Back to Journals » Cancer Management and Research » Volume 12
Prognostic Significance of Metastatic Lymph Nodes Ratio (MLNR) Combined with Protein-Tyrosine Phosphatase H1 (PTPH1) Expression in Operable Breast Invasive Ductal Carcinoma
Received 27 November 2019
Accepted for publication 12 February 2020
Published 13 March 2020 Volume 2020:12 Pages 1895—1901
DOI https://doi.org/10.2147/CMAR.S239085
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Shao Ma, Yanrong Lv, Rong Ma
Department of Breast Surgery, QiLu Hospital of Shandong University, Jinan 250012, Shandong, People’s Republic of China
Correspondence: Rong Ma
Department of Breast Surgery, QiLu Hospital of Shandong University, Jinan 250012, Shandong, People’s Republic of China
Email [email protected]
Purpose: The metastatic lymph node ratio (MLNR) is one of the most important factors in prognostic analysis of breast cancer. The objective of this study was to determine if MLNR combined with protein-tyrosine phosphatase H1 (PTPH1) pathological expression can be used to predict the prognosis of patients with breast invasive ductal carcinoma (IDC) better than one factor only.
Patients and Methods: A total of 136 patients with invasive ductal carcinoma (IDC) of breast who underwent modified radical mastectomy and were treated with chemotherapy after operation at Qilu Hospital of Shandong University from December 2008 to October 2011 were included. PTPH1 expression was evaluated by immunohistochemistry in surgical specimens retrospectively collected from patients with histologically proven invasive ductal breast cancer. Kaplan–Meier survival analysis and Cox regression analysis were performed to assess the prognostic significance of PTPH1 expression. A prognostic factor for disease-free survival (DFS) was identified by univariate and multivariate analyses. ROC analysis was used to evaluate the performance of single factors and combined feature.
Results: One hundred and thirty-six patients were included in the analysis. By cut-point survival analysis, MLNR cut-off was designed as 0.2. On multivariate analysis, a MLNR> 0.2 was associated with a worse DFS (HR=2.581, 95% CI=1.303– 5.113, P=0.007). PTPH1 overexpression is correlated with a better DFS (HR=0.391, 95% CI=0.162– 0.945, P=0.037). In addition, MLNR and PTPH1 combined feature had better performance in predicting clinical outcomes after surgery long before recurrence had occurred (Area under the curve=0.795 [95% CI=0.694– 0.896], P< 0.001).
Conclusion: These findings indicate that both PTPH1 and MLNR are accurate independent prognostic parameters in patients with IDC of the breast. Better information on IDC prognosis could be obtained from the combined feature.
Keywords: breast invasive ductal carcinoma, protein-tyrosine phosphatase H1, metastatic lymph node ratio, prognosis
Introduction
Breast cancer is one of the most common malignant tumors in women worldwide and also in China.1 It was reported that several clinical and pathologic factors are related to the breast cancer recurrence. Among these risk factors, axillary nodal status is an important prognostic factor for survival.2,4 Compared to node-negative patients, of breast cancer patients with metastatic lymph nodes, most of them are invasive ductal carcinoma (IDC) and have worse prognosis after operation. The metastatic lymph node ratio (MLNR), defined as the ratio of positive nodes to the total retrieved nodes, has been used to predict the prognosis of colorectal, esophageal, and gastric cancers.5,8 Recently, MLNR was reported to predict the prognosis of breast cancer better than the N stage.9 But there are still many patients with no metastatic lymph node who have a poor prognosis. One reason for this is the fact that cancer development and progression is determined by multiple factors rather than a single status.
Protein-tyrosine phosphatase H1 (PTPH1), also named PTPN3, is a 120-kDa protein that belongs to the non-transmembrane PTP super-family.10 PTPH1 plays a critical role in malignant progression. Previous studies showed that PTPH1 promotes Ras oncogenesis through overexpression under certain conditions.11 One recent study further demonstrated that PTPH1 increases ER stability and nuclear accumulation, and enhances breast cancer sensitivity to anti-estrogens.12 Another important report showed that PTPH1 decreases EGFR tyrosine phosphorylation, thereby regulating the ER-EGFR interaction and breast cancer sensitivity to tyrosine kinase inhibitor (TKI).13 PTPH1 is a tyrosine phosphatase and sometimes chemotherapy can be affected by phosphorylation.14 Therefore, PTPH1 expression may be a potential factor related to the prognosis of breast IDC with chemotherapy treatment after operation.
The objective of this study was to investigate the correlation from MLNR and PTPH1 to predict the prognosis for IDC of the breast. We also aimed to determine if the MLNR combined with PTPH1 expression could predict the survival of patients with breast IDC preferably.
Materials and Methods
Patients
A record of 136 patients with IDC of the breast who underwent modified radical mastectomy was included in the analysis. They were collected at Qilu Hospital of Shandong University, from December 2008 to October 2011. All of the patients received postoperative chemotherapy. Clinicopathological, tumor-specific data was obtained from the patients’ medical recording system of Qilu Hospital. Descriptive statistics were used to summarize the demographic and clinical characteristics of the patients. Patients’ histological data was assessed by at least two independent pathologists. Written informed consent was obtained from the patients and the study protocol was approved by the Institutional Review Board of Qilu Hospital of Shandong University. The study was undertaken according to the ethical standards of the World Medical Association Declaration of Helsinki.
Breast Cancer Specimens and Tissue Microarrays
Hematoxylin and eosin (HE) stained slides were examined by two independent reviewers who were not aware of the clinical characteristics or clinical outcomes. Seven tissue microarrays (TMAs) were constructed with 136 tumor tissues. Briefly, two cores were taken from each representative tumor tissue (1.5 mm in diameter for each core).
Immunohistochemistry and Evaluation of Immunohistochemical Findings
The primary antibodies for PTPH1 (sc9789, Santa Cruz, CA, dilution 1/100), the corresponding horseradish peroxidase (HRP) conjugated secondary antibody (ab6881, Abcam, UK, dilution 1/300), and diaminobenzidine (DAKO, Denmark) were obtained and validated for labeling. PTPH1 labeling was scored according to staining intensity using the following scale: 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). The percentage of positive cells was also rated using the following four categories: 0 (0%), 1 (<10%), 2 (11–50%), 3 (51–75%), and 4 (76–100%). Each case also received an immunoreactive score (IRS), which was the product of the percentage of positive cells score and the staining intensity score. IRS values ranged from 0 to 12, and the cut-off point of PTPH1 expression is 7.
Statistic Analysis
The largest Log rank test statistic was applied to detect the optimal cut-off point for the number of the lymph node ratio as predictors of survival. The MLNR was defined as the ratio of metastatic lymph node number to total dissected nodes. Then, patients were retrospectively divided into two groups according to MLNR (≤0.2 and >0.2) for analysis. The PTPH1 expression data was also obtained from the same data. The follow-up was completed in October 2019. Survival time was calculated from the date of surgery to the event or the last follow-up. Survival analyses were performed by Kaplan–Meier curves with Log rank tests for significance. Statistical analyses included univariate analysis and multivariate analysis. Univariable Cox regression analyses were performed using disease recurrence or death as the outcomes with a significance level of P<0.05. Multivariate analysis was carried out with a Cox proportional hazards model to evaluate MLNR, PTPH1, and other prognostic factors with respect to disease-free survival (DFS). Hazard ratios (HRs) and 95% confidence intervals (CI) were calculated. A value of P<0.05 was considered as statistically significant. Logistic regression was used to assess the influence of binary factors. Receiver operating characteristic curve (ROC) analysis was used to determine the predictive value of the parameters. Two sided P<0.05 was considered as statistically significant. All statistical analyses were conducted using the SPSS statistical software package (version 20.0; SPSS Inc, Chicago, IL).
Results
One hundred and thirty-six patients (100%) with IDC of the breast were included in the analysis. Some of them were found to have positive lymph node metastases after the operation. Clinicopathologic characteristics and PTPH1 expression are shown in Table 1. The median age of the participants was 49 years (range=22–83). The number of median total lymph nodes harvested was 17 (range=2–33).
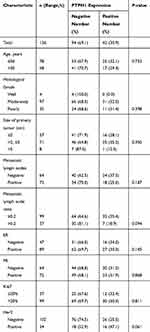 |
Table 1 Clinicopathologic Characteristics and the Expression of PTPH1 |
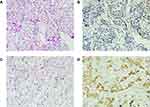
To investigate the clinical significance of PTPH1 in breast cancer, we conducted immunohistochemical staining for PTPH1 expression in the tumor samples. As shown in Figure 1, positive staining for PTPH1 protein was observed mainly in the cytoplasm of breast cancer cells, and most of the intra- or extra-tumor stromal cells were negative for PTPH1. Using the semi-quantified scoring criteria, positive PTPH1 staining was observed in 42 (30.9%) of the 136 cases (Figure 1).
After a median follow-up time of 98 months, 34 of the 136 patients experienced recurrence. The 5-year DFS rate of this cohort was 86.7%. Patients with MLNR higher than 0.2 had significantly worse DFS (P<0.05) than those with MLNR less than 0.2 . Kaplan–Meier curves of DFS based on MLNR are shown in Figure 2. Survival analysis showed a significant difference in DFS (P<0.05) among PTPH1 expression in this entire dataset. Kaplan–Meier curves of DFS based on PTPH1 expression are shown in Figure 3. PTPH1 low expression had worse DFS than PTPH1 high expression.
 |
Figure 2 Disease-free survival curve for 136 patients with IDC according to the MLNR (MLNR<0.2 and MLNR≥0.2). Abbreviation: MLNR, metastatic lymph node ratio. |
 |
Figure 3 Disease-free survival curve for 136 patients with IDC according to the PTPH1 expression (PTPH1 negative and PTPH1 positive). |
Univariate survival analysis indicated that histological grade, PTPH1 expression, metastatic lymph nodes status, and MLNR were potential prognostic factors correlated with DFS (all P<0.05, Table 2). Multivariate analyses were performed using Cox proportional-hazards regression, as shown in Table 3. Multivariate analysis demonstrated that PTPH1 and MLNR were independent prognostic factors for DFS. On multivariate analysis for DFS, MLNR>0.2 (HR=2.441, 95% CI=1.039–5.735, p=0.041) was significantly associated with a worse DFS, and PTPH1 positive (HR=0.375, 95% CI=0.144–0.975, P=0.044) was associated with a better DFS. Positive lymph nodes number and grade were not significantly associated with DFS on multivariate analysis.
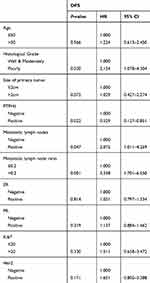 |
Table 2 Univariate Analysis of Factors Associated with DFS |
 |
Table 3 Multivariate Analysis of Factors Associated with DFS |
Three independent factors were included in the ROC analysis in this study. MLNR, PTPH1, and combined feature were shown to improve the prediction of IDC prognosis. For the combined feature, patients were divided into three subgroups according to the number of risk features (MLNR>0.2 and PTPH1 negative): group I, no risk factors were observed; group II, one risk factor was observed; and group III, two risk factors were observed. Combined features would be better to predict the clinical outcomes of IDC patients compared to other factors (Area under the curve=0.795 [95% CI=0.694–0.896], P<0.001) (Figure 4).
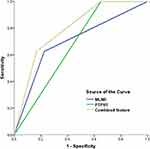 |
Figure 4 ROC analysis of 136 patients with IDC according to the MLNR, PTPH1, and combined feature. The combined feature has better prognostic performance in breast IDC patients. |
Discussion
IDC of the breast is mostly common in breast cancer. Several factors have been shown to affect prognosis in IDC.1 These factors include the number of involved metastatic nodes, size of the tumor, and pathological grade. However, axillary status is always important for predicting breast cancer prognosis. Thus, more and more attention has been focused on accurate knowledge of lymph node status in IDC. Metastatic lymph node ratio (MLNR), the ratio of positive nodes to the total number of total retrieved nodes, was defined to describe the lymph node status of patients more accurately and was used to estimate prognosis of breast cancer. In previous studies, MLNR showed better performance than metastatic lymph node number.9,15,18 However, there are still many patients with recurrence who have no metastatic lymph nodes. Increasing recognition of the active role of cancer cell signaling pathway in tumorigenesis has led to the identification of novel markers for prognostic prediction.
PTPH1, as a protein-tyrosine phosphatase, plays an important role in the development of cancer.11 In addition, PTPH1 regulates tyrosine phosphorylation and protein–protein interaction by its catalytic activity, which promotes tamoxfen and TKI induced cell growth inhibition in breast cancer.12,13 Importantly, protein phosphorylation can affect the benefit of breast cancer chemotherapy.19 So we studied PTPH1 and found it may be an independent factor for the prognosis of breast IDC. A combined analysis of new integrated tumor cell factor as a useful strategy to evaluate cancer progression and patient survival in breast IDC was based on our studies focused on the co-evolution of tumor cells and tumor pathological features.
In this study, the clinico-pathological characteristics are similar to those reported in another large series of the breast IDC population. The 5-year DFS was good but the rate continued to decline. Our results showed that the expression of PTPH1 was frequently correlated with decreased recurrence risk of patients treated with chemotherapy. We found that MLNR>0.2 was associated with a worse significant DFS in patients with breast IDC than MLNR≤0.2. This proved the prognostic value of MLNR as an independent factor in patients with IDC who underwent modified radical mastectomy. Also, PTPH1 positive expression is correlated with better DFS. Meanwhile, we compare MLNR and PTPH1 to other pathologic risk factors such as size of primary tumor>2 cm and metastatic lymph node positive. MLNR and PTPH1 showed better prognostic values in survival prediction for IDC of the breast.
Furthermore, as we all know it is difficult to demonstrate whether one factor is better than the other factors;20 we need to take the results in the exact context to evaluate. In the present study, we have figured out that both MLNR and the PTPH1 expression were associated with DFS in IDC of the breast. But after a ROC study, we found that the MLNR and PTPH1 combined factor is better to predict the prognosis of IDC. This result shows that a single factor may not be enough for predicting prognosis in breast cancer.
The combined feature included in our study showed good performance, but the promising results are based on retrospective analysis, which is the major limitation. It is a complex work for a tumor biomarker to be ready in clinical use, and a useful prognostic marker must be a proven independent and significant factor that is easy to determine and has practical impact. Prospective randomized clinical trials to evaluate the clinical utility of a prognostic or predictive biomarker are still the gold standard, but they are costly and difficult to implement. In the long-term, more efficient retrospective analysis based on the study of archived specimens would be an alternative method.
Conclusion
In conclusion, either MLNR or PTPH1 may be used as the independent prognostic parameter in patients in IDC who underwent breast modified radical mastectomy. In addition, combined MLNR and PTPH1 can predict DFS in IDC of the breast better than one factor. Further studies are needed to verify whether MLNR combined with PTPH1 could be used to select the appropriate preventive measures for an individual with poor prognosis to improve outcomes.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Institutional Review Board of Qilu Hospital of Shandong University, Jinan, China) and with the 1964 Declaration of Helsinki and its later amendments.
Acknowledgments
This work was supported by grants from China Postdoctoral Science Foundation (2019M662368), and National Natural Science Foundation of China (81602310).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yu XQ, Baade P. RE: cancer incidence and mortality in China. Cancer Lett. 2017;401(401):72–73. doi:10.1016/j.canlet.2017.04.018
2. Pilewskie ML, Morrow M. Management of the clinically node-negative axilla: what have we learned from the clinical trials? Oncology (Williston Park). 2014;28:371–378.
3. Caudle AS, Cupp JA, Kuerer HM. Management of axillary disease. Surg Oncol Clin N Am. 2014;23:473–486. doi:10.1016/j.soc.2014.03.007
4. Bachleitner-Hofmann T, Gnant M. Surgical axilla dissection. Technical standard or obsolete method? Zentralbl Chir. 2000;125:822–829. doi:10.1055/s-2000-10053
5. Park J, Byun BH, Noh WC, et al. Lymph node to primary tumor SUV ratio by 18F-FDG PET/CT and the prediction of axillary lymph node metastases in breast cancer. Clin Nucl Med. 2014;39(4):e249–e253. doi:10.1097/RLU.0b013e3182a75477
6. Lee YC, Yang PJ, Zhong Y, Clancy TE, Lin MT, Wang J. Lymph node ratio-based staging system outperforms the seventh AJCC system for gastric cancer: validation analysis with National Taiwan University Hospital Cancer Registry. Am J Clin Oncol. 2014;40:35–41.
7. Jiang K, Zhu Y, Liu Y, et al. Lymph node ratio as an independent prognostic indicator in stage III colorectal cancer: especially for fewer than 12 lymph nodes examined. Tumour Biol. 2014;35(11):11685–11690. doi:10.1007/s13277-014-2484-x
8. Chen SL, Steele SR, Eberhardt J, Zhu K, Bilchik A, Stojadinovic A. Lymph node ratio as a quality and prognostic indicator in stage III colon cancer. Ann Surg. 2011;253(1):82–87. doi:10.1097/SLA.0b013e3181ffa780
9. Ahn SH, Kim HJ, Lee JW, et al. Lymph node ratio and pN staging in patients with node-positive breast cancer: a report from the Korean breast cancer society. Breast Cancer Res Treat. 2011;130:507–515. doi:10.1007/s10549-011-1730-9
10. Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833–846. doi:10.1038/nrm2039
11. Hou SW, Zhi H, Pohl N, et al. PTPH1 dephosphorylates and cooperates with p38g MAPK to increases Ras oncogenesis through PDZ mediated interaction. Cancer Res. 2010;70:2901–2910. doi:10.1158/0008-5472.CAN-09-3229
12. Suresh PS, Ma S, Migliaccio A, Chen G. Protein-tyrosine phosphatase H1 increases breast cancer sensitivity to antiestrogens by dephosphorylating estrogen receptor at tyr537. Mol Cancer Ther. 2014;13:230–238. doi:10.1158/1535-7163.MCT-13-0610
13. Ma S, Yin N, Qi XM, et al. Tyrosine dephosphorylation enhances the therapeutic target activity of epidermal growth factor receptor (EGFR) by disrupting its interaction with estrogen receptor (ER). Oncotarget. 2015;6(15):13320–13333. doi:10.18632/oncotarget.3645
14. Sherry XY, Costantino JP, Kim C, et al. Akt phosphorylation at Ser473 predicts benefit of paclitaxel chemotherapy in node-positive breast cancer. Swain Oncotarget. 2010;28(18):2974–2981.
15. Yang SX, Costantino JP, Kim C, et al. The value of positive lymph nodes ratio combined with negative lymph node count in prediction of breast cancer survival. J Clin Oncol. 2010;28(18):2974–2981. doi:10.1200/JCO.2009.26.1602
16. Kim SI, Cho SH, Lee JS, et al. Clinical relevance of lymph node ratio in breast cancer patients with one to three positive lymph nodes. Br J Cancer. 2013;109:1165–1171. doi:10.1038/bjc.2013.465
17. Vinh-Hung V, Verkooijen HM, Fioretta G, et al. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol. 2009;27:1062–1068. doi:10.1200/JCO.2008.18.6965
18. Danko ME, Bennett KM, Zhai J, Marks JR, Olson JA
19. Ke B, Song XN, Liu N, Zhang RP, Wang CL, Liang H. Akt phosphorylation at Ser473 predicts benefit of paclitaxel chemotherapy in node-positive breast cancer. PLoS One. 2014;9(5):e96455. doi:10.1371/journal.pone.0096455
20. Chen YL, Wang CY, Wu CC, et al. Prognostic influences of lymph node ratio in major cancers of Taiwan: a longitudinal study from a single cancer center. J Cancer Res Clin Oncol. 2015;141(2):333–343. doi:10.1007/s00432-014-1810-4
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.