independent risk factor for malignancy, whereas a few studies18–20 have suggested that either the LMR harbors no prognostic significance for malignant tumors or that the prognostic significance of the LMR should be judged on a situational basis.
To our knowledge, there have been no reports to date concerning the prognostic value of the LMR in GBC patients. Hence, the aim of our study was to evaluate the role of the preoperative peripheral blood LMR on the long-term postoperative outcomes of GBC patients.
Patients and Methods
Patient Enrollment
In total, 154 GBC patients undergoing surgery at the Department of Liver Surgery at Peking Union Medical Hospital (PUMH) between January 2005 and May 2017 were enrolled in this retrospective study. Eligible subjects were included according to the following criteria: 1) age >18 years old; (2) histologically confirmed GBC; (3) no adjuvant chemotherapy or radiotherapy prior to gallbladder resection; and (4) performance status of 0 or 1. The exclusion criteria were as follows: (1) unavailable pretreatment hematologic parameters or loss during follow-up; (2) distant metastasis at the initial visit; (3) presence of other types of cancer; (4) complications with an active infection or any hematologic disease; or (5) medication with any immunosuppressive agent.
Data Collection
Patient demographics and clinicopathological characteristics, including age, sex, medical comorbidities, complete blood cell count, liver function parameters, CA199 value, histological differentiation, pathological type, T stage, lymph node involvement, distant metastasis, TNM stage, margin status, and maximal tumor diameter, were extracted from the clinical records by one researcher and reviewed by another researcher. Histopathological as well as clinical stages were determined by postoperative histopathological results and clinical evaluations according to the AJCC TNM stage (8th edition) or the UICC TNM classification. The preoperative lymphocytes and monocytes in peripheral blood were isolated from the complete peripheral blood. Moreover, the LMR was defined by dividing the lymphocyte count by the monocyte count. The definition of jaundice was a serum bilirubin concentration greater than 34.2 μmol/L.
Ethical Considerations
The current study conformed to the provisions of the Declaration of Helsinki and gained approval from the Medical Ethics Committee at Peking Union Medical College Hospital of Chinese Academy of Medical Sciences and PUMC (CAMS & PUMC). All subjects were well informed of the investigational property of this study and signed written informed consent.
LMR
Preoperative blood specimens were collected after diagnosis and before surgery. A Sysmex XE-5000 automated hematology analyzer™ (Sysmex, Kobe, Japan) was employed to analyze the differential white blood cell count in line with the manufacturer’s instructions. The LMR was calculated as described in the data collection section.
Treatment and Follow-Up
Radical cholecystectomy or palliative cholecystectomy was performed for each eligible GBC patient. Follow-up was conducted either by an outpatient visit or by a telephone interview, which was performed every three months during the first two years, every six months during the next three years, and annually after five years. The follow-up period was from the date of surgery to the date of either death or the last follow-up. Follow-up data of all enrolled patients were available.
Statistical Analysis
Continuous data are shown as the mean ± standard deviation for normally distributed data (Kolmogorov–Smirnov test, P>0.05) and as the median (min-max) for abnormally distributed data. Categorical data are shown as frequencies and percentages. The χ2 test or Fisher’s exact test was employed to determine the correlation of qualitative data with the LMR, whereas an independent Student’s t-test was utilized to analyze quantitative data. Receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cut-off values for the LMR. Overall survival (OS) was defined from the date of surgery to the date of death. OS and survival curves were plotted as Kaplan-Meier curves by using the Log rank test. The multivariate Cox regression model was carried out to evaluate the hazard ratios (HRs). SPSS version 24.0 (IBM Corp., Armonk, NY) was used for statistical analysis. A two-sided P value <0.05 was considered statistically significant.
Results
Optimal Cut-Off Value of the Continuous LMR for Survival Analysis
An ROC curve for OS was plotted to verify the optimal cut-off value for the continuous LMR (Figure 1). As shown in Figure 1, the area under the curve (AUC) was recorded as 0.701 (95% CI: 0.618–0.785), and the optimal LMR cut-off value for OS was 4.76, with the highest sensitivity and specificity being 0.647 and 0.721, respectively. Based on this value, there were 86 patients (55.8%) with an LMR ≤4.76 and 68 patients (44.2%) with an LMR >4.76 (Table 1).
 |
Table 1 Baseline Characteristics of 154 Patients Who Underwent Potential Curative Cholecystectomy |
Patient Baseline Characteristics
The median follow-up time was 17 months. There were 103 patients who died during the follow-up period, with an estimated median OS duration of 14.5 months (range: 0.5–153.0 months). The 1-year and 2-year survival rates were 55.8% and 35.7%, respectively. A total of 154 patients with primary GBC were included in our study, among whom 63 (40.9%) were male and 91 (59.1%) were female. The median age at diagnosis was 64 years (range: 29–85 years), and 98 (63.6%) patients were >60 years old. A total of 38 (24.7%) patients had a diagnosis of diabetes before surgery, and 75 (48.7%) had a history of gallstones before surgery. One hundred fifty patients (97.4%) were pathologically diagnosed with adenocarcinoma carcinoma, 3 (1.9%) with adenosquamous cell carcinoma and 1 (0.6%) with papillary carcinoma. Ninety-four (61.0%) patients were histologically diagnosed with moderate or well differentiated disease, and 60 (39.0%) were diagnosed with poorly differentiated disease. There were 58 (37.7%) patients with a positive resection margin. According to the TNM staging methods, 16 (10.4%) patients were diagnosed with stage 0 or I tumors, 16 (10.4%) with stage IIA-IIB tumors, 92 (59.7%) with stage IIIA-IIIB tumors, and 30 (19.5%) with stage IVA-IVB tumors. The detailed baseline characteristics of the patients are listed in Table 1.
Correlations Between the Preoperative LMR and Clinicopathological Factors
Based on the cut-off value, all patients were dichotomized into the low value group or the high value group. The characteristics of patients in the high and low LMR groups were compared (Table 2). We found a significant relationship between an elevated LMR and sex (P=0.032), resection margin (P<0.001), degree of differentiation (P=0.013), N stage (P=0.003), T stage (P<0.001), CA199 value (P=0.033) and TNM stage (P<0.001). In contrast, no significant associations were observed between the LMR and age, gallstone history, comorbid diabetes, pathological type, jaundice, distant metastasis, ABO blood group, or tumor size (P>0.05).
 |
Table 2 Correlation Between LMR and Clinicopathological Characteristics in GBC Patients |
Comparison of the Clinicopathological Characteristics and LMR in Relation to OS Postoperation
In the univariate Cox analysis, resection margin (HR: 3.683, 95% CI: 2.468–5.496, P<0.001), distant metastasis (HR: 2.550, 95% CI: 1.388–4.684, P=0.003), jaundice (HR: 2.598, 95% CI: 1.644–4.106, P<0.001), CA199 value (HR: 3.125, 95% CI: 2.010–4.858, P<0.001), lymph node metastasis (P<0.001), degree of differentiation (HR: 1.527, 95% CI: 1.031–2.261, P=0.035), T stage (P<0.001), TNM stage (P<0.001) and LMR (HR: 0.399, 95% CI: 0.265–0.602, P<0.001) were significant prognostic factors for OS (Table 3), whereas sex, age, gallstone history, comorbid diabetes, pathological type, ABO blood group and maximum tumor diameter were not significant predictors of OS (P>0.05; Table 3). In the multivariate Cox regression analysis, age (HR: 1.533, 95% CI: 1.015–2.374, P=0.042), margin status (HR: 2.350, 95% CI: 1.537–3.595, P<0.001), TNM stage (P=0.008) and CA199 (HR: 1.712, 95% CI: 1.074–2.727, P<0.001) were independent risk factors for poor OS (Table 4).
 |
Table 3 Univariate Analysis of Overall Survival in GBC Patients |
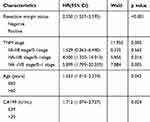 |
Table 4 Multivariate Analysis for Overall Survival in GBC Patients |
Subgroup Analysis for Prognostic Factors Influencing OS
When we divided the GBC patients according to age, we found that for the younger subgroup (≤60 years old), the LMR (HR: 0.168, 95% CI: 0.070–0.400, P<0.001), margin status (HR: 4.428, 95% CI: 2.117–9.261, P<0.001), jaundice (HR: 3.231, 95% CI: 1.480–7.053, P=0.003), CA199 value (HR: 4.722, 95% CI: 2.097–10.633, P<0.001), N stage (P=0.017), T stage (P=0.007) and TNM stage (P=0.017) were significant prognostic factors for OS in the univariate analysis (Table 5). A CA199 value >39 (HR: 2.671, 95% CI: 1.088–6.588, P=0.032) and an LMR ≤4.76 (HR: 0.272, 95% CI: 0.105–0.704, P=0.007) were independent risk factors for poor OS in the multivariate analysis (Table 5). The survival curve stratified by the LMR showed that OS was longer in GBC patients with an LMR >4.76 than in those with an LMR ≤4.76 (Figure 2). For the older subgroup (>60 years old), the univariate analysis indicated that the LMR (HR: 0.546, 95% CI: 0.337–0.885, P=0.014), margin status (HR: 3.627, 95% CI: 2.224–5.917, P<0.001), distant metastasis (HR: 2.934, 95% CI: 1.480–5.815, P=0.002), jaundice (HR: 2.279, 95% CI: 1.288–4.033, P=0.005), CA199 value (HR: 2.417, 95% CI: 1.431–4.084, P=0.001), N stage (P=0.007), T stage (P=0.027) and TNM stage (P<0.001) were significant prognostic factors for OS (Table 6). A CA199 value >39 (HR: 2.137, 95% CI: 1.250–3.651, P=0.005), margin status (HR: 3.299, 95% CI: 1.985–5.483, P<0.001), distant metastasis (HR: 3.117, 95% CI: 1.554–6.256, P=0.001), and degree of differentiation (HR: 1.726, 95% CI: 1.048–2.843, P=0.032) were independent risk factors for poor OS in the multivariate analysis (Table 6).
 |
Table 5 Univariate and Multivariate Analysis for Overall Survival in GBC Patients with Age ≤60 Years |
 |
Table 6 Univariate and Multivariate Analysis for Overall Survival in GBC Patients with Age>60 Years |
 |
Figure 2 Survival curve according to the presence of preoperative LMR. Data compares LMR>4.76 vs.≤4.76 group (P<0.05). Abbreviation: LMR, lymphocyte to monocyte ratio. |
When we divided GBC patients according to TNM stage, we found that the LMR was not an independent risk factor for patients with early-stage (0/I/II) (P=0.994) or advanced-stage (III/IV stage) disease (P=0.147) based on the multivariate Cox regression analysis.
LMR Cut-Off Value in GBC Patients >60 Years Old and Its Prognostic Significance in This Patient Subgroup
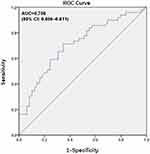
Because the LMR cut-off value of 4.76 was not significantly associated with OS (P=0.099) in patients >60 years old, we identified a new cut-off value for elderly patients according to the ROC curve for OS. As shown in Figure 3, the AUC was 0.708 (95% CI: 0.606–0.811), and the optimal LMR cut-off value for OS was 4.21, with the highest sensitivity and specificity being 0.714 and 0.653, respectively. In the multivariate analysis, an LMR ≤4.21 (HR: 0.544, 95% CI: 0.330–0.895, P=0.017) margin status (HR: 3.179, 95% CI: 1.904–5.306, P<0.001), distant metastasis (HR: 3.262, 95% CI: 1.620–6.567, P=0.001) and CA199 value >39 (HR: 2.057, 95% CI: 1.221–3.490, P=0.007) were independent risk factors for unfavorable OS (Table 7). The survival curve stratified by the LMR showed that OS was longer in GBC patients with an LMR >4.21 than in patients with an LMR ≤4.21 (Figure 4).
 |
Table 7 Multivariate Analysis for Overall Survival in GBC Patients with Age>60 Years According to the New Cut-off Value (4.21) |
 |
Figure 4 Survival curve according to the presence of preoperative LMR. Data compares LMR>4.21 vs.≤4.21 group (P<0.05). Abbreviation: LMR, lymphocyte to monocyte ratio. |
Discussion
To the best of our knowledge, this is the first retrospective study to evaluate the prognostic significance of the LMR in patients with GBC who underwent potentially curative resection and to describe this relationship with respect to the age of the GBC patients. In the present study, we found that the prognostic significance of the LMR in GBC patients was age dependent. In patients ≤60 years old, LMR values ≤4.76 were correlated with unfavorable OS, but this cut-off value was not associated with a difference in OS in patients >60 years old. However, LMR values ≤4.21 were associated with inferior OS in patients >60 years old. The optimal cut-off value in elderly patients was significantly lower than that in nonelderly patients.
The LMR is an inflammation- and immunity-related biomarker defined as the ratio of lymphocytes to monocytes (i.e., lymphocytes divided by monocytes). This ratio reflects both lymphopenia, which results from a weak, insufficient immunologic reaction to the tumor,21 and high levels of monocytes and macrophages present in the tumor microenvironment, which support cancer cell invasion, migration, intravasation, and angiogenesis and can even lead to the suppression of the antitumor immune reaction.22,23 Therefore, the LMR, which can be easily obtained from a peripheral blood test, is drawing increasing attention as a prognostic marker in multiple cancers.
In contrast to most published studies15,24-27 suggesting that the LMR is an independent prognostic factor, our study found that the prognostic value of the LMR is age dependent in GBC patients and that different age subgroups have different cut-off values. A single and unique LMR cut-off value has no predictive value for the prognosis of patients with GBC. These conclusions were consistent with the results of studies conducted by Wu et al,18 Koh et al19 and Giacomelli et al.20 Wu et al reported that the LMR could not predict the prognosis of nonmetastatic rectal cancer patients (HR: 1.034, 95% CI: 0.682–1.566, P=0.876) despite its status as an easy obtainable and highly efficient laboratory inflammatory marker. Koh et al19 described patients with diffuse large B cell lymphoma (DLBCL) and reported conclusions similar to ours, which indicated that the prognostic significance of the LMR in DLBCL patients was age dependent, as the LMR was significantly different between DLBCL patients younger than and at least 70 years of age who received rituximab plus CHOP (R-CHOP) therapy (<30.4 VS <2.36), and the LMR values were significantly lower in the ≥70-year-old group than in the <70-year-old group. Therefore, they suggested different LMR cut-off values to distinguish high-risk and low-risk groups among elderly patients with DLBCL. However, Giacomelli et al20 demonstrated that the LMR values were variable during the perioperative period in patients with pancreatic cancer and showed that changes in the LMR during the treatment course could predict OS.
The exact underlying mechanism for this phenomenon is still unclear. These observations are inconsistent with the conception that we usually hold: different subtypes of lymphocytes and monocytes have different effects on tumors. Even the same type of lymphocyte or monocyte exerts different functions. Previous studies have shown that different subtypes of lymphocytes could exert a positive,28 negative29 or neutral30 influence on nonmetastatic rectal cancer. However, some studies have indicated that monocytes and their progeny can promote31 or suppress32 the migration and invasion of tumor cells. Perhaps the unclear relationship among lymphocytes, monocytes and the prognosis of malignant cancers leads to the inability of the LMR to effectively predict the prognosis of tumor patients.
When we carefully analyzed and reviewed a meta-analysis by Nishijima et al in 201533 regarding the relationship between the LMR and tumors, we found that the optimal LMR cut-off values differed with the average ages of the study population. Among the included studies, Jiang et al34 reported a prognostic impact of the LMR in patients with nasopharyngeal carcinoma with a median age of 51 years using an LMR cut-off value of 5.22. Chang et al35 reported prognostic significance for the LMR in patients with renal cell carcinoma with a median age of 56 years using an LMR cut-off value of 4.44. Another study conducted by Temraz et al36 described the prognostic significance of the LMR in patients with bladder cancer with a median age of 65 years using the LMR cut-off value of 2.81. The results of the studies listed above, which are consistent with those of our study, indicate that the LMR value is inversely proportional to the median or mean age of the study population.
The cause of this phenomenon may be that aging could affect the volume and function of lymphocytes and monocytes. A previous study37 indicated that the total number of lymphocytes was significantly lower in elderly people than in the overall adult population; however, some studies showed that the number of monocytes was normal38 or even increased39 in elderly people relative to the respective values in normal healthy adults. The reduced lymphocyte count and increased monocyte count can inhibit immune system function, causing tumor cells to migrate, invade and metastasize.
However, there are some limitations to this study. First, this was a single-center retrospective study that lacked prospective data. Second, the sample size was relatively small, which would inevitably generate uncontrolled and unrecognized bias. Third, this study included some recent patients with only a short follow-up period. Therefore, prospective, multicenter studies with a large sample size are needed to further evaluate the clinical applicability and deficiencies of our results.
Conclusions
In conclusion, the findings of our study demonstrate that the prognostic value of the LMR in GBC patients after surgery is age dependent. The optimal cut-off value of the LMR in GBC patients is significantly different between patients ≤60 years old and those >60 years old. Therefore, we recommend different LMR cut-off values according to patient age in future clinical applications. However, the true prognostic value of the LMR in predicting the outcome of GBC patients still requires further validation.
Abbreviations
AUC, area under the curve; CAMS & PUMC, Chinese Academy of Medical Sciences and Peking Union Medical College; CI, confidence interval; DLBCL, diffuse large B cell lymphoma; GBC, gallbladder carcinoma; HR, hazard ratio; LMR, lymphocyte to monocyte ratio; OS, overall survival; ROC, receiver operating characteristic; TNM, tumor node metastasis; UICC, The Union for International Cancer Control.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics and Consent Statement
This study was approved by the hospital medical ethics committee of the Peking Union Medical College Hospital of the Chinese Academy of Medical Sciences and Peking Union Medical College (CAMS & PUMC). Every patient provided written informed consent before enrollment.
Consent for Publication
Written informed consent for publication was obtained.
Author Contributions
(I) Conception and design: W Xu; (II) Administrative support: Y Zheng, W Liu; (III) Provision of study materials or patients: Y Zheng, X Sang; (IV) Collection and assembly of data: X Wu, S Yu, G Xu, J Xiong, J Zhang; (V) Data analysis and interpretation: W Xu, X Wu, X Wang; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from the Beijing Natural Science Foundation (No. L172055), Beijing Municipal Science & Technology Commission research fund (No. Z171100000417004), WBE Liver Fibrosis Foundation (CFHPC 2020021), and Beijing Dongcheng District outstanding talent funding project.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Lin N, Yao Z, Xu M, et al. Long noncoding RNA MALAT1 potentiates growth and inhibits senescence by antagonizing ABI3BP in gallbladder cancer cells. J Exp Clin Cancer Res. 2019;38(1):244. doi:10.1186/s13046-019-1237-5
2. Hundal R, Shaffer E. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99–109. doi:10.2147/CLEP.S37357
3. Li M, Liu F, Zhang F, et al. Genomic ERBB2/ERBB3 mutations promote PD-L1-mediated immune escape in gallbladder cancer: a whole-exome sequencing analysis. Gut. 2019;68(6):1024–1033. doi:10.1136/gutjnl-2018-316039
4. Cao Y, Liang H, Zhang F, et al. Prohibitin overexpression predicts poor prognosis and promotes cell proliferation and invasion through ERK pathway activation in gallbladder cancer. J Exp Clin Cancer Res. 2016;35(1):68. doi:10.1186/s13046-016-0346-7
5. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi:10.1016/S0140-6736(00)04046-0
6. Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
7. Grivennikov SI. Immunity, Inflammation, and Cancer. Cell. 2010;6(140).
8. Lippi G, Meschi T, Nouvenne A, Mattiuzzi C, Borghi L. Neutrophil gelatinase-associated lipocalin in cancer. Adv Clin Chem. 2014;64:179–219.
9. Del Prete A, Allavena P, Santoro G, et al. Molecular pathways in cancer-related inflammation. Biochem Med (Zagreb). 2011;21(3):264–275. doi:10.11613/BM.2011.036
10. Ying H-Q, Deng Q-W, He B-S, et al. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014;31(12):305. doi:10.1007/s12032-014-0305-0
11. Deng Q, He B, Liu X, et al. Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. J Transl Med. 2015;13(1):66. doi:10.1186/s12967-015-0409-0
12. Han L-H, Jia Y-B, Song Q-X, et al. Prognostic significance of preoperative lymphocyte-monocyte ratio in patients with resectable esophageal squamous cell carcinoma. Asian Pac J Cancer Prev. 2015;16(6):2245–2250. doi:10.7314/APJCP.2015.16.6.2245
13. Qi Q, Geng Y, Sun M, et al. Clinical implications of systemic inflammatory response markers as independent prognostic factors for advanced pancreatic cancer. Pancreatology. 2015;15(2):145–150. doi:10.1016/j.pan.2014.12.004
14. Koh YW, Jung SJ, Yoon DH, et al. The absolute lymphocyte to monocyte ratio is associated with poor prognosis in classical Hodgkin lymphoma patients younger than 60 years of age. Hematol Oncol. 2015;33(3):133–140. doi:10.1002/hon.2155
15. Lin G-N, Peng J-W, Xiao -J-J, et al. Prognostic impact of circulating monocytes and lymphocyte-to-monocyte ratio on previously untreated metastatic non-small cell lung cancer patients receiving platinum-based doublet. Med Oncol. 2014;31(7):70. doi:10.1007/s12032-014-0070-0
16. Anand M, Chodda SK, Parikh PM, et al. Abnormal levels of proinflammatory cytokines in serum and monocyte cultures from patients with chronic myeloid leukemia in different stages, and their role in prognosis. Hematol Oncol. 1998;16(4):143–154. doi:10.1002/(SICI)1099-1069(199812)16:4<143::AID-HON628>3.0.CO;2-U
17. Hu P, Shen H, Wang G, et al. Prognostic significance of systemic inflammation-based lymphocyte- monocyte ratio in patients with lung cancer: based on a large cohort study. PLoS One. 2014;9(10):e108062. doi:10.1371/journal.pone.0108062
18. Wu Q-B, Wang M, Hu T, et al. Prognostic role of the lymphocyte-to-monocyte ratio in patients undergoing resection for nonmetastatic rectal cancer. Medicine (Baltimore). 2016;95(44):e4945. doi:10.1097/MD.0000000000004945
19. Koh YW, Park C-S, Yoon DH, et al. Should the cut-off values of the lymphocyte to monocyte ratio for prediction of prognosis in diffuse large B-cell lymphoma be changed in elderly patients? Eur J Haematol. 2014;93(4):340–348. doi:10.1111/ejh.12354
20. Giacomelli I, Scartoni D, Mohammadi H, et al. Does lymphocyte-to-monocyte ratio before, during, or after definitive chemoradiation for locally advanced pancreatic cancer predict for clinical outcomes? J Gastrointest Oncol. 2017;8(4):721–727. doi:10.21037/jgo.2017.06.14
21. Yoshikawa T, Saito H, Osaki T, et al. Elevated Fas expression is related to increased apoptosis of circulating CD8+ T cell in patients with gastric cancer. J Surg Res. 2008;148(2):143–151. doi:10.1016/j.jss.2007.07.011
22. Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. doi:10.1038/nrc1256
23. Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. doi:10.1016/j.cell.2006.01.007
24. Watanabe R, Tomita N, Itabashi M, et al. Peripheral blood absolute lymphocyte/monocyte ratio as a useful prognostic factor in diffuse large B-cell lymphoma in the rituximab era. Eur J Haematol. 2014;92(3):204–210. doi:10.1111/ejh.12221
25. Liu T, Fang X-C, Ding Z, et al. Pre-operative lymphocyte-to-monocyte ratio as a predictor of overall survival in patients suffering from osteosarcoma. FEBS Open Bio. 2015;5(1):682–687. doi:10.1016/j.fob.2015.08.002
26. Marín Hernández C, Piñero Madrona A, Gil Vázquez PJ, et al. Usefulness of lymphocyte-to-monocyte, neutrophil-to-monocyte and neutrophil-to-lymphocyte ratios as prognostic markers in breast cancer patients treated with neoadjuvant chemotherapy. Clin Transl Oncol. 2018;20(4):476–483. doi:10.1007/s12094-017-1732-0
27. Vassilakopoulos TP, Dimopoulou MN, Angelopoulou MK, et al. Prognostic implication of the absolute lymphocyte to absolute monocyte count ratio in patients with classical hodgkin lymphoma treated with doxorubicin, bleomycin, vinblastine, and dacarbazine or equivalent regimens. The Oncologist. 2016;21(3):343–353. doi:10.1634/theoncologist.2015-0251
28. Correale P, Rotundo MS, Del Vecchio MT, et al. Regulatory (FoxP3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother. 2010;33(4):435–441. doi:10.1097/CJI.0b013e3181d32f01
29. Yaqub S, Henjum K, Mahic M, et al. Regulatory T cells in colorectal cancer patients suppress anti-tumor immune activity in a COX-2 dependent manner. Cancer Immunol Immunother. 2008;57(6):813–821. doi:10.1007/s00262-007-0417-x
30. Sinicrope FA, Rego RL, Ansell SM, et al. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009;137(4):1270–1279. doi:10.1053/j.gastro.2009.06.053
31. Wilcox RA, Wada DA, Ziesmer SC, et al. Monocytes promote tumor cell survival in T-cell lymphoproliferative disorders and are impaired in their ability to differentiate into mature dendritic cells. Blood. 2009;114(14):2936–2944. doi:10.1182/blood-2009-05-220111
32. Jensen TO, Schmidt H, Møller HJ, et al. Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma. J Clin Oncol. 2009;27(20):3330–3337. doi:10.1200/JCO.2008.19.9919
33. Nishijima TF, Muss HB, Shachar SS, et al. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat Rev. 2015;41(10):971–978. doi:10.1016/j.ctrv.2015.10.003
34. Jiang R, Cai X-Y, Yang Z-H, et al. Elevated peripheral blood lymphocyte-to-monocyte ratio predicts a favorable prognosis in the patients with metastatic nasopharyngeal carcinoma. Chin J Cancer. 2015;34(6):237–246. doi:10.1186/s40880-015-0025-7
35. Chang Y, An H, Xu L, et al. Systemic inflammation score predicts postoperative prognosis of patients with clear-cell renal cell carcinoma. Br J Cancer. 2015;113(4):626–633. doi:10.1038/bjc.2015.241
36. Temraz S, Mukherji D, Farhat ZAA, et al. Preoperative lymphocyte-to-monocyte ratio predicts clinical outcome in patients undergoing radical cystectomy for transitional cell carcinoma of the bladder: a retrospective analysis. BMC Urol. 2014;14:76. doi:10.1186/1471-2490-14-76
37. Diaz-Jauanen E, Strickland RG, Williams RC. Studies of human lymphocytes in the newborn and the aged. Am J Med. 1975;58(5):620–628. doi:10.1016/0002-9343(75)90497-0
38. Tollerud DJ, Clark JW, Brown LM, et al. The influence of age, race, and gender on peripheral blood mononuclear-cell subsets in healthy nonsmokers. J Clin Immunol. 1989;9(3):214–222. doi:10.1007/BF00916817
39. Della Bella S, Bierti L, Presicce P, et al. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin Immunol. 2007;122(2):220–228. doi:10.1016/j.clim.2006.09.012
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.