Back to Journals » Cancer Management and Research » Volume 11
Prognostic significance of perineural invasion in vulvar squamous cell carcinoma
Authors Long Y, Yao DS, Wei YS, Wei CH, Chen XY
Received 23 December 2018
Accepted for publication 10 April 2019
Published 14 May 2019 Volume 2019:11 Pages 4461—4469
DOI https://doi.org/10.2147/CMAR.S198047
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Ying Long,1,* De-Sheng Yao,1 You-Sheng Wei,1,* Chang-Hong Wei,2 Xiao-Yu Chen2
1Department of Gynecologic Oncology, Affiliated Tumor Hospital of Guang Xi Medical University, Nanning, People’s Republic of China; 2Department of Pathology, Affiliated Tumor Hospital of Guang Xi Medical University, Nanning, People’s Republic of China
*These authors contributed equally to this work
Background: Perineural invasion (PNI) is closely associated with poor survival in several types of malignant tumours, but whether this is true in vulvar squamous cell carcinoma (VSCC) is unclear. The aims of this study were to determine the prognostic significance of PNI in patients with VSCC.
Patients and methods: We retrospectively analysed clinico-pathological data on 105 patients with VSCC (stages IB-IV) treated surgically at our medical center between 2005 and 2015.
Results: PNI was detected in 30 (28.6%) patients, and it was significantly associated with well-known clinical risk factors: large tumour size, depth of invasion, lymphatic vascular space invasion (LVSI), and intra- or extra-nodal spread. Significantly greater proportions of patients with PNI received adjuvant therapy after surgery (P=0.001) or showed local recurrence (P=0.002). Multivariable analysis indicated that risk factors for disease-free survival were tumour size (HR 3.02, 95%CI 1.75–7.75), LVSI (HR 4.82, 95%CI 1.36–17.07), depth of invasion (HR 3.11, 95%CI 1.50–6.44), lymph node metastasis (HR 3.15, 95%CI 1.14–8.96) and positive or close surgical margins (HR 4.86, 95%CI 1.67–14.19). The latter three variables were also risk factors for overall survival. PNI was associated with significantly shorter disease-free survival (DFS) (P=0.020) and overall survival (OS) (P=0.017) based on the log-rank test. Among patients who received adjuvant treatment, Kaplan-Meier curves indicated no significant differences between PNI-positive or -negative subgroups in disease-free survival (P=0.085) or overall survival (P=0.061). Based on multivariable analysis of all patients, PNI was not a significant risk factor for either type of survival .
Conclusion: PNI in VSCC is associated with significantly shorter disease-free and overall survival, though it appears to be a weak independent predictor of worse prognosis. Combining PNI with other risk factors may be useful for predicting whether postoperative adjuvant therapy will be needed.
Keywords: Perineural invasion, vulvar squamous cell carcinoma, adjuvant therapy, prognostic significance
Introduction
Vulvar carcinoma is a rare malignancy, accounting for approximately 5% of all gynaecological cancers, and the most frequent histological subtype is vulvar squamous cell carcinoma (VSCC).1 Surgical excision, ranging from wide local excision to radical vulvectomy with inguinal-femoral lymphadenectomy, is the standard treatment for most patients with vulvar cancer. Patients with strong risk factors for poor prognosis often receive adjuvant radio- or chemo-radiotherapy after surgery in order to ensure better outcomes.2,3 These risk factors typically include nodal involvement, lymphovascular space invasion (LVSI), depth invasion, and positive or close surgical margins. These factors reflect the generally accepted idea that vulvar cancer spreads directly as well as via vascular and lymphatic channels.
Although adjuvant therapies can prevent recurrence and improve outcomes, recurrence rates remain as high as 40%, and the 5-year survival rate in patients with locally advanced disease is only 30–50%.4,5 These poor outcomes may reflect the influence of risk factors yet to be characterised. Therefore, research is needed into new prognostic factors to help predict which VSCC patients will benefit from postoperative adjuvant therapy.
One potential risk factor reflects the possibility that cancer can spread via perineural invasion (PNI).6 In this process, tumours invade nervous structures and spread along nerve sheaths. PNI occurs in a substantial proportion of several types of malignant tumours, such as head and neck,7 prostate,8 and pancreas.9 In these cancers, PNI is closely associated with local tumour recurrence and poor survival.6 In cervical cancer, PNI has been linked to the presence of several risk factors indicative of cancer cell invasion and aggressiveness: depth of stromal invasion, LVSI and parametrial invasion.10–14
Few studies have examined the potential prognostic significance of PNI in vulvar cancer. One study15 identified it as a significant independent risk factor for VSCC recurrence. This makes it a possible indicator of whether a patient is likely to require adjuvant therapy after surgery. Here we further explored this possibility in a retrospective analysis of VSCC patients at our medical center. We also investigated the association of PNI with clinico-pathological features of VSCC.
Patients and methods
Patients
This study was approved by the Institutional Review Board of the Affiliated Tumour Hospital of Guangxi Medical University. Medical records and tissue specimens were analysed retrospectively for 105 consecutive VSCC patients surgically treated at our hospital between January 2005 and January 2015. Written informed consent for this retrospective study was obtained from all surviving patients or the family members of patients who died, in accordance with the Declaration of Helsinki.
To be enrolled in the study, patients had to (a) have histopathological confirmation of primary VSCC; (b) have received surgical treatment, including wide local excision or radical vulvectomy plus complete bilateral linguinofemoral lymphadenectomy; (c) have received adjuvant radio- or chemo-radiotherapy after surgery on the basis of pathological risk factors of poor prognosis; (d) be between 20 and 70 years old; (e) show normal function of major organs (heart, lung and kidney) based on standard laboratory tests, chest x-ray imaging and electrocardiography; and (f) have complete follow-up data.
Diagnosis of PNI and collection of clinico-pathological data
Examination of surgical specimens for PNI was not part of the routine clinical histopathological assessment for patients in this series, so the results of such analysis were absent from the pathology reports of some enrolled patients. Therefore, we performed immunohistochemical staining against S-100 from archived specimens of all 105 patients. This marker is clinically useful in colorectal cancer and oral cavity squamous cancer.16,17 Formalin-fixed, paraffin-embedded tissue blocks were sliced into 3-mm sections and incubated with rabbit anti-S-100 polyclonal antibody (Maixin Biotech; Fuzhou, China) according to the manufacturer’s instructions.
PNI was defined as the presence of tumour cells within any of the three layers of the nerve sheath, or as the presence of tumour in close proximity to a nerve and involving at least one-third of the nerve’s circumference.6 The presence or absence of PNI was determined independently by two expert pathologists blinded to patient outcomes. In the event of uncertain diagnosis, pathologists came to an agreement through discussion.
The following clinico-pathological data were extracted from medical records of all patients: age; tumour stage, grade, size and depth of invasion; LVSI; positive or close surgical margin; as well as lymph nodes positive or negative for tumour, number of positive nodes, intra- or extra-nodal growth, and laterality of positive nodes. To verify these data from medical records, experienced pathologists re-reviewed the archived histopathology slides.
Surgery and postoperative treatment
Wide local excision of the vulva or radical vulvectomy was performed on all patients. Dissection of the primary tumour was carried out with a macroscopic margin of at least 2 cm. The following criteria were used when deciding when patients should receive radio- or chemo-radiotherapy after surgery. Radiotherapy was recommended for patients with tumour-positive margins or close surgical margins (defined as tumour-free margin <8 mm based on histology) at the primary tumor site, and it was considered for patients possessing more than one of the following risk factors: advanced tumour stage, large tumour size, deeper invasion and LVSI. External beam radiation therapy was delivered to the entire vulva through anterior–posterior/posterior–anterior fields. From 2010 onwards, three-dimensional conformal radiation therapy (3D-CRT) or intensity-modulated radiation therapy (IMRT) was used in adjuvant treatment. Radiotherapy consisted of a total dose of 50.4 Gy in fractions of 1.8 Gy, with five fractions administered per week.
Patients with a single positive lymph node received radiotherapy to the bilateral inguinofemoral lymph nodes. Patients with two or more positive nodes, or with lymph nodes showing extracapsular spread, received radiotherapy to the bilateral inguinofemoral and lower pelvic nodes. The radiotherapy mode and dose were the same as for the vulva, except that patients were chemosensitised with weekly cisplatin treatment (40 mg/m2).
Follow-up
All patients were followed until January 2017 for a median of 45 months (range 3–200). Follow-up was conducted through routine outpatient visits, telephone calls or letters every 3 months during the first two postoperative years, then every 6 months during postoperative years 3–6, then once annually. Data on recurrence and survival were collected. Disease-free survival (DFS) was defined as the time from diagnosis to progression or last follow-up, while overall survival (OS) was the time from diagnosis until death or last follow-up. Progression in our study included local/locoregional and distant recurrence,because most of the progressive patients had local/locoregional recurrence, and the few cases of distant recurrence occurred following local recurrence.
Statistical analysis
Statistical analyses were performed using SPSS 17.0 (IBM, Chicago, IL, USA) or Graphpad Prism 6.0 (GraphPad Software, San Diego, CA, USA). Inter-group differences were assessed for significance using the chi-squared test, which was also used to test for possible associations between PNI and clinical characteristics of patients. Survival curves were generated with the Kaplan–Meier method and compared using the log-rank test. Univariable and multivariable analyses based on the Cox proportional hazard model were performed to identify factors associated with DFS and OS. When appropriate, 95% confidence intervals were calculated. Statistical significance was defined as P<0.05.
Results
Table 1 shows the characteristics of patients with or without PNI from whom surgical samples were taken. Among the 105 patients, 30 (28.6%) were PNI-positive and 75 (72.4%) were PNI-negative; the numbers of patients in clinical stages IB, II, III and IV were, respectively, 38, 18, 46 and 3; the numbers of patients with tumour grades 1–3 were, respectively, 36, 45 and 3. Nearly half of patients (42, 40.0%) had larger tumours (>4 cm), 42 (40.0%) showed deep invasion (>2 cm), 35 (33.3%) had LVSI, 48 (45.7%) showed lymph node metastasis, 17 (16.2%) showed positive or close surgical margins, 33 (31.4%) showed local/locoregional recurrence, and 65 (61.9%) received adjuvant therapy after surgery. Clinico-pathological characteristics were compared between those who were PNI-positive or -negative (Table 1). The two groups did not differ significantly in age, FIGO stage, tumour grade or positive or close surgical margins.
 | Table 1 Clinico-pathological characteristics of patients with VSCC |
Tumours in the PNI-positive group were significantly larger (>4 vs <4 cm, P=0.027), had invaded deeper (>2 vs ≤2 cm, P<0.001) and more often showed LVSI (P=0.006). The proportion of patients receiving adjuvant radiotherapy or chemo-radiotherapy was significantly higher in the PNI-positive group (26/30, 86.7% vs 39/75, 52.0%; P=0.001), which also showed a higher frequency of local recurrence (16/30, 53.3% vs 14/75, 18.7%; P=0.002).Lymph node metastasis is a well-known risk factor for worse prognosis in malignant disease. Its frequency differed significantly between patients showing intra- or extra-nodal spread (P=0.037), but not between PNI-negative or -positive patients, between patients showing different numbers of positive lymph nodes or between patients showing different laterality of lymph node metastasis (Table 2).
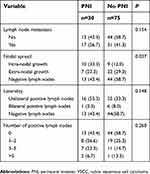 | Table 2 Lymph node status of VSCC patients with or without PNI |
We used Cox proportional hazard analyses to identify factors associated with increased risk of DFS and OS. All histopathological variables that showed significant prognostic value in univariable analysis were included in the multivariable analysis (Table 3). For DFS, univariable analysis identified the following factors as associated with recurrence: advanced stage (P=0.025), tumour size (P<0.001), depth of invasion (P<0.001), LVSI (P<0.001), lymph node metastasis (P<0.001), positive or close margins (P<0.001), and PNI (P=0.018). In multivariable analysis, the following factors predicted risk of recurrence: tumour size (HR 3.02, 95%CI 1.75–7.75), depth of invasion (HR 3.11, 95%CI 1.50–6.44), LVSI (HR 4.82, 95%CI 1.36–17.07), lymph node metastasis (HR 3.15, 95%CI 1.14–8.96), and positive or close surgical margins (HR 4.86, 95%CI 1.67–14.19).
 | Table 3 Univariable and multivariable analysis to identify factors affecting disease-free survival after surgery in VSCC patients |
For OS (Table 4), univariable analysis identified the following factors as associated with death: FIGO stage (P=0.016), tumour size (P=0.009), depth of invasion (P=0.001), LVSI (P=0.006), lymph node metastasis (P<0.001), positive or close surgical margins (P=0.001), and PNI (P=0.020). Multivariable analysis identified the following predictors of death: depth of invasion (HR 3.13, 95%CI 1.09–8.96), lymph node metastasis (HR 2.54, 95%CI 1.25–5.16), and positive or close surgical margins (HR 2.34, 95%CI 1.01–5.46).
 | Table 4 Univariable and multivariable analysis to identify factors affecting overall survival after surgery in VSCC patients |
Mean follow-up time was 45 months. Kaplan-Meier curves for DFS and OS were compared between PNI-positive and -negative groups (Figure 1). The log-rank test indicated significant differences in the curves for DFS (P=0.020) and OS (P=0.017), even though PNI was not a significant independent risk factor for recurrence (Table 3) or death (Table 4) in multivariable analysis. Among patients who received adjuvant treatment, Kaplan-Meier curves did not differ significantly between PNI-positive or -negative subgroups for DFS (P=0.085) or OS (P=0.061) based on the log-rank test (Figure 2).
Discussion
This study accomplished its aims of determining the incidence of PNI, assessing the potential association of PNI with prognostic factors in VSCC, and evaluating the prognostic significance of PNI. We found PNI in 28.6% of VSCC patients in our cohort, which is between the incidences of 53% and 7.6% reported in the only two studies of PNI prevalence in VSCC of which we are aware.15,18 This difference may reflect differences in patient populations, disease status, examination of tissue sections, or other variables. One of the challenges with understanding the prevalence of PNI in VSCC is the lack of a consensus definition of PNI. We applied the criteria of Liebig et al.6 and used anti-S100 staining for disease detection; this antibody staining is associated with higher detection rates than hematoxylin-eosin staining.15–17
We found that PNI was significantly associated with larger tumour size, depth of invasion, and LVSI. We also investigated the association between lymph nodes status and PNI since lymph node metastasis is an important risk factor for worse prognosis in vulvar cancer.19–21 Although PNI in our cohort was not significantly associated with the presence of lymph node metastases, the number of positive lymph nodes or laterality of lymph node metastasis, it was significantly associated with intra- or extra-nodal tumour spread. The latter are well-known risk factors associated with tumour cell invasion that affect prognosis in vulvar cancer patients. The association of PNI with known risk factors of poor prognosis may help explain how PNI can predict the need for adjuvant therapy: the PNI-positive group contained a significantly higher proportion of patients receiving adjuvant therapy, and a higher local recurrence rate, similar to what has been reported in cervical and other malignant cancers.7–9
Several kinds of malignant tumours show PNI, which leads to locoregional recurrence and reduces the likelihood of survival after surgery.7,8,22,23 In early-stage cervical cancer, PNI appears to be associated with negative outcomes,24 although the detailed prognostic significance is controversial.10,11,25,26 In our cohort, DFS and OS were significantly shorter in PNI-positive patients than in PNI-negative ones. Consistently, univariable analysis in a Cox proportional hazard model linked PNI with increased risk of recurrence or death. This implies that PNI is associated with more aggressive disease. However, multivariable analysis did not identify PNI as an independent risk factor for recurrence or death. In addition, when we analysed only patients who received adjuvant therapy, we found that DFS and OS did not differ significantly between PNI-positive or -negative subgroups. This result was different from the comparison in overall patients, which may mean that adjuvant therapy reduced the adverse effects of PNI on prognosis.
We conclude that independent risk factors for DFS and OS in VSCC are depth of invasion, lymph node metastasis, and positive or close surgical margins. Independent risk factors only for DFS are tumour size and LVSI. Our results on prognostic factors in vulvar cancer differ from those of Hothoff et al15 who examined a cohort from America. In their multivariable logistic regression model, PNI was an independent predictor of recurrence, whereas depth of invasion was not. We feel that the most likely explanation for the discrepant results is that those authors evaluated only two variables in their multivariable analysis. In contrast, we evaluated the variables found to be significant in univariable analysis, as well as PNI and depth of invasion. The P-value for the association between PNI and recurrence was 0.045, so it may easily rise above the significance threshold of 0.05 if the model includes different variables from ours. In addition, we complemented our modeling by plotting OS curves for PNI-negative and -positive patients, which Holthoff et al15 did not. Another study, by Salcedo et al,18 suggested that PNI is an independent predictor of poor prognosis. Similar to Holthoff et al, they included only two variables (clinical stage and PNI) in their multivariate analysis. It is possible that PNI was under-reported in that study, since pathology results were not reviewed, nor was specific staining such as immunohistochemistry performed, in contrast to the present study. We found that although PNI increased the risk of recurrence and death for vulvar carcinoma patients based on survival curves, it showed limited strength as an independent predictor of prognosis. Perhaps the power of PNI to predict poor survival in vulvar cancer reflects the combined effects of various risk factors. This should be examined in future work.
Our results should be interpreted with caution in light of some limitations. Our study was retrospective and examined a relatively small sample from a single site. Only VSCC was examined, leaving open the question of whether PNI can be used to predict prognosis in other types of vulva tumors. In addition, our patient population showed a higher incidence of adjuvant therapy than some other studies.27,28 The criteria for such treatment can vary substantially from center to center.
Conclusions
Our study provides reliable evidence that PNI incidence in vulvar cancer, at least in the Chinese population, is approximately 30%. The presence of PNI was closely associated with prognostic risk factors, and PNI-positive patients were more likely to receive radio- or chemo-radiotherapy after surgery. Even though PNI appears to be weak as an independent prognostic factor, it is associated with significantly higher risk of recurrence and shorter survival. It may be that combining PNI with other prognostic risk factors can help predict which vulvar cancer patients will need adjuvant treatment. Indeed, we found that postoperative adjuvant therapy seemed to benefit patients with PNI. PNI should be evaluated routinely and included in histopathology reports of vulvar cancer. Large, rigorously controlled studies are needed to further assess the prognostic value of PNI in vulvar cancer.
Acknowledgments
The authors thank their colleagues for helping with this research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hacker NF, Eifel PJ, van der Velden J. Cancer of the vulva. Int J Gynaecol Obstet. 2015;131(Suppl 2):S76–S83. doi:10.1016/j.ijgo.2015.06.002
2. Mahner S, Prieske K, Grimm D, et al. Systemic treatment of vulvar cancer. Expert Rev Anticancer Ther. 2015;15(6):629–637. doi:10.1586/14737140.2015.1037837
3. Stecklein SR, Frumovitz M, Klopp AH, Gunther JR, Eifel PJ. Effectiveness of definitive radiotherapy for squamous cell carcinoma of the vulva with gross inguinal lymphadenopathy. Gynecol Oncol. 2018;148(3):474–479. doi:10.1016/j.ygyno.2018.01.007
4. Maggino T, Landoni F, Sartori E, et al. Patterns of recurrence in patients with squamous cell carcinoma of the vulva. A multicenter CTF Study. Cancer. 2000;89(1):116–122.
5. Natesan D, Hong JC, Foote J, Sosa JA, Havrilesky L, Chino J. Primary versus preoperative radiation for locally advanced vulvar cancer. Int J Gynecol Cancer. 2017;27(4):794–804. doi:10.1097/IGC.0000000000000938
6. Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115(15):3379–3391. doi:10.1002/cncr.24396
7. Ju J, Li Y, Chai J, et al. The role of perineural invasion on head and neck adenoid cystic carcinoma prognosis: a systematic review and meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(6):691–701. doi:10.1016/j.oooo.2016.08.008
8. Harnden P, Shelley MD, Clements H, et al. The prognostic significance of perineural invasion in prostatic cancer biopsies: a systematic review. Cancer. 2007;109(1):13–24. doi:10.1002/cncr.22388
9. Zhang JF, Hua R, Sun YW, et al. Influence of perineural invasion on survival and recurrence in patients with resected pancreatic cancer. Asian Pac J Cancer Prev. 2013;14(9):5133–5139.
10. Memarzadeh S, Natarajan S, Dandade DP, et al. Lymphovascular and perineural invasion in the parametria: a prognostic factor for early-stage cervical cancer. Obstet Gynecol. 2003;102(3):612–619.
11. Elsahwi KS, Barber E, Illuzzi J, et al. The significance of perineural invasion in early-stage cervical cancer. Gynecol Oncol. 2011;123(3):561–564. doi:10.1016/j.ygyno.2011.08.028
12. Cho HC, Kim H, Cho HY, Kim K, No JH, Kim YB. Prognostic significance of perineural invasion in cervical cancer. Int J Gynecol Pathol. 2013;32(2):228–233. doi:10.1097/PGP.0b013e318257df5f
13. Wei YS, Yao DS, Long Y. Evaluation of the association between perineural invasion and clinical and histopathological features of cervical cancer. Mol Clin Oncol. 2016;5(3):307–311. doi:10.3892/mco.2016.941
14. Zhu Y, Zhang G, Yang Y, et al. Perineural invasion in early-stage cervical cancer and its relevance following surgery. Oncol Lett. 2018;15(5):6555–6561. doi:10.3892/ol.2018.8116
15. Holthoff ER, Jeffus SK, Gehlot A, et al. Perineural invasion is an independent pathologic indicator of recurrence in vulvar squamous cell carcinoma. Am J Surg Pathol. 2015;39(8):1070–1074. doi:10.1097/PAS.0000000000000422
16. Kurtz KA, Hoffman HT, Zimmerman MB, Robinson RA. Perineural and vascular invasion in oral cavity squamous carcinoma: increased incidence on re-review of slides and by using immunohistochemical enhancement. Arch Pathol Lab Med. 2005;129(3):354–359. doi:10.1043/1543-2165(2005)129<354:PAVIIO>2.0.CO;2
17. Shimada Y, Kido T, Kameyama H, et al. Clinical significance of perineural invasion diagnosed by immunohistochemistry with anti-S100 antibody in Stage I-III colorectal cancer. Surg Today. 2015;45(12):1493–1500. doi:10.1007/s00595-014-1096-9
18. Salcedo MP, Sood AK, Dos Reis R, et al. Perineural invasion (PNI) in vulvar carcinoma: a review of 421 cases. Gynecol Oncol. 2019;152(1):101–105. doi:10.1016/j.ygyno.2018.10.035
19. Raspagliesi F, Hanozet F, Ditto A, et al. Clinical and pathological prognostic factors in squamous cell carcinoma of the vulva. Gynecol Oncol. 2006;102(2):333–337. doi:10.1016/j.ygyno.2005.12.027
20. Panici PB, Tomao F, Domenici L, et al. Prognostic role of inguinal lymphadenectomy in vulvar squamous carcinoma: younger and older patients should be equally treated. A prospective study and literature review. Gynecol Oncol. 2015;137(3):373–379. doi:10.1016/j.ygyno.2015.03.013
21. Te GNC, Pouwer AW, de Bock GH, et al. Prognostic factors for local recurrence of squamous cell carcinoma of the vulva: a systematic review. Gynecol Oncol. 2018;148(3):622–631. doi:10.1016/j.ygyno.2017.11.006
22. Deng J, You Q, Gao Y, et al. Prognostic value of perineural invasion in gastric cancer: a systematic review and meta-analysis. PLoS One. 2014;9(2):e88907. doi:10.1371/journal.pone.0088907
23. Knijn N, Mogk SC, Teerenstra S, Simmer F, Nagtegaal ID. Perineural invasion is a strong prognostic factor in colorectal cancer: a systematic review. Am J Surg Pathol. 2016;40(1):103–112. doi:10.1097/PAS.0000000000000518
24. Cui L, Shi Y, Zhang GN. Perineural invasion as a prognostic factor for cervical cancer: a systematic review and meta-analysis. Arch Gynecol Obstet. 2015;292(1):13–19. doi:10.1007/s00404-015-3627-z
25. Horn LC, Meinel A, Fischer U, Bilek K, Hentschel B. Perineural invasion in carcinoma of the cervix uteri–prognostic impact. J Cancer Res Clin Oncol. 2010;136(10):1557–1562. doi:10.1007/s00432-010-0813-z
26. Tian N, Kuerban G. The significance of perineural invasion as a prognostic factor in cervical cancer patients of different ethnicities. Int J Clin Exp Med. 2016;9(10):19634–19643.
27. Nooij LS, van der Slot MA, Dekkers OM, et al. Tumour-free margins in vulvar squamous cell carcinoma: does distance really matter. Eur J Cancer. 2016;65:139–149. doi:10.1016/j.ejca.2016.07.006
28. Aragona AM, Cuneo NA, Soderini AH, Alcoba EB. An analysis of reported independent prognostic factors for survival in squamous cell carcinoma of the vulva: is tumor size significance being underrated. Gynecol Oncol. 2014;132(3):643–648. doi:10.1016/j.ygyno.2013.12.022
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


