Back to Journals » OncoTargets and Therapy » Volume 12
Prognostic Significance of FKBP14 in Gastric Cancer
Authors Ghoorun RA, Wu XH , Chen HL, Ren DL, Wu XB
Received 5 July 2019
Accepted for publication 2 December 2019
Published 30 December 2019 Volume 2019:12 Pages 11567—11577
DOI https://doi.org/10.2147/OTT.S221943
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nicola Silvestris
Roshan Ara Ghoorun,1,* Xiao-Hua Wu,2,* Hong-Lei Chen,3 Dong-Lin Ren,1 Xiao-Bin Wu4
1Department of Colorectal Surgery, The Sixth Affiliated Hospital of Sun Yat-Sen University, Guangzhou, Guangdong, People’s Republic of China; 2Department of Oncology, Longhu People’s Hospital, Shantou, Guangdong, People’s Republic of China; 3Department of Endoscopy, The Eighth Affiliated Hospital of Sun Yat-Sen University, Shenzhen 518033, People’s Republic of China; 4Department of Gastrointestinal Surgery, The Eighth Affiliated Hospital of Sun Yat-Sen University, Shenzhen 518033, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiao-Bin Wu
Department of Gastrointestinal Surgery, The Eighth Affiliated Hospital of Sun Yat-Sen University, No. 3025 Shennan Middle Road, Shenzhen 518033, People’s Republic of China
Email [email protected]
Dong-Lin Ren
Department of Colorectal Surgery, The Sixth Affiliated Hospital of Sun Yat-Sen University, No. 26 Yuancun Er Heng Rd, Tianhe District, Guangzhou, Guangdong 510655, People’s Republic of China
Tel +86-20-38254151
Email [email protected]
Introduction: Although our understanding on gastric cancer biology is better than a decade ago, its practical effect on screening and diagnosis remains limited. Moreover, there are no markers that can be accurately used in the clinic to diagnose early-stage gastric cancer or monitor the patient’s response to therapy. Herein, we investigate whether FKBP14 is involved in the progression of gastric cancer.
Methods: The AGS cell line was chosen for over-expression analysis, whereas the SGC-7901 cell line was selected for knock-down analysis. AGS cells were transfected with an FKBP14 overexpression plasmid (AGS-PLV.O-FLAG). The expression pattern of FKBP14 in both cell lines was determined by Western blot and RT-PCR. Cell proliferation was assessed using Cell Counting Kit-8, whereas apoptosis was performed using flow cytometry. The expression of FKBP14 in 70 Chinese patients with gastric cancer was also investigated using tissue microarrays and compared with gastric cancer patients from The Cancer Genome Atlas.
Results: FKBP14 was highly expressed in SGC7901 and had a relatively low expression in AGS cells. Upregulation of FKBP14 in AGS cells promoted migration and invasion and inhibits apoptosis. Knock-down of FKBP14 resulted in a suppression in migration and invasion and promoted apoptosis in the SGC-7901 cell line. Effectively, gastric cancer patients had a higher expression of FKBP14, with a lower survival rate (P = 0.028). Patients with a high expression of FKBP14 were significantly correlated with lymph node metastasis (P =0.016), and an advanced histologic grade (P =0.021).
Conclusion: FKBP14 is often up-regulated in gastric cancer. Patients with a high expression of FKBP14 are usually associated with worse overall survival. FKBP14 is an oncogene in gastric cancer, and is a potential biomarker for GC diagnosis, invasion, and prognosis.
Keywords: FKBP14, gastric cancer, in vitro, prognosis, The Cancer Genome Atlas
Introduction
Despite the global decreased incidence since 1950s,1 gastric cancer (GC) continues to be rampant in many regions around the world. Recent epidemiological studies report GC as the most commonly diagnosed cancer, and the third cancer-leading cause of death worldwide.2 Although our understanding on GC biology is better than a decade ago, its practical effect on screening and diagnosis remains limited. Moreover, until now, there are no markers that can be accurately used in the clinic to diagnose early-stage GC or monitor the patient’s response to therapy. It is therefore necessary to discover new and appropriate markers, which can differentiate between the different stages of cancer spread.
FKBP14 is a member of the FKBP family. In humans, this family consists of 16 proteins, ranging from 12 to 135 kDa, which are dispersed in several tissues and subcellular compartments. FKBPs are involved in multiple cellular tasks such as T-cell activation with immunosuppressive ligands, protein folding, protein stability, kinase activity, and receptor signaling.3 Lately, the expression levels of FKBPs have also been shown to be mutated in both cancer cell lines and in human cancer tissues.4
Previous studies have demonstrated the role of FKBP14 in Ehlers-Danlos syndrome,5 ovarian cancer,6 cervical cancer,7 and osteosarcoma.8 However, the expression of FKBP14 in the invasion and metastasis of GC cells is poorly understood. In this study, we investigate the expression of FKBP14 in the GC and explore its function in regulating cell migration, invasion, and apoptosis. We also evaluate the correlation of FKBP14 with clinicopathologic factors and overall survival (OS).
Materials and Methods
GC Cell Lines
The cell lines were purchased from the Culture Collection of the Chinese Academy of Science (Shanghai, China). Prior to the investigations, all cell lines tested negative for Mycoplasma. The expression of FKBP14 was investigated in the following cell lines: GES-1, HGC-27, BGC-823, SGC-7901, and AGS. The latter was chosen for over-expression analysis, whereas the SGC-7901 cell line was selected for knock-down analysis. The cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen Life Technologies), at 37°C with 5% CO2. We also supplemented the cells with 10% fetal bovine serum, 2 mML glutamine, 100 units/mL penicillin-G, and 100 mg/mL streptomycin.
RNA Extraction and qRT-PCR
Total RNA was isolated from cell lines using Trizol (Invitrogen, Carlsbad, CA, USA). β-Actin was used as the control throughout this study. Real-time (RT) PCR was carried out in trio on an ABI 7300 machine (Applied Biosystems, Foster City, CA, USA), utilizing the SYBR Green PCR Master Mix (TOYOBO, Osaka, Japan).
RT-PCR assays for detecting FKBP14 expression level in the cell lines were performed with a specific primer for FKBP14. The specific primer for the latter was F:AGAAGGAAAAGGTAAAATTCCCCC; R: TTCATTCACCACCGCACCAT, whereas the specific primer for β-actin was F:AGATCAAGATCATTGCTCCTCCT; R: ACGCAGCTCAGTAACAGTCC.
Cell Transfection
Cell transfection was performed as per the manufacturer’s protocols. AGS cells were transfected with an FKBP14 overexpression plasmid (AGS-PLV.O-FLAG) for the cells that have a low level of endogenous FKBP14. AGS-PLV.O-FLAG and AGS-PLV.O-FLAG-FKBP14 cells were provided by the Cell Construction Group of Guangzhou Huiyuanyuan Pharmaceutical Technology Co., Ltd. (Guangzhou, China). We first seeded AGS cells at a density of 1×105 cells utilizing a serum-free RPMI1640 medium. Thereafter, the AGS-NC-FLAG empty vector and AGS-FKBPP14-FLAG overexpression vector were used for transfection. For knock-down analysis, we transfected SGC7901 cells with rabbit FKBP14 siRNA (Bioss antibodies, Beijing China). In the experimental group (SGC7901-si-FKBP14-001), SGC7901 cells were transfected with anti-FKBP14 cells, whereas in the control group (SGC7901-si-NC), SGC7901 cells were transfected with FKBP14’s negative control. The expression status of all transfectants was confirmed using Western Blot.
Western Blot Analysis
Western blot was performed as per the manufacturer’s protocols (Beyotime, Jiangsu, China). The relative expression of FKBP14 in AGS, AGS-NC-FLAG, AGS-FKBP14-FLAG, SGC7901-si-NC, SGC7901-si-FKBP14 was analyzed using a lysis buffer. The antibodies against FLAG, FKBP14, and β-actin were acquired from Bioss antibodies (Beijing, China).
Cell Proliferation Assay
Cell proliferation was assessed using Cell Counting Kit-8 (CCK-8: Beyotime) as per the manufacturer’s directions. Cells were first digested and counted. The cell suspension (200 μL/well) was prepared according to the counting conditions and inoculum size (5000/well). For inoculation and culture, the prepared cell suspension was inoculated into a 96-well plate, and the plate was pre-cultured (37°C, 5% CO2) for 4 hrs, 24 hrs, 48 hrs and 72 hrs in an incubator. Using the CCK8 detection kit, 20 μL of CCK8 solution was added to each well. Care was taken to not generate bubbles in the pores. The culture plate was then incubated for 2 hrs. A microplate reader was used to measure absorbance at 450nm.
Wound Healing Assay
Cells suspension was prepared at a concentration of 1×106/mL, and uniformly inoculated into a 6-well culture plate and cultured in a 37° C, 5% CO2 incubator. The monolayer cells were evenly spread on the bottom of the plate. Thereafter, the cells were changed and treated with mitomycin (4 μg/mL) for 2 hrs to inhibit cell division. Scratches were made along the cells using a 10μL pipette. The cell culture medium was aspirated and rinsed three times in PBS to wash away the cell debris generated by the scratch. The cells were photographed at 0hr, 6hrs, and 24hrs. The cell migration distance was calculated using mage Pro-Plus 6.0 software.
Transwell Assay
The cell culture was rinsed with PBS and then re-suspended with a serum-free medium. 5×104 cells were inoculated in the upper chamber supplemented with 500 μL of complete medium in the lower chamber. The plate was then incubated for 48 hrs. The next day, after rinsing the cells, 0.1% crystal violet dye solution was added for 20 mins. The stained cells were observed under the microscope and photographed.
Cell Apoptosis Analysis
The harvested cells were re-suspended with binding buffer. Care was taken to ensure the number of cells per tube remained at 1×105. 5 μL of Annexin V-APC was added to the cells, gently vortexed, and incubated at room temperature for 15 mins. After resuspension, apoptosis was detected using Flowjo analysis.
Pathological Specimens
All patients provided a written informed consent form prior to the experiment. The Ethics committee of the Sixth Affiliated hospital of Sun Yat-Sen University approved of this investigation. At the time of tissue collection, a fraction was immediately frozen in liquid nitrogen and stored at −80°C until ready for RNA extraction while the other fraction was formalin-fixed, and paraffin embedded for histopathology diagnosis and immunohistochemical study.
Immunohistochemistry and Immunohistochemical Analyses
Tissue microarrays (TMA) were used for the immunohistochemistry analysis. 70 gastric cancer tissues and 10 non-cancer gastric tissue were utilized to construct the TMA. We excluded patients who underwent any neoadjuvant chemotherapy or radiotherapy. We also further retrieved clinical information for 414 gastric cancer tissues from The Cancer Genome Atlas; the expression of FKBP14 at mRNA level was analyzed, and a survival analysis was performed. The detailed information on the clinical features of all patients in this study is classified in Table 1.
 |
Table 1 Correlation of FKBP14 Expression with Clinicopathological Characteristics in Patients with Gastric Cancer (*P<0.05) |
Immunohistochemistry Analysis
Immunohistochemistry was performed as previously described.9 As per the manufacturer’s protocols, we cut the paraffin-embedded tissues at 4μm, and performed negative controls by omitting out the primary antibody. In brief, Paraffin was gradually removed from the tissues using Xylene, and then rehydrated for further peroxidase (DAB) immunohistochemistry staining. The latter was done utilizing the DAKO EnVision System (Dako Diagnostics, Switzerland).
After the short proteolytic digestion, peroxidase was added to the slides. The latter were then incubated overnight with the primary antibody against FKBP14 (rabbit polyclonal antibody, bs-16092R, Bioss, lnc. CN) at a dilution of 1: 600, at 4°C. We then utilized a peroxidase-labeled polymer and a substrate-chromogen to analyze FKBP14 in GC tissues.
Evaluation of Immunostaining Results
Two experienced pathologists independently scored the immunostaining intensity. None of the pathologists knew the clinicopathological data or clinical outcomes of the patients. Any discrepancy was reevaluated by the consultant pathologist. They evaluated the immunolabeling of cancer cells as previously described.
In brief, the two pathologists separately scored and stratified the staining intensity as follows: no dyeing (score point = 0), mild dyeing (score point = 1), moderate dyeing (score point = 2), and strong dyeing (score point = 3). The area covered by the immunoreactive tumor cells was calculated as follows: 0% (score point = 0), 1–10% (score point = 1), 11–50% (score point = 2), 51–75% (score point = 3), and >75% (score point = 4). The ultimate immunoreactivity scores (IRS) of each case were totaled by adding up the two obtained scores.
Statistical Analysis
We used SPSS 23.0 (SPSS, Inc, Chicago, Illinois) to carry out the statistical analysis. Student’s t-test was used to assess continuous variables, whereas we used the Chi-square test or Fisher’s exact test to correlate categorical variables. We assessed survival time starting from the date of surgery to the date of death due to any cause or until the last day of follow-up. We used the Kaplan-Meier method to perform survival analysis; correlation was done using the log-rank test. We also used the Cox proportional hazards model to assess multivariate survival analysis. A P value less than 0.05 was deemed as statistically significant.
Results
Expression of FKBP14 in Gastric Cancer Cells
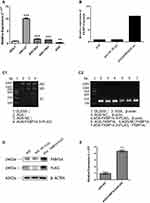
We used the SGC7901 and the AGS cell line to assess the role of FKBP14 in GC. FKBP14 was highly expressed in SGC7901 and had a relatively low expression in AGS cells. Therefore, the SGC7901 cell line was used for knockdown experiments whereas the AGS cell line was employed for over-expression experiments owing to the relatively poor expression (Figure 1A).
Over-Expression of FKBP14 Intensifies Cell Growth in GC
To assess whether FKBP14 over-expression advocates cell growth in GC, we infected the AGS cell line with an over-expression plasmid (AGS-PLV.O-FLAG) (Figure 1B) FKBP14 over-expression was confirmed by qPCR (Figure 1C) and Western blot analysis (Figure 1D and E).
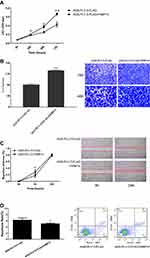
CCK-8 assay (Figure 2A), transwell assay (Figure 2B), wound healing assay (Figure 2C) showed that FKBP14 hastened the cell growth rates and slows down the rate of apoptosis in AGS-PLV.O-FLAG-FKBP14 cells (Figure 2D).
Knock-Down of FKBP14 in SGC7901 Cells
A stable cell line silencing FKBP14 (SGC7901-SI-FKBP14-002) was generated in the SGC-7901 cell line using anti-FKBP14 antibodies. Western blot was used to confirm FKBP14 was knocked down from the cell line (Figure 3A). The rate of proliferation, invasion, and apoptosis were investigated to assess the effect of FKBP14 knockdown in GC. Our results showed that FKBP14 knockdown effectively slowed down cell growth (Figure 3B and C), and increases apoptosis relative to control cells (Figure 3D). These findings collectively suggested that FKBP14 knock-down significantly inhibits cell proliferation of GC cells.
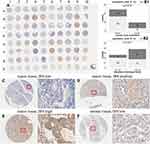
FKBP14 Protein Is Up-Regulated in Human Gastric Cancer Tissues
FKBP14 protein expression was first detected in Tissue micro-arrays (TMA) (Table 1) using immunohistochemistry (Figure 4A). FKBP14 immunostainings occurred more strongly in the cytoplasm of gastric cancer tissues (Figure 4C–F). We also observe that an overexpression of FKBP14 protein was significantly associated with lymph node metastasis (P =0.016) (Figure 4B1), and neoplasm histologic Grade (P=0.021) (Figure 4B2). However, high FKBP14 levels were not associated with tissue type, age, gender, tumor stage, distant metastasis, TNM Stage (all P > 0.05) (Table 1).
Increased Expression of FKBP14 Is Associated with Lymph Node Metastasis, TNM Stage and Histologic Grade of Gastric Cancer Tissues in TCGA Database
An open database (The Cancer Genome Atlas database) was used to confirm our results. In this database, we analyzed the sequencing data of 414 gastric cancer tissues. Similar to our results, FKBP14 was upregulated upon lymph node metastasis (N2, N3, or N4) (P = 0.036) and when the histological grade was G3 or G4 (0.001). However, unlike our results, statistical significance was also observed for patients in TNM Stage III-IV in the TCGA database (0.031).
FKBP14 Is an Independent Prognostic Factor in Gastric Cancer
The effect of FKBP14 expression status on overall survival (OS) was assessed. Kaplan-Meier survival curves showed that a high expression of FKBP14 expression was significantly correlated to poor survival (P = 0.028) (Figure 5). Multivariate analysis using Cox proportional hazards model revealed that high FKBP14 expression was independent of lymph node metastasis (0.006) and of TNM disease stage (<0.001) (Table 2).
 |
Table 2 Prognostic Value of FKBP14 Expression for Survival Rate by Cox Proportional Hazards Model (*P <0.05; **P<0.01; ***P<0.0001) |
 |
Figure 5 The Kaplan-Meier survival curve from the 70 gastric cancer patients used in this study shows a high expression of FKBP14 expression is significantly correlated to poor survival (P < 0.05). |
Discussion
The low survival rate of GC is attributed to the following factors: (1) inadequate diagnostic tools for early detection and progression of GC; (2) unsuitable prognostic indicators; (3) low efficacy of current treatment modalities (including surgery and chemotherapy) in advanced GC; (4) lack of understanding in treatment resistance and tumor progression. All these issues can be fixed upon the development of adequate tools which can both diagnose and monitor GC to improve the clinical outcome of patients.
Herein, we initially observe that FKBP14 can effectively speed up cell growth and slow down apoptosis in the GC, which is in accordance with the study performed by Wang et al.10 Using the SGC7901 cell line, we observe that KBP14 knockdown significantly inhibits cell proliferation of GC cells. Most patients die of GC as the disease has already metastasized at the time of presentation. Cell invasion is a significant feature associated with cancer metastasis. One way through which cancer cells can form new tumors is through cell adhesion. Herein, we observe that FKBP14 can hasten cell growth and slows down apoptosis. Silencing of FKBP14 effectively suppressed the adhesion and invasion of GC cells. These results illustrate that FKBP14 is associated with GC metastasis.
By conducting Kaplan-Meier survival curves, we observe that a high FKBP14 expression is significantly associated with unfavourable survival. Univariate and multivariate analyses further confirm that FKBP14 is an independent predictor of lymph node metastasis and TNM stage, and that it may serve as a predictive biomarker of patient prognosis. Subgroup analyses further revealed that patients with a high expression of FKBP14 were significantly correlated with lymph node metastasis (P =0.016), and an advanced histologic grade (P =0.021). However, unlike the results of The Cancer Genome Atlas database, an overexpression of FKBP14 was not associated with an advanced TNM stage in the Chinese population.
Various genetic and epigenetic mutations are responsible for oncogenesis and cancer progression. Recently, there has been a strong association between FKBPs and cancer. This is mainly due to the increased activity of mammalian target of rapamycin (mTOR) by FKBPs, particularly in cells with no functional PTEN.11 Favorable management of cancer using immunosuppressants FK 506 and rapamycin highlights the likelihood of targeting FKBPs in cancer treatment.12 In leukemia, inhibition of FKBP51 has been shown to promote drug-induced apoptosis.13 Few reports have showed that FKBP14 is mutated in several malignancies. In ovarian cancer, knockdown of FKBP14-inhibited cell growth.7 In osteosarcoma, an increased expression of FKBP14 was correlated with metastasis and tumor stage.8 In vitro experiments showed that an under-expression of FKBP14 weakened cell invasion and inhibited the expression level of PCNA, CDK1, and CCNB1.14
This study is, to our knowledge, the first to investigate the expression level and effects of knocking down FKBP14 in the SGC7901 cell line, as well as correlate the clinicopathological factors and prognosis of FKBP14 in GC. Since FKBP14 is strongly associated with lymph node metastasis and TNM stage, our study implicates FKBP14 can be used to monitor disease progression. Few limitations need to be noted in our study. We did not investigate the signaling pathway of FKBP14 in GC. Also, we did not analyze any association between FKBP14 and chemoresistance. Further studies should address these issues.
Conclusion
Our study suggests patients exhibiting an overexpression of FKBP14 in GC promotes cell proliferation and migration. Moreover, high expressions of FKBP14 in GC are correlated with poor clinicopathological factors of GC and forecast a low OS for patients with advanced GC. Our results suggest overexpression of FKBP14 in GC is a potential biomarker for GC diagnosis, invasion, and prognosis.
Abbreviations
GC, Gastric cancer; OS, Overall Survival; IRS, Immunoreactivity scores; TMA, Tissue micro-arrays; TCGA, The Cancer Genome Atlas database.
Acknowledgement
The authors acknowledge Callum M.G. Walker for proof-editing.
Author Contributions
Roshan Ara Ghoorun and Xiao-Hua Wu: conception and design, data acquisition, and manuscript drafting. Hong-Lei Chen, Dong-Lin Ren, and Xiao-Bin Wu: data analysis and interpretation and critical revision of the manuscript. All authors have approved the final version of the manuscript to be published and have agreed to be accountable for all aspects of this work in ensuring the accuracy and integrity of this work.
Ethics Approval and Consent to Participate
Ethics approval was granted by the Sixth Affiliated Hospital of the Sun Yat-Sen University and the Eighth Affiliated Hospital of Sun Yat-Sen University.
Patient Consent for Publication
Only patients from the Sixth Affiliated Hospital of Sun Yat-Sen University were enrolled in this study. All patients provided a written consent form prior to the experiment. The Ethics Committee of the Sixth Affiliated Hospital of Sun Yat-Sen University approved this investigation.
Disclosure
All authors declare that they have no financial relationships to disclose and they report no conflicts of interest in this work.
References
1. Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of eighteen major cancers in 1985. Int J Cancer. 1993;54:594–606. doi:10.1002/(ISSN)1097-0215
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.v68.6
3. Ghartey-Kwansah G, Li Z, Feng R, et al. Comparative analysis of FKBP family protein: evaluation, structure, and function in mammals and Drosophila melanogaster. BMC Dev Biol. 2018;18(1):7. doi:10.1186/s12861-018-0167-3
4. Solassol J, Mange A, Maudelonde T. FKBP family proteins as promising new biomarkers for cancer. Curr Opin Pharmacol. 2011;11(4):320–325. doi:10.1016/j.coph.2011.03.012
5. Bursztejn AC, Baumann M, Lipsker D. Ehlers-Danlos syndrome related to FKBP14 mutations: detailed cutaneous phenotype. Clin Exp Dermatol. 2017;42(1):64–67. doi:10.1111/ced.2017.42.issue-1
6. Lu M, Miao Y, Qi L, Bai M, Zhang J, Feng Y. RNAi-mediated downregulation of FKBP14 suppresses the growth of human ovarian cancer cells. Oncol Res. 2016;23(6):267–274. doi:10.3727/096504016X14549667333963
7. Sun LY, Tao JZ, Yan B, Lin JS. Inhibitory effects of FKBP14 on human cervical cancer cells. Mol Med Rep. 2017;16(4):4265–4272. doi:10.3892/mmr.2017.7043
8. Wang K, Qi XJ, Liu HZ, Su H. MiR-361 inhibits osteosarcoma cell lines invasion and proliferation by targeting FKBP14. Eur Rev Med Pharmacol Sci. 2018;22(1):79–86. doi:10.26355/eurrev_201801_14103
9. Yang Z, Ghoorun RA, Fan X, et al. High expression of Beclin-1 predicts favorable prognosis for patients with colorectal cancer. Clin Res Hepatol Gastroenterol. 2015;39(1):98–106. doi:10.1016/j.clinre.2014.06.014
10. Wang R, Fang H, Fang Q. Downregulation of FKBP14 by RNA interference inhibits the proliferation, adhesion and invasion of gastric cancer cells. Oncol Lett. 2017;13(4):2811–2816. doi:10.3892/ol.2017.5781
11. Wang X, Proud CG. mTORC1 signaling: what we still don’t know. J Mol Cell Biol. 2011;3:206–220.
12. Kozany C, März A, Kress C, Hausch F. Fluorescent probes to characterise FK506-binding proteins. Chembiochem. 2009;10:1402–1410. doi:10.1002/cbic.v10:8
13. Tissing WJ, Meijerink JP, den Boer ML, Brinkhof B, Pieters R. mRNA expression levels of (co) chaperone molecules of the glucocorticoid receptor are not involved in glucocorticoid resistance in pediatric ALL. Leukemia. 2005;19(5):727–733. doi:10.1038/sj.leu.2403681
14. Huang Z, Li J, Du S, et al. FKBP14 overexpression contributes to osteosarcoma carcinogenesis and indicates poor survivaloutcome. Oncotarget. 2016;7(26):39872–39884. doi:10.18632/oncotarget.9524
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.