Back to Journals » Cancer Management and Research » Volume 12
Prognostic Significance of Log(CA125)/PCI for the Resectability of Epithelial Ovarian Cancer: A Retrospective Study
Authors He C , Thapa N , Wang Y, Song Z, Yang J , Xu M, Zuo N , Cai H
Received 19 July 2019
Accepted for publication 17 February 2020
Published 25 March 2020 Volume 2020:12 Pages 2223—2230
DOI https://doi.org/10.2147/CMAR.S223519
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Can He,1 Niresh Thapa,1,2 Yang Wang,1 Ziye Song,1 Jing Yang,1 Mengfei Xu,1 Na Zuo,1 Hongbing Cai1
1Department of Gynecological Oncology, Zhongnan Hospital of Wuhan University, Hubei Cancer Clinical Study Center, Hubei Key Laboratory of Tumor Biological Behaviors, Wuhan 430071, Hubei, People’s Republic of China; 2Karnali Academy of Health Sciences, Jumla, Nepal
Correspondence: Can He; Hongbing Cai Email [email protected]; [email protected]
Objective: This study aimed to evaluate the roles of the ratio of log(serum CA125 level)/PCI in epithelial ovarian cancer.
Methods: This is a retrospective study. Data were retrieved for patients with epithelial ovarian cancer who received primary debulking surgeries (PDS) between January 2014 and December 2017 in Zhongnan Hospital of Wuhan University. The PCI and CA125 were determined retrospectively using surgical reports, histological findings, and intraoperative photographic documentation. Survival analysis and ROC curves were applied to evaluate the roles of the ratio of log(serum CA125 level)/PCI in epithelial ovarian cancer.
Results: A total of 69 patients were included. Of these, serous ovarian cancer and mucinous carcinoma accounted for 63.8% (n=44) and 31.9% (n=22), respectively. The remaining patients had clear cell carcinoma (2.9%, n=2) and endometrioid carcinoma ( 1.4%, n= 1). Kaplan–Meier survival analysis showed that log(serum CA125 level)/PCI (log-rank p=0.018) were prognostic factors for OS. Cox regression analysis, otherwise, suggested that only stages were an independent factor of PFS (P=0.02, 95% CI 0.043– 0.763); outcomes of cytoreductive surgery could only affect OS significantly (P=0.009, 95% CI 1.639– 31.016). Binary logistic regression discovered that only log(serum CA125 level)/PCI was an independent risk factor of PDS. We further used the ROC curve to find that log(serum CA125 level)/PCI could correctly predict the resectability of PDS with AUC 0.781.
Conclusion: The ratio of log(CA125)/PCI that combined the tumor burden and characteristics of peritoneal carcinoma of ovarian origin can predict the resectability of PDS in epithelial ovarian cancer.
Keywords: epithelial ovarian cancer, peritoneal carcinomatosis index, resectability, cancer antigen 125, primary debulking surgery
Introduction
Ovarian cancer, as the sixth leading cause of female cancer and the most lethal gynecological cancer, kills nearly 140,000 women all over the world every year.1 Most epithelial ovarian cancer(EOC) patients are detected at advanced stages, with extensive metastasis throughout the abdomen and pelvis, and have a 5-year survival rate of less than 30%.2 The National Comprehensive Cancer Network (NCCN) recommended primary debulking surgery (PDS) followed by adjuvant platinum-based chemotherapy as the first choice for the treatment of EOC; those who cannot achieve the satisfactory outcome of PDS (size of residual tumor <1 cm) could use neoadjuvant chemotherapy (NACT) to shrink the tumor.3 Some studies demonstrated that NACT combined with interval debulking surgery had fewer complications and shorter operative time, and had similar median overall survival with PDS.4 Forde et al compared the cost and effectiveness of PDS and NACT and found that for the patients at stage IIIC, PDS was significantly more cost-effective compared to NACT; in women with stage IV EOC, PDS was also more cost-effective although the quality-adjusted life-years gained were much more costly and exceed a $50,000 willingness to pay.5 How to evaluate the extent of ovarian cancer accurately and predict the outcomes before PDS is related to patients’ cost and survival.
Strategies for patients diagnosed with EOC are made together by a multidisciplinary team (MDT) which consists of gynecological physicians, radiologists, anesthetists, oncologists, sonographers, and so on. The group commonly relies on the scan of computerized tomography (CT) or magnetic resonance imaging to predict the resectability of EOC patients. Thus, numerous studies have tried to find the most accurate predictive model of CT/MRI, such as Nelson criteria, Bristow criteria, Dowdy criteria, Qayyum criteria, and so on. The accuracy of these criteria was 71–93%. But there still remains lots of work to get consensus results. Laparoscopy may be the most precise method to evaluate the metastasis of ovarian cancer, but there are few patients who benefit from laparoscopy.6 Tumor markers, like serum markers of cancer antigen 125 (CA125) and human epididymis protein 4 (HE4), are reliable tumor markers to distinguish benign and malignant ovarian tumors. The latest research demonstrated that serum CA125 was not recommended to predict the resectability of PDS (grade A), nor was HE4.7 This research indeed mentioned that the peritoneal carcinoma index (PCI) was recommended for the prediction of PDS.
Although PCI can provide precise extensive information on peritoneal carcinoma patients, some studies showed that different original peritoneal carcinoma had different biological characteristics. Also, it cannot be directly correlated with resectability, in that a low PCI score may be associated with unresectable disease at crucial anatomic sites such as the telophragma.2 A study suggested that the ratio of CEA/PCI was a prognostic factor for colorectal cancer patients: the lower the ratio of CEA/PCI, the longer the overall survival (OS) and progression free survival (PFS).8 Whether the relationship between tumor markers and PCI still works in EOC remains a puzzle.
This study aims to assess the prognostic impact of CA125 in relation to tumor volume, as depicted by the peritoneal carcinoma index (PCI) for patients with EOC.
Methods
Ethical Statement for Collecting Clinical Information
This was a retrospective study. The study was carried out according to the Declaration of Helsinki, and written informed consent was obtained from all patients or their legal guardians. Ethical approval was obtained from the Institutional Review Board of the Zhongnan Hospital of Wuhan University.
Study Design and Patient Selection
Thiswas a retrospective study. Institutional ethical board approval was obtained for this study. Data were retrieved forthe patients who had PDS for EOC in Zhongnan Hospital of Wuhan University between January 2014 and December 2017. The inclusion criteria were: all patients with serum tumor marker (including CA125) test before surgery, all patients who were evaluated for the size of residual tumors after PDS, confirmed pathological diagnosis of EOC, and patients who received 6–8 cycles of platinum-based chemotherapy after PDS. Exclusion criteria were: patients who had extra-abdominal disease, who had incomplete data, and who had a pathological diagnosis other than EOC. Informed consent was obtained from all patients before the treatment and they were followed up with tumor markers and imaging scans.
We collected the surgical information containing residual metastasis diameters. Less than 1 cm of the residual lesion after operation was regarded as satisfactory cytoreduction, otherwise regarded as unsatisfactory cytoreduction. We noted the ascites, more than 200 mL of volume, as positive; FIGO stages, the grades of ovarian cancer, and histological types were also analyzed.
PDS and the PCI
PDS was performed by surgeons of the Department of Gynecological Oncology at Zhongnan Hospital, Wuhan University. The PCI was calculated according to the description by Jacquet and Sugarbaker.9 The abdomen and pelvis are divided into 13 regions. Each of these regions received a “Lesion size score”, ranging from LS 0 to LS 3: LS 0 indicates no tumor in this region; LS 1 indicated tumor mass up to 0.5 cm; LS 2 indicated tumor mass up to 5 cm; and LS 3 indicated tumor mass greater than 5 cm or confluent tumor masses, which have caused the adherence of neighboring structures.
Follow-Up
All patients were followed up at monthly intervals in the first 3 months after surgery and 6 months after chemotherapy treatment until the last time of contact or death. Therefore, we got the time of progression free survival (PFS) and overall survival (OS). The follow-up review mainly contained clinical examination, measurement of tumor markers test, and imaging, like CT/MRI scans with or without PET scans.
Statistical Analysis
All statistical analyses were performed using IBM SPSS for Windows version 19.0. Where necessary, log-transformation of data (serum level of CA125) was performed to achieve a normal distribution. Median survival was calculated based on the date of death or last follow-up in months. Survival analysis was performed using Kaplan–Meier curves and the log-rank test for comparison. Univariate and multivariate logistic regression analyses were performed to find the risk factors of PDS. ROC curves were performed to find the specificity and sensitivity of risk factors affecting PDS. All P-values <0.05 were regarded as statistically significant.
Results
Patient Demographics
A total of 69 patients with EOC underwent primary CRS during the above-mentioned time frame. An overview of the demographics is shown in Table 1. Median age was 51.5 years (SD=9.9998), The overall cohort median PCI was 9 (SD=5.68). The majority of histopathology type was serous carcinoma (n=44, 63.8%), and the second was mucinous carcinoma (n=20, 29.0%). We noticed that FIGO stage I–II of patients accounted for 24.6% (n=17) of the total patients; stage IIIb accounted for 4.3% (n=3); stage IIIC was 62.3% (n=43); stage IV was 8.7% (n=6). The majority of patients with EOC (68.7%) had significant ascites positive. In total, 97.1% (n=67) of patients’ serum CA125 level was above 35, and 79.7% (n=55) of patients achieved optimal PDS (Table 1).
 |
Table 1 Background and Characteristics of Patients |
Linear Correlation Analysis of Log(Serum CA125 Level) with PCI
In this analysis, we found a significant linear correlation between log(serum CA125 level) and the PCI (95% CI 0.017–0.07, P=0.002, R2=0.152) (Figure 1). Despite this linear relationship, it turned out that a large proportion of EOC patients presented an opposite relationship between log(CA125) and the PCI; like one patient showed PCI=22 and log(CA125) was 3.0, while another patient showed PCI=9 and log(CA125) was 3.18. Hence, these two opposite findings inspired us to analyze the ratio of log(serum CA125 level) to the PCI in an attempt to find a variate which not only presents the volume of disease but also shows the tumor biological activity.
Survival Analysis
We divided the PCI and log(CA125)/PCI into two groups according to their median values respectively. Kaplan–Meier curves demonstrated that the PCI significantly affected both PFS (stratified by median value, log-rank P=0.016, lower PCI group vs higher PCI group with median survival time 26.5 months vs 14.5 months) and OS (log-rank P=0.04, median survival 35 months vs 52 months); optimal PDS and log(CA125)/PCI were prognostic factors of OS (log-rank p=0.000 and 0.018 respectively) (Figures 2–5).
 |
Figure 4 Kaplan–Meier curves of log(CA125) (A, B). Log(CA125) can neither affect the progression free survival time (P>0.05) nor the overall survival time (P>0.05). |
Univariate Cox regression analysis suggested that stages and the PCI were the factors affecting PFS with P=0.009 and 0.019 respectively. On the other hand, residual tumor size (p<0.001), the PCI (p=0.037), and log(CA125)/PCI (p=0.007) were the factors which had a significant effect on OS of EOC patients (Table 2). Multivariate Cox regression analysis found that only the FIGO stage was an independent factor affecting PFS (p=0.02) and the outcome of PDS was an independent factor on OS (P=0.009), which means when the tumor residual less than 1 cm, patients had longer survival.
 |
Table 2 Univariate Cox Regression Analysis |
Logistic Analysis About PDS
We later decided to perform logistic analysis about residual tumor size (whether it can attain 1 cm or below) since Kaplan–Meier curves and univariate Cox regression analysis results were different from multivariate Cox regression results. There may exist some linkage between residual tumor size of PDS and hte PCI or log(CA125)/PCI. We ultimately used logistic regression analysis to find the risk factors affecting residual tumor size after PDS, and the results suggested that log(CA125)/PCI was an independent risk factor to affect the outcomes of PDS (adjusted P=0.021, adjusted OR=1918.713), which means the higher log(CA125)/PCI, the more difficult it is to achieve optimal PDS (Table 3).
 |
Table 3 Logistic Regression Analysis |
ROC Curves
Receiver operating characteristic curves were used to graph the combination of sensitivity and specificity regarding the residual tumor size of PDS and log(CA125)/PCI. An area under the curve (AUC) of 0.781 demonstrated the high precision in discrimination with which log(CA125)/PCI could correctly predict the outcome of PDS (Figure 6).
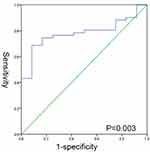 |
Figure 6 The ROC curve of log(CA125)/PCI. Area under the curve (AUC) of 0.781 with P<0.05. |
Discussion
This study revealed that despite a statistically significant linear relationship between serum tumor markers and the PCI, a large proportion of patients with EOC will present with divergent tumor marker and PCI values. Furthermore, these patients can accurately be characterized through the calculation of a log(CA125)/PCI ratio, which allows for the simultaneous assessment of tumor activity and volume. Our research revealed that the ratio of log(CA125)/PCI was a prognostic factor of the resectability of PDS. Also, it was an independent prognostic factor of overall survival.
Since the PCI score is established in general surgery, it seems applicable to all peritoneal metastasizing tumors. It is markedly precise and all abdominal and pelvic regions are weighted equally in the description. Although some studies demonstrated that the outcomes of PDS are dependent on the skills of the surgeons, there is still a lack of an accurate coherent index to predict the resectability of PDS. Many studies focused on the roles that the PCI played for all of the origins of peritoneal carcinoma, and therefore it could be a producible tool to estimate the outcomes pre-operation. Our study, otherwise, suggested that the PCI was an inadequate prediction factor as logistic regression about PDS found that the PCI had no significant relationship with residual tumor of PDS, and thus implied that only the tumor burden was not enough to give clues about the outcomes of PDS. Also, different origins of peritoneal carcinoma had different risk regions, such as diaphragmatic involvement, and the mesenteric root involved heralds unresectability in EOC patients,10 which also means that different origins of peritoneal carcinoma had different activities. When we evaluate the whole metastasis, we should combine these two indexes together.
Tumor markers are not only used in screening ovarian cancer; some studies found that CA125 showed good performance in ovarian cancer with BRCA1/2 mutation detection as a single marker (AUC=0.799).11 This reminded us that tumor markers may represent the characteristics to some degree. Unfortunately, our results suggested that CA125, the most commonly used tumor marker in ovarian cancer, had no significant effect on survival or on the outcomes of PDS. Research also tested that there were no tumor markers to predict the survival of EOC patients alone, and our results were consistent with other studies. Consistent with other studies, our results suggested that whether EOC patients achieved no residual tumor was an independent factor of overall survival. Therefore, finding the factors which affect the outcomes of PDS before surgery means a lot. Our results showed that ascites, FIGO stage, the PCI, and CA125 were not risk factors of PDS, while log(CA125)/PCI had a significant relationship with PDS. Meanwhile, we also found that with an equal PCI value for two EOC patients, the lower the serum CA125 level, the more likely to achieve residual tumors less than 1 cm.
For all this, our study had many limitations: it was a retrospective study, there may exist some bias, and the group of patients was small; especially, the number of patients who had accepted PDS with residual tumors more than 1 cm was much smaller than patients whose residual tumors were less than 1 cm. This phenomenon may be the reason for the large OR value of log(CA125)/PCI in the logistic regression analysis. In the meantime, we are not sure whether HE4 had better performance than CA125 since it was reported that HE4 had better sensitivity and specificity in screening ovarian cancer.
Another limitation was that we did not assess any imaging tools to evaluate the PCI before surgery. Mazzei et al evaluated the accuracy of MDCT in the preoperative definition of the PCI in patients with advanced ovarian cancer and their results encouraged the use of MDCT as the only technique sufficient to select patients with peritoneal carcinomatosis for cytoreductive surgery and HIPEC.12 We committed to finding the most accurate imaging methods to evaluate PCI pre-operation, and compared the accuracy of different ratios of tumor markers combined with the PCI in predicting the outcomes of PDS.
Conclusion
The ratio of log(CA125)/PCI is an independent prognostic factor for resectability of PDS in patients with EOC. This novel approach to considering serum tumor marker levels allows both tumor activity and tumor volume to be accounted for in one index, and may provide a more accurate indication of the biological behavior of the tumors being treated.
Acknowledgments
We thank the staff of the hospital departments caring for patients with EOC for their continuous effort and contribution to acquisition of the data in the Department of Gynecological Oncology in Zhongnan Hospital Affiliated Wuhan University. This work was also supported by funds from the Wuhan University Research Council (1606/240100167. PI: Hongbing Cai).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gupta KK, Gupta VK, Naumann RW. Ovarian cancer: screening and future directions. Int J Gynecol Cancer. 2019;29(1):195–200. doi:10.1136/ijgc-2018-000016
2. Spiliotis J, Halkia EE, Kalantzi N, et al. Mapping the location of peritoneal metastases using the peritoneal cancer index and the correlation with overall survival: a retrospective study. J BUON. 2015;20(1 Suppl):S64–S70.
3. Karlsen MA, Fago-Olsen C, Hogdall E, et al. A novel index for preoperative, non-invasive prediction of macro-radical primary surgery in patients with stage IIIC-IV ovarian cancer-a part of the Danish prospective pelvic mass study. Tumour Biol. 2016;37(9):12619–12626. doi:10.1007/s13277-016-5166-z
4. Fago-olsen CL, Ottesen B, Kehlet H, et al. Does neoadjuvant chemotherapy impair long-term survival for ovarian cancer patients? A nationwide Danish study. Gynecol Oncol. 2014;132(2):292–298. doi:10.1016/j.ygyno.2013.11.035
5. Forde GK, Chang J, Ziogas A. Cost-effectiveness of primary debulking surgery when compared to neoadjuvant chemotherapy in the management of stage IIIC and IV epithelial ovarian cancer. Clinicoecon Outcomes Res. 2016;8:397–406. doi:10.2147/CEOR
6. Gemer O, Gdalevich M, Ravid M, et al. A multicenter validation of computerized tomography models as predictors of non- optimal primary cytoreduction of advanced epithelial ovarian cancer. Eur J Surg Oncol. 2009;35(10):1109–1112. doi:10.1016/j.ejso.2009.03.002
7. Bendifallah S, Body G, Darai E, Ouldamer L. [Diagnostic and prognostic value of tumor markers, scores (clinical and biological) algorithms, in front of an ovarian mass suspected of an epithelial ovarian cancer: article drafted from the French Guidelines in oncology entitled “Initial management of patients with epithelial ovarian cancer” developed by FRANCOGYN, CNGOF, SFOG, GINECO-ARCAGY under the aegis of CNGOF and endorsed by INCa]. Gynecol Obstet Fertil Senol. 2019;47(2):134–154. French. doi:10.1016/j.gofs.2018.12.013
8. Kozman MA, Fisher OM, Rebolledo BJ, et al. CEA to peritoneal carcinomatosis index (PCI) ratio is prognostic in patients with colorectal cancer peritoneal carcinomatosis undergoing cytoreduction surgery and intraperitoneal chemotherapy: a retrospective cohort study. J Surg Oncol. 2018;117(4):725–736. doi:10.1002/jso.24911
9. Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374.
10. Llueca A, Serra A, Herraiz JL, et al. Peritoneal carcinomatosis index as a predictor of diaphragmatic involvement in stage III and IV ovarian cancer. Onco Targets Ther. 2018;11:2771–2777. doi:10.2147/OTT
11. Deng H, Chen M, Guo X, et al. Comprehensive analysis of serum tumor markers and BRCA1/2 germline mutations in Chinese ovarian cancer patients. Mol Genet Genomic Med. 2019;7(6):e672.
12. Mazzei MA, Khader L, Cirigliano A, et al. Accuracy of MDCT in the preoperative definition of Peritoneal Cancer Index (PCI) in patients with advanced ovarian cancer who underwent peritonectomy and hyperthermic intraperitoneal chemotherapy (HIPEC). Abdom Imaging. 2013;38(6):1422–1430. doi:10.1007/s00261-013-0013-9
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




