Back to Journals » OncoTargets and Therapy » Volume 12
Prognostic Significance of LDH Ratio in Serum/Cerebral Spinal Fluid of Patients with Primary Testicular Diffuse Large B-Cell Lymphoma
Authors Liu YZ , Luo P , Liu C, Xue K, Jin J, Xia ZG, Liu XJ, Zhang QL, Cao JN, Hong XN, Lv FF
Received 26 August 2019
Accepted for publication 21 November 2019
Published 2 December 2019 Volume 2019:12 Pages 10469—10475
DOI https://doi.org/10.2147/OTT.S228746
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Yi-Zhen Liu,1,* Pan Luo,2,* Cheng Liu,3 Kai Xue,1 Jia Jin,1 Zu-Guang Xia,1 Xiao-Jian Liu,1 Qun-Ling Zhang,1 Jun-Ning Cao,1 Xiao-Nan Hong,1 Fang-Fang Lv1
1Department of Medical Oncology, Fudan University Shanghai Cancer Center; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, People’s Republic of China; 2Department of Medical Oncology, First People’s Hospital of Yueyang, Yueyang 414000, People’s Republic of China; 3Department of Nuclear Medicine, Fudan University Shanghai Cancer Center, Shanghai 200032, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fang-Fang Lv
Department of Medical Oncology, Fudan University Shanghai Cancer Center, 270 Dongan Road, Xuhui District, Shanghai 200032, People’s Republic of China
Tel +86 21 64175590
Email [email protected]
Objective: Primary testicular diffuse large B-cell lymphoma (PT-DLBCL) is relatively rare, and risk factors of this disease are still not well understood. This study aims to identify clinical features and prognostic factors of PT-DLBCL patients.
Methods: Thirty-two patients were included in this retrospective study who were diagnosed as PT-DLBCL and treated in Fudan University Shanghai Cancer Center between November 2010 and May 2018. The demographic details, clinico-pathological characteristics of the patients were summarized, and the impact on progression-free survival (PFS) and overall survival (OS) was analyzed.
Results: The median age of the patients was 57 (range 36–76) years old. All patients received rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for 4–6 cycles and central nervous system (CNS) prophylaxis, with a CR rate 87.5% and an ORR 96.9%. Nineteen patients continued prophylactic contralateral testis radiation therapy (PCTRT) in our hospital. The 3-year PFS and OS rates were 79% and 92%, respectively. None of the 19 patients who received PCTRT experienced local recurrence. All three patients who suffered from CNS relapse were germinal center B-cell subtype. Kaplan–Meier analyses showed that PT-DLBCL patients with late-stage (Stage IV) (P =0.022), higher IPI score (IPI≥ 2) (P =0.017), B symptoms (P =0.004), and elevated LDH level (P =0.03) had a shorter PFS. More importantly, we found that patients with the ratio of the LDH level in serum to that in CSF ≥ 6.5 suffered from a worse PFS (P =0.028).
Conclusion: Our work revealed that staging IV, IPI score ≥2, having B symptoms and elevated LDH level were risk factors for PT-DLBCL patients. Significantly, the PT-DLBCL patients with a high ratio of LDH level in serum to that in CSF were indicated to have a worse PFS.
Keywords: primary testicular diffuse large B-cell lymphoma, clinical feature, prognosis
Introduction
Primary testicular lymphoma (PTL) accounts for 1–2% of all non-Hodgkin’s lymphomas (NHLs), and 1–9% of testicular malignancies.1,2 The most common histological subtype of PTL is diffuse large B-cell lymphoma (DLBCL).3 Since patients with primary testicular diffuse large B-cell lymphoma (PT-DLBCL) usually relapse in contralateral testis and in the central nervous system (CNS), multiple modality treatment including chemotherapy (R-CHOP), prophylactic contralateral testicle radiation therapy (PCTRT), and prophylactic intrathecal chemotherapy are extremely important.4,5 Nowadays, indicators of therapeutic response remain unavailable. To identify the subtype of PT-DLBCL patients with aggressive behaviors, predictive or prognostic factors that could indicate precise stratification and proper treatment modalities are required in clinical practice.
Some studies have shown that age, Ann Arbor stage, Eastern Cooperative Oncology Group (ECOG) performance status and treatment might be prognostic factors of PT-DLBCL.6,7 However, certain biochemical markers with high accuracy to predict disease progression are still needed. Little is known about the prognostic significance of biochemical factors in the cerebral spinal fluid (CSF). At present, CSF examination is a routine clinical procedure for most PT-DLBCL patients. This approach is able to measure biomolecules such as glucose, protein, and miRNAs in CSF, which may help to improve the accuracy of CNS involvement diagnosis and prognostic estimation.8
In the current study, we retrospectively reviewed a cohort of PT-DLBCL patients treated in our center. Through integrative prognostic analyses, we seek to find out key clinico-pathological or biochemical factors capable of predicting outcome and therapy efficiency of PT-DLBCL patients.
Materials and Methods
Patients and Samples
A total of 32 patients with newly diagnosed, PT-DLBCL were included in this retrospective study. All the patients were treated in Shanghai Cancer Center, China during November 2010 to May 2018. All patients were pathologically confirmed as DLBCL and all pathological results were reviewed by experienced pathologists in our center. Our study was approved by the Institutional Review Board of the Fudan University Shanghai Cancer Center, and the research was conducted in accordance with the Declaration of Helsinki. Informed consent was not required as the study was based on retrospective anonymous patient data and was not involved with patient intervention or the use of human tissue samples. The demographic details, clinical and laboratory features of the patients are summarized in Table 1. For each patient, the following data before treatment were collected: patient demographics, subtype of germinal center B-cell-like (GCB) or non-GCB, Ann Arbor stage, IPI score, Eastern Cooperative Oncology Group (ECOG) performance score, laboratory tests, type of treatment, and survival status. All patients underwent prophylactic intrathecal chemotherapy during treatment. Baseline CSF factors such as ALB levels, LDH, white-blood cells, red blood cells and glucose, chlorine were also routinely detected and recorded. The cytology examination of CSF at baseline in all patients was negative for malignant cells.
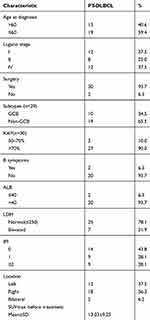 |
Table 1 Baseline Characteristics of 32 PT-DLBCL Patients |
Statistical Analysis
All analyses were performed using PASW Statistics 18 (SPSS Inc., Chicago). Kaplan–Meier survival curves were constructed for survival analyses, and differences were tested by the log-rank test. Overall survival (OS) was defined as the time between the date of diagnosis and the date of death or the date of the last follow-up time. Progression-free survival (PFS) referred to the period from the date of diagnosis to the observed progression of the disease or the occurrence of death for any reason. The data of patients alive at the end of the study were censored. Univariate and multiple Cox proportional hazards regression (backward, stepwise) were performed to identify the independent factors with a significant impact on patient survival. The hazard ratios (HRs) and 95% confidence intervals of the prognostic factors were calculated. All P values were two-sided, and the results were considered significant if P < 0.05.
Results
Patient Characteristics
All 32 cases enrolled in this study were from hospitalized patients. The median age was 57 (range 36–76) years. According to the Ann Arbor staging system, 20 patients (62.5%) and 12 (37.5%) patients were diagnosed with stage I/II and stage IV disease, respectively. The left testicle was involved in 12 (37.5%) patients, the right testicle in 18 (56.3%). Bilateral testicular involvement was observed in two patients (6.2%). There were ten patients (34.5%) who were diagnosed with the germinal center B-cell-like (GCB) and 19 (65.5%) with the non-GCB subtype. The mean Ki67 index was 80% (range 50–95%) (Table 1).
Treatment Modalities of PT-DLBCL Patients
All patients except two underwent orchiectomy at diagnosis. All the patients received 4–6 cycles of R-CHOP regimens (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone), and were managed with prophylactic intrathecal chemotherapy (12mg methotrexate and 5mg dexamethasone for 4–6 cycles). Nineteen patients underwent PCTRT after chemotherapy in our hospital. Twenty-eight patients (87.5%) achieved complete response (CR), three (9.4%) achieved partial response (PR), and only one (3.1%) patient had progressive disease (PD). With a median follow-up time 946 days (range 156–2,976 days), two (6.2%) patients died of the disease. None of the 19 patients who received PCTRT experienced relapse in the contralateral testicle. Three patients (9.4%) had central nervous system relapse and all of them had GCB subtype. Median OS was not reached at the time of reporting. The estimated 3-year PFS and OS rate were 79% and 92%, respectively (Figure 1).
 |
Figure 1 Progression-free survival (PFS) and overall survival (OS) of PT-DLBCL patients in our study. Kaplan–Meier curves showing the PFS (A) and OS (B) of PT-DLBCL patients in our study. |
Effect of Clinico-Pathological and Biochemical Parameters on OS and PFS
To screen out the clinico-pathological and biochemical parameters linked to the outcome of PT-DLBCL, we firstly incorporated patients’ basic information, histological characters, as well as the examination results of serum and CSF. Then, all available factors were subjected to Kaplan–Meier analyses. The results showed that PT-DLBCL patients characterized by late-stage (Stage IV) (P =0.022), higher IPI score (IPI ≥ 2) (P =0.017), having B symptoms (P =0.004), elevated LDH level (P =0.03) had lower progression-free survival (PFS) rates (Figure 2). Since only a few patients reached the endpoint in overall survival analysis, all factors failed to effectively separate the patients with distinguished OS rates in the present cohort.
Likewise, the prognostic value of those relapse predictors was also corroborated by the Cox regression model. The univariate Cox regression analyses indicated that late-stage, higher IPI score, having B symptoms, and elevated LDH levels were contributing factors to lower PFS rate of patients. Multivariate Cox proportional hazards model suggested that IPI score and B symptoms were independent prognostic factors in PT-DLBCL patients (Table 2).
 |
Table 2 Univariate and Multivariate Analysis of Progression-Free Survival in PT-DLBCL Patients |
LDH Ratio Indicates Recurrence of PT-DLBCL Patients
LDH levels were conventionally tested both in the serum and CSF. But the clinical significance of the LDH ratio is unclear. Intriguingly, followed by a cutoff value derived from the ROC curve, our results revealed that the ratio of LDH level in serum to that in CSF was positively correlated with patients’ recurrence (P =0.028). The relapses of PT-DLBCL patients were more likely to occur in those with the serum LDH/CSF LDH ratio ≥6.5 (Figure 3).
Discussion
Central nervous system recurrence is a devastating event for DLBCL patients. Certain extranodal sites involvement such as testis, adrenal, paranasal sinus, and breast confers increased risk of central nervous system recurrence. It has been reported that the CNS relapse rate of PT-DLBCL patients was 12–25%, even early-stage PT-DLBCL patients who received complete resection also took the risk of recurrence.9,10 One way to minimize the incidence of CNS relapse is CNS prophylaxis. All the patients in our study received CNS prophylaxis and had their baseline CSF examination just before initial intrathecal injection. The CNS relapse rate in our study was lower than those previously reported,11–13 all three patients who suffered from CNS relapse had the GCB subtype of DLBCL, which was in consistent with other investigation.14 The estimated 3-year PFS and OS rates in our study were slightly higher than previously reported, which may be attributed to the combination treatment modalities. Nevertheless, there are still some patients suffered from unfavorable outcomes in clinical practice, even after CNS prophylaxis. Therefore, to forecast the effectiveness of clinical management, a set of predictive markers is still merit exploration.
Among clinical parameters, stage is firstly concerned. In our study, 20 patients (62.5%) were diagnosed with early stage (stage I and II), which was similar to the previous report. We found that patients with late-stage (stage IV) disease had poorer PFS (P=0.022), which was in consistent with previous publications.6,15 The correlation between tumor location and survival of PT-DLBCL patients has been controversial in previous literatures. Some studies showed that left testicular involvement was associated with worse PFS,7 while others found that the difference was of no statistical significance.15,16 Our results are in accord with the latter one, showing no statistical difference among patients with left, right or bilateral testicular involvement.
In our study, we found that higher IPI score, advanced stage, having B symptoms, and elevated LDH level were risk factors for a shorter PFS. Previous report also suggested that patients who did not receive anthracycline-containing chemotherapy and who did not receive rituximab were associated with an increased risk of relapse.15 However, all patients in our study received anthracycline-based and rituximab containing chemoimmunotherapy.
The highlight in our study was that we took into account some parameters in CSF and/or the relative levels to those in serum. The results suggested that the PT-DLBCL patients with higher ratio of LDH level in serum to that in CSF were indicated to have a worse PFS. The serum level of LDH has long been appreciated as a prognostic factor for survival in multiple types of malignancies. It may reflect tumor burden, growth and invasive potential.17 High serum LDH concentration was also reported to serve as the predictive factor for CNS involvement for NHL patients.18 In our study, the LDH level alone in CSF did not show a statistically significant difference with PFS, which was in agreement with previous report.19 Interestingly, the ratio of LDH level in serum to that in CSF showed promising significant value with PFS, which had not been previously reported. Our work may provide new insights about the evaluation of CSF based on routinely tested parameters. The high ratio of LDH level in serum to that in the CSF might indicate patients at higher risk of recurrence, and these patients might need more intense chemotherapy. We hope that findings would guide the patients’ treatment plan, and assist in clinical decision-making. Prospective studies aiming to reduce relapse in high-risk PT-DLBCL patients either through additional CNS prophylaxis or systemic intensification therapy are needed.
We cannot deny some limitations of this study currently, such as the non-involved MYD88 mutation status and relatively small sample size. The morbidity of PT-DLBCL is low in China, which limit us to enroll a huge number of patients. Nonetheless, we engaged to perform this retrospective investigation in the hope of providing some clinical insight about the disease. Our results may shed light on novel ways in the evaluation of PT-DLBCLs and inspired us to explore optimal treatment modalities in clinical management of this disease.
Acknowledgments
This work was supported by National Science Fund (81702259), and Shanghai Municipal Commission of Health and Family Planning Fund (20174Y0075).
Disclosure
All the authors in this study have declared that no competing interest exists.
References
1. Gundrum JD, Mathiason MA, Moore DB, Go RS. Primary testicular diffuse large B-cell lymphoma: a population-based study on the incidence, natural history, and survival comparison with primary nodal counterpart before and after the introduction of rituximab. J Clin Oncol. 2009;27:5227–5232. doi:10.1200/JCO.2009.22.5896
2. Moller MB, d’Amore F, Christensen BE. Testicular lymphoma: a population-based study of incidence, clinicopathological correlations and prognosis. The Danish Lymphoma Study Group, LYFO. Eur J Cancer. 1994;30A:1760–1764. doi:10.1016/0959-8049(94)00311-R
3. Menter T, Ernst M, Drachneris J, et al. Phenotype profiling of primary testicular diffuse large B-cell lymphomas. Hematol Oncol. 2014;32:72–81. doi:10.1002/hon.v32.2
4. Mazloom A, Fowler N, Medeiros LJ, Iyengar P, Horace P, Dabaja BS. Outcome of patients with diffuse large B-cell lymphoma of the testis by era of treatment: the M. D. Anderson Cancer Center experience. Leuk Lymphoma. 2010;51:1217–1224. doi:10.3109/10428191003793358
5. Vitolo U, Chiappella A, Ferreri AJ, et al. First-line treatment for primary testicular diffuse large B-cell lymphoma with rituximab-CHOP, CNS prophylaxis, and contralateral testis irradiation: final results of an international phase II trial. J Clin Oncol. 2011;29:2766–2772. doi:10.1200/JCO.2010.31.4187
6. Xu H, Yao F. Primary testicular lymphoma: a SEER analysis of 1,169 cases. Oncol Lett. 2019;17:3113–3124. doi:10.3892/ol.2019.9953
7. Cao B, Ji DM, Zhou XY, et al. A clinical analysis of primary testicular diffuse large B-cell lymphoma in China. Hematology. 2011;16:291–297. doi:10.1179/102453311X13085644680221
8. Yang K, Wang S, Cheng Y, Tian Y, Hou J. Role of miRNA-21 in the diagnosis and prediction of treatment efficacy of primary central nervous system lymphoma. Oncol Lett. 2019;17:3475–3481. doi:10.3892/ol.2019.9941
9. Kridel R, Telio D, Villa D, et al. Diffuse large B-cell lymphoma with testicular involvement: outcome and risk of CNS relapse in the rituximab era. Br J Haematol. 2017;176:210–221. doi:10.1111/bjh.14392
10. Fonseca R, Habermann TM, Colgan JP, et al. Testicular lymphoma is associated with a high incidence of extranodal recurrence. Cancer. 2000;88:154–161. doi:10.1002/(ISSN)1097-0142
11. Lantz AG, Power N, Hutton B, Gupta R. Malignant lymphoma of the testis: a study of 12 cases. Can Urol Assoc J. 2009;3:393–398. doi:10.5489/cuaj.1153
12. Zucca E, Conconi A, Mughal TI, et al. Patterns of outcome and prognostic factors in primary large-cell lymphoma of the testis in a survey by the International Extranodal Lymphoma Study Group. J Clin Oncol. 2003;21:20–27. doi:10.1200/JCO.2003.11.141
13. Park BB, Kim JG, Sohn SK, et al. Consideration of aggressive therapeutic strategies for primary testicular lymphoma. Am J Hematol. 2007;82:840–845. doi:10.1002/ajh.20973
14. Zhou BC, Ye X, Zhu L, et al. Clinical and histological features of primary testicular diffuse large B-cell lymphoma: a single center experience in China. Oncotarget. 2017;8:112384–112389. doi:10.18632/oncotarget.19736
15. Deng L, Xu-Monette ZY, Loghavi S, et al. Primary testicular diffuse large B-cell lymphoma displays distinct clinical and biological features for treatment failure in rituximab era: a report from the International PTL Consortium. Leukemia. 2016;30:361–372. doi:10.1038/leu.2015.237
16. Ma RZ, Tian L, Tao LY, et al. The survival and prognostic factors of primary testicular lymphoma: two-decade single-center experience. Asian J Androl. 2018;20:615–620. doi:10.4103/aja.aja_73_18
17. Suh SY, Ahn HY. Lactate dehydrogenase as a prognostic factor for survival time of terminally ill cancer patients: a preliminary study. Eur J Cancer. 2007;43:1051–1059. doi:10.1016/j.ejca.2007.01.031
18. Tomita N, Kodama F, Sakai R, et al. Predictive factors for central nervous system involvement in non-Hodgkin’s lymphoma: significance of very high serum LDH concentrations. Leuk Lymphoma. 2000;38:335–343. doi:10.3109/10428190009087024
19. Salzburg J, Burkhardt B, Zimmermann M, et al. Prevalence, clinical pattern, and outcome of CNS involvement in childhood and adolescent non-Hodgkin’s lymphoma differ by non-Hodgkin’s lymphoma subtype: a Berlin-Frankfurt-Munster Group Report. J Clin Oncol. 2007;25:3915–3922. doi:10.1200/JCO.2007.11.0700
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


