Back to Journals » Cancer Management and Research » Volume 11
Prognostic Significance Of The Inflammatory Index-Based Scoring System In Patients Preliminarily Diagnosed With Multiple Myeloma In The Bortezomib-Based Chemotherapy Era
Authors Liu S, Shi J, Guo H , Xu F , Wei M , Sun K , Chen Y
Received 17 August 2019
Accepted for publication 10 October 2019
Published 5 November 2019 Volume 2019:11 Pages 9409—9420
DOI https://doi.org/10.2147/CMAR.S227671
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Eileen O'Reilly
Siwei Liu,1 Jie Shi,2 Honggang Guo,2 Fangfang Xu,2 Min Wei,1 Kai Sun,2 Yuqing Chen2
1Department of Hematology, Henan University People’s Hospital, School of Clinical Medicine, Henan University, Zhengzhou 450003, Henan, People’s Republic of China; 2Department of Hematology, Henan Provincial People’s Hospital, Zhengzhou 450003, Henan, People’s Republic of China
Correspondence: Yuqing Chen
Department of Hematology, Zhengzhou University People’s Hospital, Henan Provincial People’s Hospital, #7 Weiwu Road, Zhengzhou, Henan 450003, People’s Republic of China
Tel +86-13633812912
Fax +86371-65580875
Email [email protected]
Kai Sun
Department of Hematology, Zhengzhou University People’s Hospital, Henan Provincial People’s Hospital, #7 Weiwu Road, Zhengzhou, Henan 450003, People’s Republic of China
Tel +86-18237110038
Email [email protected]
Purpose: Red blood cell distribution width (RDW), neutrophil-to-lymphocyte ratio (NLR), and platelet count (PLT) have been reported to be associated with the prognosis of malignancies; this study aimed to evaluate the prognostic significance of the inflammatory prognostic scoring index (IPSI), comprised of RDW, N LR, and PLT for overall survival (OS) in newly diagnosed multiple myeloma patients in the bortezomib-based chemotherapy era.
Patients and methods: The prognostic significance of variables associated with the OS of 175 newly diagnosed multiple myeloma patients was evaluated through univariate and multivariate analyses. The cut-off values of RDW, NLR, and PLT were obtained from references. Patients with high RDW (RDW>14) were given a score of 1; patients with high NLR (NLR>2) or low PLT (PLT≤150) were given a score of 2. According to the obtained scores, the inflammatory prognostic scoring index (IPSI) was formed, in which patients were grouped into high-risk group (4–5 points), intermediate-risk group (3 points) and low-risk group (0–2 points).
Results: OS varied significantly in different IPSI groups (P< 0.001). On multivariate analysis, the IPSI was an independent prognostic factor for OS (intermediate-risk group HR 2.89, 95% CI 1.60–5.22, high risk-group HR 14.50, 95% CI 7.26–28.93, P<0.001). Importantly, with IPSI as supplement to the International Staging System (ISS), a significant difference in OS was observed among IPSI subgroups (ISS I, P<0.001; ISS II, P=0.008; ISS III, P<0.001).
Conclusion: The IPSI, comprised of RDW, NLR, and PLT, played specific role in the prognosis of patients preliminarily diagnosed with multiple myeloma in the bortezomib-based chemotherapy era and could be a beneficial supplement for ISS staging.
Keywords: multiple myeloma, red blood cell distribution width, neutrophil-to-lymphocyte ratio, platelet count, inflammatory prognostic scoring index
Introduction
Multiple myeloma (MM) is a malignant plasmocyte disease that accounts for 10% of hematopoietic tissue tumors and 1% of all malignancies.1 It is the common malignant hematologic tumor.2 MM is characterized by clonal proliferation of malignant plasma cells in bone marrow and excessive secretion of monoclonal immunoglobulins in the blood and/or in urine.3 In recent years, with advancing biological-targeted therapy and the development of new drugs, the treatment and prognosis of MM has improved greatly. In particular, the wide application of lenalidomide and bortezomib increased the 5-year survivorship from 25% to 47%.4–6 Currently, tumor burden and malignancy grade based on the Revised International Staging System (R-ISS) is the most widely applied MM prognosis system.7 However, MM shows significant heterogeneity in disease progression and prognosis. Clinically, some patients considered low risk on the R-ISS may also present poor prognosis. A possible explanation might be that tumor survival not only depends on tumor burden and malignancy degree but is also closely related to factors such as patients’ performance status and inflammatory state.8 Thus, even if the current staging could systematically evaluate tumor burden and malignancy degree, determining a novel index that reflects the patient’s inflammatory status and facilitates a more precise prognostic diagnosis and individualized therapy is necessary.
A close relationship was noted between tumor and inflammation. Chronic inflammation is an important inducer of tumors. In an environment rich in inflammatory factors, growth factors, and activated matrix, continuous cell proliferation increases neoplastic risk.9 Inflammatory factors are essential for tumor cell proliferation, angiogenesis, invasion, and migration. These factors also regulate the communication between tumor and cell matrix, as well as its interaction with extracellular matrix.10,11
Recently, the number of studies on inflammatory factors and tumor prognosis has been increasing. Moreover, the red blood cell distribution width (RDW) is reported to be instructional in the prognosis of laryngeal squamous cell carcinoma and nasopharyngeal carcinoma.12,13 The neutrophil-to-lymphocyte ratio (NLR) is instructional in the prognosis of head and neck tumors and intrahepatic cholangiocarcinoma,14,15 and platelet count (PLT) is instructional in the prognosis of esophageal cancer and lung cancer.16,17 Further, studies have indicated that RDW, NLR, and PLT may be clinically instructive for the prognosis of MM patients.18–20
However, the significance of inflammatory indicators in the prognosis of MM patients still lacks support from large-scale retrospective data, and whether the comprehensive evaluation of inflammatory indicators could complement the existing staging system and refine the risk stratification remains inconclusive. Thus, this study aimed to determine inflammatory indicators, including the inflammatory prognostic scoring index (IPSI) which is composed of RDW, NLR, PLT, and the risk ratio of these three indexes; to evaluate the role of the IPSI in the prognosis of patients initially diagnosed with MM; and to determine whether the IPSI is supplemental to ISS staging.
Materials And Methods
This retrospective study enrolled a total of 175 MM patients who were newly diagnosed in Henan Provincial People’s Hospital, Kaifeng Central Hospital, and Xinxiang Central Hospital from October 2012 to February 2019. The study was approved by the Ethical Committee of Henan Provincial People’s Hospital and all patients involved have signed informed consent. The defining characteristics of MM were based on the 2014 International Myeloma Working Group.
The exclusion criteria were patients diagnosed with reactive plasmacytosis, monoclonal gammopathy of unknown significance, Waldenstrom macroglobulinemia, amyloidosis, and plasma cell leukemia. In addition, as a result of poor compliance, limitation of physical fitness and economics, there were only eight cases of autologous stem cell transplantation (ASCT), which could not make effective statistics possible. Therefore, these eight ASCT cases were excluded from this study. Accordingly, a total of 205 patients were identified. Among them, 30 patients (14.63%) were lost to follow-up, finally, 175 patients were included in the analysis.
All 175 patients received bortezomib-based regimen chemotherapy, including bortezomib, 1.3 mg/m2, subcutaneous injection at days 1, 8, 15, and 22; dexamethasone, 40 mg, PO at days 1, 8, 15, and 22 (BD). bortezomib, 1.3 mg/m2, subcutaneous injection at days 1, 8, 15, and 22; cyclophosphamide, 300 mg/m2, oral administration at days 1, 8, 15, and 22; dexamethasone, 40 mg PO at days 1, 8, 15, and 22 (VCD). Patients’ physical fitness and tolerance were taken into consideration for discretionary reduction. The treatment duration was 4 weeks, with a total of 6–8 courses. Maintenance therapy was conducted according to ISS and lactate dehydrogenase (LDH), and BD regimen was adopted to relatively high-risk population (ISS III and/or LDH > 250U/L), and immunomodulator such as Lenalidomide or thalidomide was adopted to relatively middle- and low-risk population (ISS I or II and LDH≤250U/L).
Clinical data collected were sex, age, and date of diagnosis. One week prior to the patient’s first chemotherapy session, laboratory data including bone marrow plasma cell count, peripheral blood neutrophil count, lymphocyte count, RDW, hemoglobin, PLT, serum calcium ion, serum creatinine, serum LDH, serum albumin, β2 microglobulin, and M protein type were recorded. Peripheral blood samples were collected from the anterior elbow vein. The M protein type was obtained by blood immunofixation electrophoresis. The NLR was obtained by dividing the absolute neutrophil count by the absolute lymphocyte count.
For the three indexes of the IPSI, i.e., RDW, NLR, and PLT, the optimal RDW cut-off value was determined according to Di Zhou et al,21 and patients were divided into the low RDW group (RDW≤14) and high RDW group (RDW>14). The optimal cut-off value of NLR was determined according to Kelkitli et al,19 and patients were divided into the low NLR group (NLR ≤2) and high NLR group (NLR >2). The optimal cut-off value of PLT was determined according to Kim et al,20 and patients were divided into the low PLT group (PLT ≤150) and high PLT group (PLT >150).
Follow-up assessment was performed every 3 months in the first year and every 6 months thereafter. Each assessment included physical examination, laboratory examination, with or without bone marrow aspiration according to the patients’ wishes. The last follow-up was performed in April 2019. Overall survival (OS) was defined as the time from diagnosis to death or the last follow-up.
Statistical Analysis
Data analysis was performed using SPSS software version 22.0 (IBM Corp., Armonk, NY, USA). Patients were divided into groups based on RDW, NLR, and PLT. The Student’s t-test or chi-square test was used to compare baseline data characteristics from different groups. The Kaplan–Meier method and Cox regression model were used to perform univariate and multivariate survival analyses. P<0.05 was considered statistically significant.
Results
Patients’ Baseline Characteristics
Of the 175 MM patients, 95 (54.29%) were men and 80 (45.71%) were women. The average age was 61 years (61.29±9.38). There were 60 (34.29%) monoclonal immunoglobulin subtype IgG patients, 57 (32.57%) IgA patients, 23 (13.14%) IgD patients, and 35 (20.00%) light chain patients. There were 23 (13.14%) cases of ISS stage I, 44 (25.14%) cases of stage II, and 108 (61.71%) cases of stage III (Table 1).
 |
Table 1 Patients’ Baseline Characteristics |
For RDW, there was no significant difference in age, sex, bone marrow plasma cell count, ISS stage, monoclonal immunoglobulin subtype, serum calcium level, serum creatinine level, serum LDH, and β2 microglobulin between the low RDW group and high RDW group. However, in the high RDW group, the hemoglobin level was significantly lower than that in the low RDW group (79.19±20.43 vs 90.85±22.91, P=0.001) (Table 1).
With regard to NLR, there was no significant difference in age, sex, bone marrow plasma cell count, monoclonal immunoglobulin subtype, and hemoglobin level between the high NLR and low NLR group. However, in the high NLR group, significantly more patients presented with ISS III, serum calcium >2.65 mmol/L, serum creatinine >177 µmol/L, serum LDH>250 U/L, and β2 microglobulin ≥5.5 mg/L than in the low NLR group (73.30% vs 49.40%, P<0.001; 31.10% vs 16.50%, P=0.023; 44.40% vs 25.90%, P=0.010; 22.20% vs 8.20%, P=0.010; 73.70% vs 49.40%, P=0.001) (Table 1).
In the analysis for PLT, there was no significant difference in age, sex, monoclonal immunoglobulin subtype, serum creatinine level, and serum LDH level between the high PLT and low PLT groups. However, the proportion of patients in the low PLT group who presented with ISS III, bone marrow plasma cell count >40%, serum calcium >2.65 mmol/L, and β2 microglobulin ≥5.5 mg/L was significantly larger than that of the high PLT group (75.30% vs 52.00%, P=0.004; 56.20% vs 38.20%, P=0.019; 34.20% vs 16.70%, P=0.007; 75.30% vs 52.00%, P =0.002). The hemoglobin level in the low PLT group was significantly lower than that in the high PLT group (76.97±20.59 vs 89.12±22.02, P<0.001) (Table 1).
Prognostic Factor Analysis For OS
The final follow-up time in this study was April 2019, with a median follow-up of 33.63 (2.17–79.33) months. At the last follow-up, 90 (51.43%) patients had died, with a median OS of 29.33 (23.51–35.49) months. The univariate analysis indicated that significant OS prognostic factors were age (P=0.020), number of bone marrow plasma cells (P=0.005), serum calcium (P=0.002), serum creatinine (P=0.048), serum LDH (P<0.001), β2 microglobulin (P<0.001), RDW (P<0.001), NLR (P<0.001), PLT (P<0.001), ISS stage (P<0.001), and maintenance therapy (P<0.001). The multivariate analysis showed that serum LDH (P=0.004), RDW (P=0.016), NLR (P<0.001), PLT (P<0.001), and ISS stage (P=0.008) were independent prognostic factors for OS (Table 2).
 |
Table 2 Univariate Analysis And Multivariate Analysis For Overall Survival |
Establishment Of The IPSI
In the multivariate analysis, inflammation-related indicators RDW (P=0.016), NLR (P<0.001), and PLT (P<0.001) were independent prognostic factors for OS and were included into the IPSI. The risk scores of RDW, NLR, and PLT against OS in the multivariate analysis were calculated according to their risk ratio. The coefficient with the lowest absolute value was used as the basis, the coefficient of other variables was divided by this coefficient, and the obtained value was rounded to the closest integer to obtain the risk score of the variable. Accordingly, at initial diagnosis, RDW>14 (HR 1.85) was recorded for 1 point, NLR>2 (HR 3.80) for 2 points, and PLT≤150 (HR 3.99) for 2 points. Then, 0, 1, 2, 3, 4, and 5 score groups were established based on the inflammation index score (P<0.0001), where there were no significant differences in the OS between the 0 score group and 1 score group, 1 score group and 2 score group, and 4 score group and 5 score group (P=0.084, P=0.222, P=0.198). Different score groups that were statistically the same were combined to form the IPSI, which categorizes patients into the low-risk group (0–2 points), intermediate-risk group (3 points), and high-risk group (4–5 points). In the group analysis, a significant difference in OS was found among different IPSI groups (P<0.001), and the OS in the high-risk group was significantly lower than those in the intermediate-risk group and low-risk group [8.43 (5.43–11.44) vs 26.43 (21.88–30.99), P<0.001; 8.43 (5.43–11.44) vs 46.40 (37.03–55.77), P<0.001]. In the intermediate-risk group, the OS was significantly lower than that in the low-risk group [26.43 (21.88–30.99) vs 46.40 (37.03–55.77), P<0.001] (Figure 1). In the multivariate analysis, the IPSI was an independent prognostic factor for OS (intermediate-risk group HR 2.89, 95% CI 1.60–5.22, high-risk group HR 14.50, 95% CI 7.26–28.93, P < 0.001) (Table 3).
 |
Table 3 Multivariate Analysis For Overall Survival Including IPSI |
 |
Figure 1 Survival curve according to IPSI in all patients. |
Subgroup Analysis
The IPSI could be used for further risk stratification in the ISS I, II, and III groups. The results demonstrated significant differences in different IPSI stratifications in the ISS I, ISS II, and ISS III groups (ISS I group P<0.001, ISS II Group P=0.008, ISS III group P<0.001) (Figure 2A–C).
 |
Figure 2 (A) Survival curve of IPSI in patients with ISS I. (B) Survival curve of IPSI in patients with ISS II. (C) Survival curve of IPSI in patients with ISS III. |
Significant differences were also found in the IPSI risk stratification among different groups on age, serum creatinine, serum calcium levels, and maintenance treatment (P<0.001 for age ≤65 years, P<0.001 for age >65 years; P<0.001 for serum creatinine ≤177 µmol/L group, P<0.001 for serum creatinine >177 µmol/L group; P<0.001 for serum calcium ion ≤2.65 µmol/L group, P = 0.002 for serum calcium ion >2.65 mmol/L group; P<0.001 for BD group, P<0.001 for Immunomodulator group) (Figure 3A and B, Figure 4A and B, Figure 5A and B, Figure 6A and B).
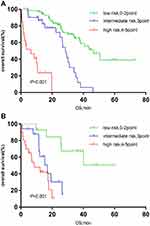 |
Figure 3 (A) Survival curve of IPSI in patients with young age (age≤65 years). (B) Survival curve of IPSI in patients with old age (age>65 years). |
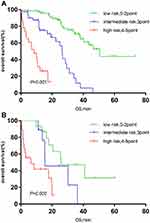 |
Figure 5 (A) Survival curve of IPSI in patients with low serum calcium (calcium ≤2.65mmol/L). (B) Survival curve of IPSI in patients with high serum calcium (calcium>2.65mmol/L). |
 |
Figure 6 (A) Survival curve of IPSI in patients with bortezomib-based maintenance therapy. (B) Survival curve of IPSI in patients with immunomodulator-based maintenance therapy. |
Discussion
The results showed that the IPSI, comprised of RDW, NLR, and PLT, played a specific role in the prognosis of patients preliminarily diagnosed with multiple myeloma in the bortezomib-based chemotherapy era. Inflammatory response has been identified as a risk prognostic factor for various tumors.22–24 The presence of inflammatory factors in tumors promotes tumorigenesis, development, and immunosuppression. Besides, tumor susceptibility may be associated with functional polymorphism in inflammatory factor genes. In vitro experiments have shown that loss or inhibition of inflammatory factors could reduce tumor occurrence and progression.25 Therefore, the level of inflammatory factors in tumor cells is instructive and meaningful for cell survival, proliferation, differentiation, and mutation. Based on these theories, several effective indicators were already adopted to reflect the inflammatory state of cancer patients and further evaluate the prognosis.
RDW is a parameter of RBC volume heterogeneity, which mainly reflects the proportion of heterogeneous RBC in peripheral blood, thereby predicting the efficiency of RBC production. RDW increase is usually induced by anemia caused by insufficient hematopoietic materials or increased RBC destruction. Therefore, RDW is mainly used for the diagnosis of anemia, especially iron deficiency anemia and megaloblastic anemia. Recently, RDW has been recognized as an inflammation marker, as inflammation could increase RDW by affecting iron metabolism and inhibiting the body’s response against erythropoietin.26 It is closely related to common markers such as C-reactive protein, erythrocyte sedimentation rate, soluble tumor necrosis factor, and interleukin-6 in the acute phase during inflammation.27 Studies have shown that high RDW is an independent prognostic risk factor for various solid tumors such as lung cancer,28 prostate cancer,29 cardiovascular disease,30–32 and chronic lymphocytic leukemia.33 In MM patients, high RDW also suggests poor prognosis.34
The systemic inflammatory response of the tumor could be manifested as a relative change in leukocyte levels in the circulation, which is an increase in neutrophil counts and a decrease in relative lymphocyte counts.35–37 Neutrophil increase is caused by de-margination of neutrophils, delayed apoptosis, and stimulation of progenitor cells by growth factors. Lymphocyte decrease is caused by the redistribution of lymphocytes in the lymphatic system and acceleration of apoptosis.38 The survival of tumor cells depends on the proliferative signaling and anti-apoptotic signaling from the tumor microenvironment, which is mainly composed of immune cells and cell matrices.39 As members of the immune cell family, neutrophils and lymphocytes play essential roles in tumor invasion, recurrence, and metastasis. Souto et al pointed out that neutrophils could slow tumor progression through cytotoxicity and reactive oxygen species secretion during Fas/Fas ligand interactions.40 However, studies have indicated that neutrophils could also promote the progression of malignant tumors by releasing growth stimuli, matrix-degrading proteases, and angiogenic mediators.41 The prognostic effect of lymphocytic infiltration on tumors depends entirely on the phenotype, location, and function of lymphocytes. CD4+T regulatory cells (T-reg) infiltration suggests poor prognosis, while CD8+ cytotoxic effector cell infiltration suggests better prognosis.42 Given the complexity of immune cell population and its synergistic and inhibitory effects during tumor infiltration, the interaction mechanism between tumor cells and immune cells and the role of immune cell subsets in tumorigenesis remain to be illuminated, which would become an interesting topic in future cancer research.
NLR could effectively represent the changing proportion in neutrophil and lymphocyte levels, which could partially indicate the influence of tumor inflammatory response on the immune system. Recently, several studies have found that high NLR is an independent prognostic risk factor for patients with gastric cancer,43 colorectal cancer,44 post-transplantation hepatocellular carcinoma,45 and diffuse large B-cell lymphoma.46 In MM patients, a high NLR also suggests poor prognosis.19,20
Platelets are important components of the inflammatory response and could act directly on tumor cells. Platelets contain various factors that promote tumor growth, invasion, and angiogenesis.47 They protect tumor cells from natural killer cell-mediated cleavage, thereby promoting the metastasis and growth of tumor cells.48 A high PLT is reported to be significantly associated with poor prognosis in colorectal cancer,49 gastric cancer,50 and non-small cell lung cancer.51 However, in MM patients, a low PLT is an independent predictor of poor prognosis.20,52 The reason for the contrary prediction from solid tumors may be the fact that malignant plasma cells of MM are produced in the bone marrow, and platelet production is inhibited along with the accumulation of plasma cells in the medullary cavity. Moreover, studies have shown that the half-life of platelet was significantly shortened in MM patients.53
Recent studies have shown that prognostic scoring systems based on inflammation, such as the Glasgow Outcome Score and the Lung Cancer Inflammation Index, are effective prognostic models for colorectal metastases and small cell lung cancer.54,55 However, an inflammation-based prognostic scoring system is rarely used for survival prediction in MM patients.
This study demonstrated the significance of RDW, NLR, and PLT in the prognosis of MM patients using univariate and multivariate analyses retrospectively in a multi-center cohort. RDW, NLR, and PLT were included in the IPSI for MM prognostic scoring, and its prognostic significance was evaluated. In this study, hemoglobin levels were significantly lower in the high RDW group than in the low RDW group. Compared to the low NLR group, a significantly larger proportion of patients in the high NLR group presented with serum calcium >2.65 mmol/L, serum creatinine >177 µmol/L, serum LDH>250 U/L, and β2 microglobulin ≥5.5mg/L. Moreover, compared to the high PLT group, a significantly larger proportion of patients in the low PLT group presented with bone marrow plasma cell count >40%, serum calcium >2.65 mmol/L, and β2 microglobulin ≥5.5 mg/L. Anemia, high calcium, renal dysfunction, high bone marrow plasma cell count, high LDH level and β2 microglobulin were some of the poor indicators in MM prognosis. High RDW, high NLR, and low PLT were significant factors in the poor prognosis of MM in both univariate and multivariate analyses. There were significant differences in OS among IPSI stratifications (low-risk, intermediate-risk, and high-risk groups). In the multivariate analysis, the IPSI was an independent prognostic factor for OS. Compared with the single inflammatory prognostic indicator, the IPSI was able to stratify MM patients more accurately.
We further performed IPSI risk analysis on different subgroups of ISS staging to determine whether the IPSI scoring system was a useful complement to the ISS staging system. Results indicated significant statistical differences in different IPSI stratifications among different subgroups of ISS staging.
Therefore, our multi-center cohort study suggested that the inclusion of the IPSI to assess patient’s inflammatory status contributes to a more comprehensive and accurate prognostic stratification of MM patients, and the IPSI is expected to be a useful complement to ISS staging. Previously, Kim DS et al.20 used a myeloma prognostic index and concluded similar outcomes using CRP, NLR, and PLT. In the present study, we used RDW instead of CRP which is closely related to acute-phase reactants such as CRP. However, clinically, RDW is much easier to obtain than CRP because almost everyone gets a hematological test. Therefore in the real world, the inflammatory prognostic scoring index (IPSI) composed of RDW, NLR and PLT is more economical and practical. Perhaps with the continuous improvement of this study, we can make a baseline risk stratification among MM patients through a simple hematological test. Although most studies indicate that inflammatory responses may be associated with poor prognosis, few studies suggest that cancer infiltration by inflammatory cells may correlate with a better prognosis in breast56 and colorectal cancer.57 This difference may be related to the cancer type, the different patterns of inflammation, and the quantification of inflammatory cell reaction. The possible explanation may be that inflammation might represent an immune response against the tumor, which can cause tumor regression. As the molecular mechanism of a highly systemic inflammatory response remains unclear, further research is needed. If the inflammatory pathway or a signal abnormality could be proven in a tumor patient with a high inflammatory response, the inflammatory scoring system could then be more instructive in further inflammation mechanism research and the development of new anti-inflammatory drugs.
Certain limitations still exist in this study. First, the IPSI was determined by retrospective data analysis, and only 175 cases were included. Second, due to experimental or economic constraints, most patients did not undergo chromosomal fluorescence in situ hybridization; thus, the cytogenetic risk was not included in the univariate and multivariate analyses of OS. This study did not use R-ISS staging and could not completely rule out the potential influence of cytogenetic abnormalities in different IPSI subgroups. In further studies, patients with complete cytogenetic data will be collected continuously and repeat replication of the IPSI would be added in the R-ISS staging system for the prognostic evaluation of MM patients. In addition, given the poor compliance some experimental data were incomplete. Moreover, because of limited physical activity and economics, none of patients included in our study received ASCT; thus, the significance of the IPSI for progression-free survival and the effect of transplantation were not assessed. Therefore, the significance of the IPSI for the prognosis of MM patients still needs further validation in large clinical independent cohort.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374(9686):324–339. doi:10.1016/S0140-6736(09)60221-X
2. Romano A, Laura Parrinello N, Cerchione C, et al. The NLR and LMR ratio in newly diagnosed MM patients treated upfront with novel agents. Blood Cancer J. 2017;7(12):649. doi:10.1038/s41408-017-0019-6
3. Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111(6):2962–2972. doi:10.1182/blood-2007-10-078022
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi:10.3322/caac.21254
5. Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. New Engl J Med. 2014;371(10):906–917. doi:10.1056/NEJMoa1402551
6. Gay F, Oliva S, Petrucci MT, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015;16(16):1617–1629. doi:10.1016/S1470-2045(15)00389-7
7. Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised international staging system for multiple myeloma: a report from international myeloma working group. J Clin Oncol. 2015;33(26):2863. doi:10.1200/JCO.2015.61.2267
8. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
9. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi:10.1038/nature01322
10. Mantovani A. Cancer – inflammation by remote control. Nature. 2005;435(7043):752–753. doi:10.1038/435752a
11. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi:10.1016/j.cell.2010.01.025
12. Fu Y, Mao YZ, Chen SQ, Yang AK, Zhang Q, Gao J-X. A novel inflammation- and nutrition-based prognostic system for patients with laryngeal squamous cell carcinoma: combination of Red Blood Cell Distribution Width and Body Mass Index (COR-BMI). PLoS One. 2016;11(9):e0163282. doi:10.1371/journal.pone.0163282
13. Wang Y, He SS, Cai XY, et al. The Novel Prognostic Score Combining Red Blood Cell Distribution Width and Body Mass Index (COR-BMI) has prognostic impact for survival outcomes in nasopharyngeal carcinoma. J Cancer. 2018;9(13):2295–2301. doi:10.7150/jca.24838
14. Cho Y, Kim JW, Yoon HI, Lee CG, Keum KC, Lee IJ. The prognostic significance of neutrophil-to-lymphocyte ratio in head and neck cancer patients treated with radiotherapy. J Clin Med. 2018;7(12):512. doi:10.3390/jcm7120512
15. Chen Q, Yang LX, Li XD, et al. The elevated preoperative neutrophil-to-lymphocyte ratio predicts poor prognosis in intrahepatic cholangiocarcinoma patients undergoing hepatectomy. Tumor Biol. 2015;36(7):5283–5289. doi:10.1007/s13277-015-3188-6
16. Shimada H, Oohira G, Okazumi S, et al. Thrombocytosis associated with poor prognosis in patients with esophageal carcinoma. J Am Coll Surgeons. 2004;198(5):737–741. doi:10.1016/j.jamcollsurg.2004.01.022
17. Aoe K, Hiraki A, Ueoka H, et al. Thrombocytosis as a useful prognostic indicator in patients with lung cancer. Respiration. 2004;71(2):170–173. doi:10.1159/000076679
18. Sun C, Ye J-N, Wang H, Zhu J-W, Zhou X, Li J-Y. [Prognostic value of red blood cell distribution width in senile patients with non-trans planted multiple myeloma]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2019;27(1):115–122. doi:10.7534/j.issn.1009-2137.2019.01.019
19. Kelkitli E, Atay H, Cilingir F, et al. Predicting survival for multiple myeloma patients using baseline neutrophil/lymphocyte ratio. Ann Hematol. 2014;93(5):841–846. doi:10.1007/s00277-013-1978-8
20. Kim DS, Yu ES, Kang KW, et al. Myeloma prognostic index at diagnosis might be a prognostic marker in patients newly diagnosed with multiple myeloma. Korean J Intern Med. 2017;32(4):711–721. doi:10.3904/kjim.2016.054
21. Zhou D, Xu PP, Peng MX, et al. Pre-treatment red blood cell distribution width provides prognostic information in multiple myeloma. Clin Chim Acta. 2018;481:34–41. doi:10.1016/j.cca.2018.02.009
22. Lee SM, Russell A, Hellawell G. Predictive value of pretreatment inflammation-based prognostic scores (neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio) for invasive bladder carcinoma. Korean J Urol. 2015;56(11):749–755. doi:10.4111/kju.2015.56.11.749
23. Jia W, Wu J, Jia H, et al. The peripheral blood neutrophil-to-lymphocyte ratio is superior to the lymphocyte-to-monocyte ratio for predicting the long-term survival of triple-negative breast cancer patients. PLoS One. 2015;10(11):e0143061. doi:10.1371/journal.pone.0143061
24. Roxburgh CSD, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6(1):149–163. doi:10.2217/fon.09.136
25. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi:10.1016/S0140-6736(00)04046-0
26. Thompson WG, Meola T, Lipkin M
27. Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133(4):628–632.
28. Warwick R, Mediratta N, Shackcloth M, Shaw M, McShane J, Poullis M. Preoperative red cell distribution width in patients undergoing pulmonary resections for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2014;45(1):108–113. doi:10.1093/ejcts/ezt275
29. Albayrak S, Zengin K, Tanik S, Bakirtas H, Imamoglu A, Gurdal M. Red cell distribution width as a predictor of prostate cancer progression. Asian Pac J Cancer Prev. 2014;15(18):7781–7784. doi:10.7314/apjcp.2014.15.18.7781
30. Arbel Y, Weitzman D, Raz R, et al. Red blood cell distribution width and the risk of cardiovascular morbidity and all-cause mortality. A population-based study. Thromb Haemost. 2014;111(2):300–307. doi:10.1160/TH13-07-0567
31. Solak Y, Yilmaz MI, Saglam M, et al. Red cell distribution width is independently related to endothelial dysfunction in patients with chronic kidney disease. Am J Med Sci. 2014;347(2):118–124. doi:10.1097/MAJ.0b013e3182996a96
32. Balta S, Aydogan M, Demirkol S, Cakar M, Akgul EO, Sarlak H. Red cell distribution width: a novel and simple predictor of mortality in chronic obstructive pulmonary disease. Copd. 2014;11(4):475–476. doi:10.3109/15412555.2013.813449
33. Podhorecka M, Halicka D, Szymczyk A, et al. Assessment of red blood cell distribution width as a prognostic marker in chronic lymphocytic leukemia. Oncotarget. 2016;7(22):32846–32853. doi:10.1038/nature01322
34. Lee H, Kong SY, Sohn JY, et al. Elevated red blood cell distribution width as a simple prognostic factor in patients with symptomatic multiple myeloma. Biomed Res Int. 2014;2014:145619.
35. Jilma B, Blann A, Pernerstorfer T, et al. Regulation of adhesion molecules during human endotoxemia. No acute effects of aspirin. Am J Respir Crit Care Med. 1999;159(3):857–863. doi:10.1164/ajrccm.159.3.9805087
36. Dionigi R, Dominioni L, Benevento A, et al. Effects of surgical trauma of laparoscopic vs. open cholecystectomy. Hepatogastroenterology. 1994;41(5):471–476.
37. O’Mahony JB, Palder SB, Wood JJ, et al. Depression of cellular immunity after multiple trauma in the absence of sepsis. J Trauma. 1984;24(10):869–875. doi:10.1097/00005373-198410000-00001
38. Zahorec R. Ratio of neutrophil to lymphocyte counts – rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102(1):5–14.
39. Carreau NA, Diefenbach CS. Immune targeting of the microenvironment in classical Hodgkin’s lymphoma: insights for the hematologist. Ther Adv Hematol. 2019;10:2040620719846451. doi:10.1177/2040620719846451
40. Souto JC, Vila L, Bru A. Polymorphonuclear neutrophils and cancer: intense and sustained neutrophilia as a treatment against solid tumors. Med Res Rev. 2011;31(3):311–363. doi:10.1002/med.20185
41. Piccard H, Muschel RJ, Opdenakker G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol. 2012;82(3):296–309. doi:10.1016/j.critrevonc.2011.06.004
42. Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26(3–4):373–400. doi:10.1007/s10555-007-9072-0
43. Mohri Y, Tanaka K, Ohi M, Yokoe T, Miki C, Kusunoki M. Prognostic significance of host- and tumor-related factors in patients with gastric cancer. World J Surg. 2010;34(2):285–290. doi:10.1007/s00268-009-0302-1
44. Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91(3):181–184. doi:10.1002/jso.20329
45. Halazun KJ, Hardy MA, Rana AA, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250(1):141–151. doi:10.1097/SLA.0b013e3181a77e59
46. Porrata LF, Ristow K, Habermann T, Inwards DJ, Micallef IN, Markovic SN. Predicting survival for diffuse large B-cell lymphoma patients using baseline neutrophil/lymphocyte ratio. Am J Hematol. 2010;85(11):896–899. doi:10.1002/ajh.21849
47. Jain S, Harris J, Ware J. Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol. 2010;30(12):2362–2367. doi:10.1161/ATVBAHA.110.207514
48. Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59(6):1295–1300.
49. Monreal M, Fernandez-Llamazares J, Pinol M, et al. Platelet count and survival in patients with colorectal cancer – a preliminary study. Thromb Haemost. 1998;79(5):916–918.
50. Takahashi Y, Bucana CD, Akagi Y, et al. Significance of platelet-derived endothelial cell growth factor in the angiogenesis of human gastric cancer. Clin Cancer Res. 1998;4(2):429–434.
51. Yu D, Liu B, Zhang L, Du K. Platelet count predicts prognosis in operable non-small cell lung cancer. Exp Ther Med. 2013;5(5):1351–1354. doi:10.3892/etm.2013.1003
52. Kim SJ, Kim K, Kim BS, et al. Clinical features and survival outcomes in patients with multiple myeloma: analysis of web-based data from the Korean Myeloma Registry. Acta Haematol. 2009;122(4):200–210. doi:10.1159/000253027
53. Fritz E, Ludwig H, Scheithauer W, Sinzinger H. Shortened platelet half-life in multiple myeloma. Blood. 1986;68(2):514–520.
54. Kobayashi T, Kawakamil M, Hara Y, et al. Combined evaluation of the glasgow prognostic score and carcinoembryonic antigen concentration prior to hepatectomy predicts postoperative outcomes in patients with liver metastasis from colorectal cancer. Hepato-Gastroenterology. 2014;61(133):1359–1362.
55. Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer. 2013;13:158. doi:10.1186/1471-2407-13-158
56. Lee AH, Gillett CE, Ryder K, Fentiman IS, Miles DW, Millis RR. Different patterns of inflammation and prognosis in invasive carcinoma of the breast. Histopathology. 2006;48(6):692–701. doi:10.1111/j.1365-2559.2006.02410.x
57. Klintrup K, Makinen JM, Kauppila S, et al. Inflammation and prognosis in colorectal cancer. Eur J Cancer. 2005;41(17):2645–2654. doi:10.1016/j.ejca.2005.07.017
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

