Back to Journals » Cancer Management and Research » Volume 11
Prognostic significance of FA score based on plasma fibrinogen and serum albumin in patients with epithelial ovarian cancer
Authors Li Y , Yang JN, Cheng SS, Wang Y
Received 8 April 2019
Accepted for publication 19 June 2019
Published 14 August 2019 Volume 2019:11 Pages 7697—7705
DOI https://doi.org/10.2147/CMAR.S211524
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lu-Zhe Sun
Yuan Li, Jia-Ni Yang, Shan-Shan Cheng, Yu Wang
Department of Gynecology and Obstetrics, The Affiliated Renji Hospital of Shanghai Jiaotong University of Medical College, Shanghai 200000, People’s Republic of China
Objective: To evaluate the significance of fibrinogenand albumin (FA) score based on preoperative peripheral blood plasma fibrinogen and serum albumin in the prognosis of patients with epithelial ovarian cancer (EOC).
Methods: Patients’ clinicopathological data of 186 cases of EOC were retrospectively collected, and these patients were divided into three groups according to their FA scores (both plasma fibrinogen and serum albumin abnormal were allocated a score of 2; one of them abnormal were allocated a score of 1; neither of them abnormal were allocated a score of 0; optimal cut-off point is taken as the critical point whether the value is abnormal or not). Correlation between FA score in patients with EOC as well as clinicopathological features and overall survival (OS) was analyzed.
Results: (1) Receiver operating characteristic curve showed that the optimal cut-off point of plasma fibrinogen in the preoperative peripheral blood of patients with EOC was 3.63 g/L. The optimal cut-off point for serum albumin level was 42.45 g/L. (2) There was no significant difference in age, tumor size, neutrophil count, lymphocyte count, C reactive protein and preoperative tumor marker CA125 between the three groups (FA score=0, FA score=1, FA score=2) (P>0.05). However, there was statistically significant difference in tumor grade, tumor stage and the presence of lymph node metastasis between different FA scoring groups (P<0.05). (3) Univariate and multivariate analyses showed that tumor size, tumor grade, tumor stage, plasma fibrinogen, serum albumin, FA score and tumor marker CA125 were statistically correlated with OS of EOC patients after surgery (P<0.05). The complex index FA score is superior to the single plasma fibrinogen and serum albumin when it comes to predicting prognosis. (4) FA score can better predict the prognosis of postoperative patients with EOC whose tumor size is ≥6 cm, whose EOC is advanced (stages III–IV) (P=0.0138) and whose tumor stage is medium or high grade (P=0.0005).
Conclusion: FA score is closely related to the clinicopathological characteristics and OS of patients with EOC and is an independent risk factor indicating the prognosis of EOC patients.
Keywords: epithelial ovarian cancer, fibrinogen, albumin, prognosis
Introduction
Ovarian cancer (OC) is the most lethal gynecological cancer and a leading cause of cancer death among women around the world.1 It is demonstrated that the age‑standardized incidence rate of OC increased substantially by 28.6% from 1990 to 2016.2 Although much progress in optimization of current treatment has emerged over the past decade, high-resource countries such as the United States and Canada, and remain at only 47% 5 years after diagnosis.3 Moreover, OC has a high recurrence rate and lymph node metastasis. Lack of sophisticated approaches for early diagnosis of OC, most patients are found to have OC with advanced stage which might count much for poor prognosis. Therefore, it is necessary to further seekfor the effective biomarkers to identify patients who may have poor clinical outcomes after surgical treatment.
Increasing evidence shows that inflammation plays a significant role in cancer progression4 and metastasis5 of a variety of solid tumors, and several inflammatory markers have been reported to be associated with outcomes of malignant tumor patients, such as lymphocyte-to-monocyte ratio6 C-reactive protein (CRP) to albumin ratio7 and platelet-to-lymphocyte ratio.8 Fibrinogen,9 a 340-kDa liver glycoprotein, which has been reported to play a vital part in inflammatory and clot formation, as well as tumor cell proliferation, migration and angiogenesis, can independently predict prognosis in various human cancers.10–12 Serum albumin levels, labeled as important indicator of systemic inflammatory response and nutritional status have also been regarded as prognosis indicators in specific solid tumors.13–16
Recently, a novel cumulative prognostic score, combination of fibrinogen and albumin has been proposed by Matsuda et al as a more effective scoring system to predict prognosis of solid tumors such as non-small cell lung cancer,17 high-grade gliomas,18 esophageal cancer,19 and upper urinary tract urothelial carcinoma.20
To the best of our knowledge, though many prognostic indicators based on systemic inflammation levels and nutritional conditions of patients with OC are published,7,12,21,22 the value of fibrinogen and albumin (FA) score has not been used to detect in patients with epithelial ovarian cancer (EOC). Thus, we decided to clarify the prognostic value of preoperative FA score in a cohort of Chinese patients with EOC.
Materials and methods
Patients
Between May 2008 and October 2013, 186 patients with pathologically diagnosed EOC who underwent standardized cytoreductive surgery at the Department of Obstetrics and Gynecology, Renji Hospital of Shanghai Jiaotong University School of Medicine were retrospectively reviewed in our cohort. Patients were excluded from our research if they: 1) underwent neoadjuvant therapy; 2) had ≥2 primary tumors; 3) had coagulation disorders or metabolic diseases before; 4) were lost to follow-up; and 5) perioperative surgery-related mortality. All clinicopathological features were obtained from the patients’ records. Pathological stages of patients were classified by the International Federation of Gynecology and Obstetrics (Edition, 2014). The laboratory hematological data of patients were tested routinely within 3 days prior to surgery. This study was ratified by the Ethics Committee of Renji Hospital of Shanghai Jiaotong University School of Medicine. Written informed consent was obtained from all the patients in this study.
Follow-up
For all the patients, follow-up started from the date of surgery. Patients were generally followed quarterly for the first year, semi-annually for the following 2 years and annually thereafter. Follow-up of patients included blood tests, urine tests, computed tomography, and physical examination. The last follow-up was until October 31, 2018, including verification of the clinical attendance records and direct telecommunications with the patients or their families. Overall survival (OS) was measured from the date of surgery to the date of death from any cause, or the date of last follow-up visit. Progression or relapse was identified according to the latest radiographic evidence.
Fibrinogen and albumin measurement (FA score)
The pretreatment plasma fibrinogen and serum albumin levels were examined in samples obtained before breakfast within 3 days prior to surgery. In terms of the FA score, patients with elevated fibrinogen and decreased albumin levels were allocated a score of 2, those with only one of these abnormalities were allocated a score of 1, and those with neither of these abnormalities were allocated a score of 0. The fibrinogen and albumin cut-off value were determined from receiver operating characteristic (ROC) curves. The fibrinogen cut-off value was defined as 3.63 g/L and albumin as 42.45 g/L.
Statistical analysis
Differences of clinicopathological parameters and relative prognostic parameters between three groups were evaluated by independent-samples Student's t-test or Fisher’s exact test according to the distribution of data. The survival curves were calculated using the Kaplan–Meier method. ROC analysis was performed to determine possible cut-off values of the continuous serum fibrinogen, albumin. The significant parameters identified by univariate analysis were evaluated using multivariate analysis employing the Cox proportional hazards model. All reported p-values were two-sided. P<0.05 was considered significant, and 95% CIs were calculated. All analyses were performed using the SPSS Statistics version 22.0 (IBM Corporation, Armonk, NY, USA).
Results
Baseline clinicopathological characteristics
Baseline pathological characteristics of 186 patients included in this study were summarized and presented in Table 1. The mean age of these patients was 59.2 years. All of them were pathologically diagnosed as EOC. There were 136 patients with middle- and high-level tumor grade, accounting for 73.1% of the total number; 123 patients were found with tumor diameter ≥6 cm during the operation, accounting for 66.1%. There were 134 patients with advanced tumors (stages III–IV), accounting for 72.0%. A total of 120 patients were diagnosed with lymph node metastasis (64.5%).
 |
Table 1 Clinicopathological features of 186 patients with epithelial ovarian cancer |
Table 1 also shows the relationship between the clinicopathological characteristics and patients with different FA scores. The results showed that there was no significant difference between the three groups in age (P=0.200), tumor size (P=0.738), lymphocyte count (P=0.078), CRP (P=0.126), neutrophil count (P=0.274) and tumor marker CA125 (P=0.463). However, tumor grade (P=0.006), tumor stage (P=0.000) and the presence of lymph node metastasis (P=0.014) were statistically different between different FA scoring groups (P<0.05). The higher the FA score, the worser the tumor differentiation and the higher the tumor stage.
Determination of the optimal cut-off value
As can be calculated from Figure 1, the optimal cut-off point of preoperative peripheral fibrinogen in patients with EOC was 3.63 g/L and the area under the curve (AUC) was 0.654 accordingly. The optimal cut-off point of peripheral albumin level was 42.45 g/L, and the AUC was 0.628. The AUC of tumor marker CA125 was 0.614. The AUC of complex indicator FA score was 0.69, so FA score was selected as the best indicator to evaluate the prognosis of patients among the four indicators. Patients were divided into three groups based on the FA score and the corresponding FA scores were 0, 1, 2. As we can see in Figure 2, there were 38 patients (20.4%) whose score was 0, 66 patients (35.4%) with a score of 1 and 82 patients (44.2%) with a score of 2 accordingly.
 |
Figure 1 Optimal cut-off point of plasma fibrinogen and serum albumin was determined by receiver operating characteristic (ROC) curve. |
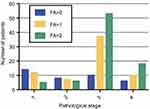 |
Figure 2 Correlation between preoperative FA score and postoperative pathological stage. |
Univariate and multivariate survival analysis
At the end of patient follow-up, 83 (44.62%) of the 186 patients died. OS ranged from 2.0 to 99.1 months, with a median survival of 45.5 months.
The results of univariate and multivariate analyses of OS are summarized in Table 2. By univariate analysis, we found that the following factors were associated with short OS in patients with EOC: higher tumor grade, higher tumor stage, higher plasma fibrinogen, lower serum albumin, higher preoperative FA score, and higher preoperative level of tumor marker CA125 (all P≤0.05). We conducted multivariate survival analysis using Cox regression model, and the relevant results are also shown in Table 2. It is demonstrated that single plasma fibrinogen and single serum albumin both can be an independent factor to predict prognosis. Moreover, the FA scoring system (1 point relative to 0 point, P=0.047, risk ratio (HR) = 1.058; 2 points relative to 0, P=0.033, HR=2.051) can better be used as an independent factor to guide postoperative prognosis of patients with EOC. We can conduct that the complicated new index FA score is superior to single index of plasma fibrinogen or serum albumin when it comes to predicting prognosis. Tumor marker CA125 (P=0.012, HR=1.934, and 95% CI=1.197–2.234), tumor grade (middle and high grade versus low grade P=0.008, HR=1.855, 95% CI=1.473–2.547) and tumor stage (stages III–IV versus stages I–II P=0.026, HR=1.602, 95% CI=1.031–2.289) can also be independent factors affecting OS.
 |
Table 2 Univariate and multivariate survival analysis of overall survival (OS) in 186 patients with ovarian cancer |
Kaplan–Meier survival analysis
In Figure 3, we found that the FA score could better predict the OS of OC patients (P<0.0001) compared with cancer-specific survival (CSS) (P=0.0002). For patients with tumor size ≥6 cm (P=0.0005), FA score can better indicate prognosis than patients with tumor size <6 cm (P=0.0352). It is found in Figure 4 that FA scoring system could not predict the prognosis of patients in the early stage of epithelial ovarian tumor (The International Federation of Gynecology and Obstetrics [FIGO] stages I–II) (P=0.1409). For patients with advanced epithelial ovarian tumor (FIGO stages III–IV), FA scoring system can predict the prognosis of OC patients (P=0.0138) with statistical significance. FA scoring system can better predict the prognosis of patients with middle/high-grade tumors compared with that of low-grade tumors.
Discussion
Ovarian cancer is one of the three most common gynecological malignancies with the highest mortality rate.23,24 Its clinical symptoms are insidious, so most patients are already in the advanced stage when they are diagnosed. With rapid tumor progression, invasion and metastasis, as well as high recurrence rate, studies have shown that 70% of newly diagnosed patients with OC have developed an advanced stage (FIGO stages III–IV) and the 5-year OS rate of these patients is only 45%.25,26 Over 75% of patients with late-stage OC die of their disease.27 For OC patients, even among individuals with the same pathological stage and receiving the same treatment, the prognosis varies widely. With the rapid development of modern molecular biotechnology, it is an attractive prospect to accurately evaluate the prognosis of malignant tumor patients which can help to guide individualized clinical treatment in patients with cancer. Reliable markers for screening the patients whose prognosis is poor or at high risk of easy relapse can remind doctors to provide prompt postoperative adjuvant therapy and immune-mediated gene therapy, in order to achieve a better prognosis.
At present, a number of basic experiments and clinical researches worldwide have been conducted to find exact serological laboratory indicators in the hope of a more reliable and accurate judgment of patients with various malignant tumors. Neutrophil/lymphocyte ratio,28 lymphocyte/monocyte ratio,29 serum albumin30 and plasma fibrinogen31 have been confirmed to have an association with prognosis of special kinds of cancers.
As is well known, nutrition status and inflammation level have been a hot spot in tumorigenesis, metastasis and recurrence.32–34 Activated coagulation and inflammation which are found to promote tumor invasion and metastasis are commonly related to poor prognosis. Plasma fibrinogen is considered to be a key factor in the regulation of inflammatory status and cancer progression by mediating the proliferation and migration of tumor cells and angiogenesis.35 As a molecular bridge, plasma fibrinogen can stabilize the adhesion between tumor cells, platelets, and endothelial cells.36–38 In addition, there is another potential influence mechanism that fibrinogen can block natural killer of tumor cells or cytotoxic agents that target tumor cells to kill tumor cells and help tumor cells escape immune surveillance, thereby promoting tumor cell growth. Low serum albumin may represent the impaired nutritional status of the host. This state of malnutrition can weaken human defense mechanisms, including cellular immunity, humoral immunity, and phagocytic function.39 Studies have shown that hypoalbuminemia is associated with poor prognosis and short OS in a variety of malignancies.14,15,40 A recent systematic review and dose–response meta-analysis which included 12 cohort studies have convinced that preoperative serum albumin can be used as an independent prognostic predictor of OS in EOC patients.
FA score is a complicated indicator composed of plasma fibrinogen and serum albumin. On the one hand, the index can represent the body’s inflammatory and blood coagulation system level, on the other hand, it can also reflect the patient’s nutritional status. It can reflect the body status three-dimensionally which is better than single index fibrinogen or albumin. It is reasonable to speculate FA score as a prognostic scoring system in EOC patients.
In our clinical study, 186 patients with EOC who were admitted to Renji hospital from May 2008 to December 2013 were retrospectively selected to analyze the correlation between FA score and their clinicopathological characteristics and OS.
This study showed that a higher FA score was significantly correlated with poor tumor differentiation, higher stage and the presence of lymph node metastasis in patients with EOC. Furthermore, both univariate and multivariate analyses indicated that FA score could be used as an independent predictor of OS in patients with EOC. We also carried out subgroup analysis according to different histological types, tumor size, grading, staging of EOC, in order to explore the specific value of FA score in predicting the prognosis of patients with malignant tumors in specific level. Firstly, we found that FA score was a better predictor of OS than CSS. For patients with EOC whose tumor size is ≥6 cm, FA score indicates prognosis better compared with those with tumor size <6 cm. In terms of tumor stage, FA scoring system could not predict the prognosis of patients in the subgroup of early stage of EOC (FIGO stages I–II). For patients with advanced EOC (FIGO stages III–IV), the advantage of the FA scoring system is demonstrated, which can better predict the prognosis of OC patients. For patients with a definite diagnosis of a medium/high-grade ovarian tumor, the FA scoring system is a better predictor of OS.
In conclusion, the nutrition and coagulation-based FA score might be a more promising prognostic predictor than CA125 in EOC patients. Measurements of fibrinogen and albumin were carried out at low cost, were less traumatic, and were easily accessible in clinical practice. Through the detection of preoperative plasma fibrinogen and serum albumin in the patients’ peripheral blood, FA score was established to guide the prognosis of patients according to its score, which is expected to be an effective method for prognosis and guiding diagnosis and treatment of target OC patients.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi:10.3322/caac.21332
2. Lan CY, Wang Y, Xiong Y, et al. Apatinib combined with oral etoposide in patients with platinum-resistant or platinum-refractory ovarian cancer (AEROC): a phase 2, single-arm, prospective study. Lancet Oncol. 2018;19(9):1239–1246. doi:10.1016/S1470-2045(18)30349-8
3. Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin. 2019. doi:10.3322/caac.21559
4. Ma M, Wang J, Hu Y, Weng M, Liu X, Wang Y. Prognostic value of inflammatory biomarkers in gastric cancer patients and the construction of a predictive model. Dig Surg. 2018;1–10. doi:10.1159/000493432
5. Zhang LX, Wei ZJ, Xu AM, Zang JH. Can the neutrophil-lymphocyte ratio and platelet-lymphocyte ratio be beneficial in predicting lymph node metastasis and promising prognostic markers of gastric cancer patients? Tumor maker retrospective study. Int J Surg. 2018;56:320–327. doi:10.1016/j.ijsu.2018.06.037
6. Lin GN, Peng JW, Liu DY, Xiao JJ, Chen YQ, Chen XQ. Increased lymphocyte to monocyte ratio is associated with better prognosis in patients with newly diagnosed metastatic nasopharyngeal carcinoma receiving chemotherapy. Tumour Biol. 2014;35(11):10849–10854. doi:10.1007/s13277-014-2362-6
7. Liu Y, Chen S, Zheng C, et al. The prognostic value of the preoperative C-reactive protein/albumin ratio in ovarian cancer. BMC Cancer. 2017;17(1):285. doi:10.1186/s12885-017-3220-x
8. Zhang M, Huang XZ, Song YX, Gao P, Sun JX, Wang ZN. High platelet-to-lymphocyte ratio predicts poor prognosis and clinicopathological characteristics in patients with breast cancer: a meta-analysis. Biomed Res Int. 2017;2017:9503025. doi:10.1155/2017/9503025
9. Luo Y, Kim HS, Kim M, Lee M, Song YS. Elevated plasma fibrinogen levels and prognosis of epithelial ovarian cancer: a cohort study and meta-analysis. J Gynecol Oncol. 2017;28(3):e36. doi:10.3802/jgo.2017.28.e36
10. Shehata AMF, Aldesoky AI, Gohar SF. Plasma fibrinogen level as possible prognostic biomarker in diffuse large B-cell lymphoma. Hematology. 2019;24(1):103–107. doi:10.1080/10245332.2018.1519932
11. Liang Y, Wang W, Que Y, et al. Prognostic value of the fibrinogen/albumin ratio (FAR) in patients with operable soft tissue sarcoma. BMC Cancer. 2018;18(1):942. doi:10.1186/s12885-018-4242-8
12. Marchetti C, Romito A, Musella A, et al. Combined plasma fibrinogen and neutrophil lymphocyte ratio in ovarian cancer prognosis may play a role? Int J Gynecol Cancer. 2018;28(5):939–944. doi:10.1097/IGC.0000000000001233
13. Muller C, Stift A, Argeny S, et al. Delta albumin is a better prognostic marker for complications following laparoscopic intestinal resection for Crohn’s disease than albumin alone – a retrospective cohort study. PLoS One. 2018;13(11):e0206911. doi:10.1371/journal.pone.0206911
14. Li C, Zhang XY, Peng W, et al. Preoperative albumin-bilirubin grade plus platelet-to-lymphocyte ratio predict the outcomes of patients with BCLC stage A hepatocellular carcinoma after liver resection. Medicine (Baltimore). 2018;97(29):e11599. doi:10.1097/MD.0000000000011599
15. Huang QX, Ma J, Wang YS. Significance of preoperative ischemia- modified albumin in operable and advanced gastric cancer. Cancer Biomark. 2018;22(3):477–485. doi:10.3233/CBM-171090
16. Yamamoto M, Saito H, Uejima C, et al. Combination of serum albumin and cholinesterase levels as prognostic indicator in patients ith colorectal cancer. Anticancer Res. 2019;39(2):1085–1090. doi:10.21873/anticanres.13217
17. Chen P, Wang C, Cheng B, et al. Plasma fibrinogen and serum albumin levels (FA score) act as a promising prognostic indicator in non-small cell lung cancer. Onco Targets Ther. 2017;10:3107–3118. doi:10.2147/OTT.S138854
18. He ZQ, Duan H, Ke C, et al. Evaluation of cumulative prognostic score based on pretreatment plasma fibrinogen and serum albumin levels in patients with newly diagnosed high-grade gliomas. Oncotarget. 2017;8(30):49605–49614. doi:10.18632/oncotarget.17849
19. Matsuda S, Takeuchi H, Kawakubo H, et al. Prognostic impact of change in the fibrinogen and albumin score during preoperative treatment in esophageal cancer patients. World J Surg. 2017;41(11):2788–2795. doi:10.1007/s00268-017-4074-8
20. Cui J, Yu M, Zhang N, et al. Prognostic scores based on the preoperative plasma fibrinogen and serum albumin level as a prognostic factor in patients with upper urinary tract urothelial carcinoma. Oncotarget. 2017;8(40):68964–68973. doi:10.18632/oncotarget.16483
21. Ayhan A, Gunakan E, Alyazici I, Haberal N, Altundag O, Dursun P. The preoperative albumin level is an independent prognostic factor for optimally debulked epithelial ovarian cancer. Arch Gynecol Obstet. 2017;296(5):989–995. doi:10.1007/s00404-017-4511-9
22. Huang QT, Zhou L, Zeng WJ, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in ovarian cancer: a systematic review and meta-analysis of observational studies. Cell Physiol Biochem. 2017;41(6):2411–2418. doi:10.1159/000475911
23. Suszynska M, Klonowska K, Jasinska AJ, Kozlowski P. Large-scale meta-analysis of mutations identified in panels of breast/ovarian cancer-related genes – providing evidence of cancer predisposition genes. Gynecol Oncol. 2019. doi:10.1016/j.ygyno.2019.01.027
24. Beamer LC, Breast H. Hereditary ovarian cancer: implications for the oncology nurse. Semin Oncol Nurs. 2019;35(1):47–57. doi:10.1016/j.soncn.2018.12.001
25. Vergote I. Role of surgery in ovarian cancer: an update. Acta Chir Belg. 2004;104(3):246–256.
26. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
27. Banerjee S, Kaye SB. New strategies in the treatment of ovarian cancer: current clinical perspectives and future potential. Clin Cancer Res. 2013;19(5):961–968. doi:10.1158/1078-0432.CCR-12-2243
28. Wang S, Ma Y, Sun L, et al. Prognostic significance of pretreatment neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in patients with diffuse large B-cell lymphoma. Biomed Res Int. 2018;2018:9651254. doi:10.1155/2018/9651254
29. Luo P, Cai W, Yang L, et al. Prognostic significance of pretreatment lymphocyte/monocyte ratio in retroperitoneal liposarcoma patients after radical resection. Cancer Manag Res. 2018;10:4727–4734. doi:10.2147/CMAR.S171602
30. Wei Y, Xu H, Dai J, et al. Prognostic significance of serum lactic acid, lactate dehydrogenase, and albumin levels in patients with metastatic colorectal cancer. Biomed Res Int. 2018;2018:1804086. doi:10.1155/2018/1804086
31. Song H, Kuang G, Zhang Z, Ma B, Jin J, Zhang Q. The prognostic value of pretreatment plasma fibrinogen in urological cancers: a systematic review and meta-analysis. Journal of Cancer. 2019;10(2):479–487. doi:10.7150/jca.26989
32. Arthur R, Williams R, Garmo H, et al. Serum inflammatory markers in relation to prostate cancer severity and death in the Swedish AMORIS study. Int J Cancer. 2018;142(11):2254–2262. doi:10.1002/ijc.31256
33. Sun KY, Xu JB, Chen SL, et al. Novel immunological and nutritional-based prognostic index for gastric cancer. World J Gastroenterol. 2015;21(19):5961–5971. doi:10.3748/wjg.v21.i19.5961
34. Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6(10):715–727. doi:10.1038/nri1936
35. Li W, Tang Y, Song Y, et al. Prognostic role of pretreatment plasma d-dimer in patients with solid tumors: a systematic review and meta-analysis. Cell Physiol Biochem. 2018;45(4):1663–1676. doi:10.1159/000487734
36. Savage B, Cattaneo M, Ruggeri ZM. Mechanisms of platelet aggregation. Curr Opin Hematol. 2001;8(5):270–276.
37. Qiu J, Yu Y, Fu Y, Ye F, Xie X, Lu W. Preoperative plasma fibrinogen, platelet count and prognosis in epithelial ovarian cancer. J Obstet Gynaecol Res. 2012;38(4):651–657. doi:10.1111/j.1447-0756.2011.01780.x
38. Palumbo JS, Potter JM, Kaplan LS, Talmage K, Jackson DG, Degen JL. Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen-deficient mice. Cancer Res. 2002;62(23):6966–6972.
39. Ataseven B, du Bois A, Reinthaller A, et al. Pre-operative serum albumin is associated with post-operative complication rate and overall survival in patients with epithelial ovarian cancer undergoing cytoreductive surgery. Gynecol Oncol. 2015;138(3):560–565. doi:10.1016/j.ygyno.2015.07.005
40. Borg N, Guilfoyle MR, Greenberg DC, Watts C, Thomson S. Serum albumin and survival in glioblastoma multiforme. J Neurooncol. 2011;105(1):77–81. doi:10.1007/s11060-011-0562-0
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


