Back to Journals » OncoTargets and Therapy » Volume 13
Prognostic Significance of Elevated Preoperative Serum CA125 Levels After Curative Hepatectomy for Hepatocellular Carcinoma
Authors Huang Y , Zeng J, Liu T, Lin X, Guo P, Zeng J, Zhou W, Liu J
Received 29 October 2019
Accepted for publication 7 May 2020
Published 22 May 2020 Volume 2020:13 Pages 4559—4567
DOI https://doi.org/10.2147/OTT.S236475
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianmin Xu
Yao Huang,1– 3 Jianxing Zeng,2,4 Teng Liu,2 Xinju Lin,2 Pengfei Guo,4 Jinhua Zeng,1– 3 Weiping Zhou,5 Jingfeng Liu1– 4
1Department of Hepatobiliary Surgery, The First Affiliated Hospital of Fujian Medical University, Fuzhou 350005, People’s Republic of China; 2Department of Hepatic Surgery, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou 350025, People’s Republic of China; 3The Liver Center of Fujian Province, Fujian Medical University, Fuzhou 350025, People’s Republic of China; 4Southeast Big Data Institute of Hepatobiliary Health, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou 350025, People’s Republic of China; 5The Third Department of Hepatic Surgery, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai 200438, People’s Republic of China
Correspondence: Jingfeng Liu
Department of Hepatobiliary Surgery, The First Affiliated Hospital of Fujian Medical University, Fuzhou 350005, People’s Republic of China
Tel/ Fax +86 591 8370 5927
Email [email protected]
Objective: The aim of this study was to investigate predictive and prognostic significance of elevated carbohydrate antigen 125 (CA125) serum level preoperatively.
Methods: A total of 3440 HCC patients were retrospectively enrolled into this study, and all of them underwent curative hepatectomy. The clinical and pathological variables together with CA125, AFP serum level were collected at diagnosis and postoperative care stages. A chi-square test was used to compare the differences between variables. Overall survival (OS) and recurrence-free survival (RFS) were measured with the Kaplan–Meier method. To estimate prognostic factors, a multivariate Cox regression analysis was performed.
Results: Of the 3440 enrolled patients, 409 (11.9%) exhibited elevated preoperative serum CA125 level, and high preoperative serum CA125 level was significantly associated with younger age, female, higher ALBI grade, higher serum AFP level, blood transfusion, more operative bleeding loss, larger tumor size, multiple tumor, increased macro- or micro-vascular invasion, Edmondson grade III–IV, absence of tumor capsular, satellite nodules, liver cirrhosis, more advanced TNM stages and BCLC stages. HCC patients with high preoperative serum CA125 level usually had a shorter OS rate and experienced a higher probability of recurrence than those with normal preoperative serum level of CA125 (p< 0.0001). The multivariate analysis suggested that elevated serum CA125 level serves as an independent predictor of OS and RFS in HCC patients after surgical resection.
Conclusion: Elevated preoperative serum CA125 correlated with many malignant characterizations of HCC and served as an independent prognostic factor of OS and RFS.
Keywords: CA125, hepatocellular carcinoma, prognosis, hepatectomy
Introduction
As the fifth most common and malignant tumor in the world, hepatocellular carcinoma (HCC) is deemed as the second cause of cancer-related death.1,2 In China more than 400 million persons are suffering from liver disease,3,4 contributing to 450,000 new cases of HCC and 383,000 patients death each year.3,5 During the past decades, curative hepatectomy has been widely applied and considered as the first choice to treat HCC.6 However, HCC patients frequently subject to recurrence/metastasis postoperatively in clinical practice. So that, only 25–40% patients survive more than 5 years, and 70% of them suffers from recurrence in 5 years after curative hepatectomy.7–9 Therefore, it remains valuable to discover and identify novel biomarker and prognostic factor to diagnosis or predict the outcome of HCC patients postoperatively. Carbohydrate antigen 125 (CA125) is one of the widely accepted serum cancer biomarkers and has been identified to be associated with worse prognosis in ovarian cancer, gastric cardia cancer,10 pancreatic adenocarcinoma,11 colorectal cancer,12 and uterine cancer.13 In clinic, elevated serum CA125 is frequently identified in hepatocellular carcinoma. Unfortunately, the prognostic significance of serum CA125 has not been well elucidated so far. Therefore, investigating the clinical significance of serum CA125 and evaluating its prognostic value are helpful to identify a new prognostic predictor of HCC patients after hepatectomy.
Methods
Patients and Study Design
This study was conducted to the ethical guideline of the 1975 Declaration of Helsinki and was approved by the institutional ethics committee of Mengchao Hepatobiliary Hospital of Fujian Medical University. Informed consent was obtained from each patient for their data to be used for research purposes. HCC patients who underwent hepatectomy between January 2008 and December 2015 were extracted from primary liver cancer big data (PLCBD) by an IT engineer, and then was verified by three independent researchers (Yao Huang, Jianxing Zeng, Teng Liu) in this study. Patient information included demographic information, surgical factors, laboratory parameters and tumor characteristics.
The inclusion criteria were set as follows: (1) 0 to 1 score of performance status;14 (2) well liver function; (3) no evidence of extrahepatic metastasis and distant metastasis; (4) no history of preoperative anticancer treatment; (5) no history of other malignancies, and (6) curative hepatectomy with tumor-negative resection margins (R0 resection).15 Meanwhile, patients who received palliative tumor resection, received recurrent tumor resection, had incomplete clinical data, died of severe surgical complications, and lost to follow-up within 60 days after discharge were excluded.
All enrolled patients received routine serological examination, imaging examination, and histopathological diagnosis. Histologic grading of HCC was based on the Edmondson-Steiner classification.16 The criterion of the American Association for the Study of Liver Diseases was used for preoperative clinical diagnosis of HCC.17 All patients were staged using the American Joint Committee on Cancer (AJCC) staging system17 and Barcelona Clinic Liver Cancer (BCLC) staging classification.18
The cut-off of CA125 was based on normal reference value. We defined CA125 ≤ 35kU/L as normal CA125 group (N-CA125) and CA125 >35kU/L as high CA125 group (H-CA125). The clinicopathological features and the prognosis of HCC patients in N-CA125 group were compared with those in H-CA125 group.
Follow-Up
Patients’ follow-up was strictly performed every 3 months in the first 2 years after surgery and later every 3 to 6 months. In each time of the follow-up, patients still received the following examination: liver function, serum AFP and imaging. The diagnostic criteria for tumor recurrence were the same as for the initial diagnosis.17 The follow-up was censored on 30th October 2018. The end-points of the study were overall survival (OS) and recurrence-free survival (RFS). OS was defined as the interval between the date of surgery and the date of death or the last follow-up. And RFS was calculated as the time ranged from the date of surgery to the date of recurrence or death, or the date of last follow-up.
Statistical Analysis
Categorical variables were grouped on the basis of normal reference value or clinical judgment. Categorical variables were compared using the chi-square test or Fisher exact test. Continuous variables were compared using the Student’s t-test for variables with a normal distribution or Mann–Whitney U-test for variables with an abnormal distribution. Kaplan–Meier method was used to estimate the OS and RFS rate, and differences between two groups were analyzed by Log-rank test. Subgroup analyzes were conducted based on the Cox model. All statistical tests were 2-tailed and a p-value of less than 0.05 was considered statistically significant. All statistical analysis was performed with R version 3.5.2 (http://www.r-project.org/).
Categorical variables are presented as no. (%); the continuous variables with normal distribution were presented with Mean (SD); and the continuous variables with abnormal distribution were showed as median (interquartile range).
Results
Comparison of Clinicopathological Features Among HCC Patients with Normal and High Serum CA125 Levels
During the study period, there were a total of 6623 patients with HCC. 3183 patients were excluded because of extrahepatic metastasis (n=207), preoperative anticancer treatment (n =464), recurrent tumor resection (n=256), history of other malignancies (n =56), palliative tumor resection (n=174), incomplete clinical data (n=1739), perioperative death (n=33), and early loss to follow-up after discharge (n=254) (Figure 1).
 |
Figure 1 The flow chart of selected patients. Abbreviations: HCC, hepatocellular carcinoma; CA125, carbohydrate antigen 125. |
According to our inclusion and exclusion criteria, 3440 patients with median age of 50.0 years old (ranged from 41 to 60 years old) were enrolled in this study. Among these patients, 3031 HCC patients were divided into N-CA125 group, whereas 409 HCC patients were distributed to H-CA125 group.
As summarized in Table 1, HCC patients in H-CA125 group were younger and higher probability of female comparing to the patients in N-CA125 group. Moreover, higher serum CA125 level was significantly associated with higher ALBI grade, higher serum AFP level, blood transfusion, more operative bleeding loss, larger tumor size, multiple tumor, increased macro- or micro-vascular invasion, Edmondson grade III–IV, absence of tumor capsulation, satellite nodules, liver cirrhosis, more advanced TNM stages and BCLC stages.
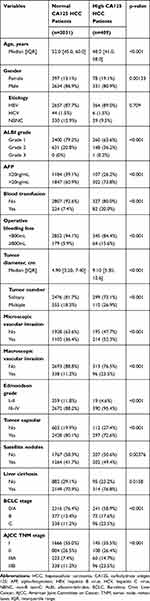 |
Table 1 Comparison of Clinicopathological Features Between Normal CA125 and High CA125 HCC Patients Who Underwent Curative Hepatectomy |
Comparison of Prognosis Between Normal CA125 and High CA125 HCC Patients
Kaplan–Meier survival curve was applied to evaluate the survival state of enrollments in this study. As shown in Figure 2A, the OS ratio at 1, 3, 5 year was 70.8%, 45.3% and 29.5%, respectively, for HCC patients in H-CA125 group and 87.9%, 66.2% and 48.4%, respectively, for HCC patients in N-CA125 group (p<0.0001, Figure 2A). Additionally, the RFS ratio at 1, 3, 5 year was 45.0%, 28.0% and 17.8%, respectively, for HCC patients in H-CA125 group and 66.8%, 44.0% and 30.2%, respectively, for HCC patients in N-CA125 group (p<0.0001, Figure 2B). These data reflected that HCC patients in H-CA125 group usually suffered from a worse OS and RFS than the patients in N-CA125 group after surgery.
After stratification according to AFP levels, HCC patients in H-CA125 group had a significantly worse prognosis than the patients in N-CA125 group (Figure 3).
Risk Factors Associated with Overall Survival and Tumor Recurrence After Curative Hepatectomy
Fifteen variables were statistically analyzed as risk factors for overall survival and tumor recurrence using a univariate analysis (Table 2). The multivariate model identified 10 variables together with serum CA125 as independent prognostic factors associated with overall survival (Table 3). Eleven variables including CA125 were the independent risk factors associated with tumor recurrence by multivariate analysis (Table 3). The multivariate analysis revealed that HCC patients in N-CA125 group had a significantly better OS and RFS rate than the patients in H-CA125 group [hazard ratio (HR) for OS = 1.267; 95% confidence interval (CI): 1.104–1.454; p = 0.001; hazard ratio (HR) for RFS = 1.172; 95% confidence interval (CI): 1.036–1.327; p = 0.012].
 |
Table 2 Univariate Analysis of Factors Associated with Overall Survival and Tumor Recurrence After Curative Hepatectomy |
 |
Table 3 Multivariate Analysis of Factors Associated with Overall Survival and Tumor Recurrence After Curative Hepatectomy |
Discussion
Serum CA125 is a “classical” biomarker of ovarian cancer, but its diagnostic and prognostic value in HCC has not been well clarified. In the present study, a statistical analysis of a big cohort of HCC patients with long term follow-up was performed and indicated that preoperative serum CA125 is closely associated with OS and RFS of HCC patients after curative hepatectomy.
CA125 was discovered for the first time and identified as a tumor marker of ovarian cancer in 1981 by Niloff et al.19 Nowadays, serum CA125 is not only used in the diagnosis of many kinds of tumors, but also an effective biomarker to predict the prognosis of tumors. It can predict the recurrence of various cancers, such as ovarian cancer,20 breast cancer,21 lung cancer,22 and gastric cancer.23,24 Therefore, the clinical significance of CA125 has extensively accepted, which indicated that CA125 might also be a HCC biomarker.
CA125 is a macromolecule glycoprotein synthesized and stored in coelomic epithelial cells, which is produced by MUC16 gene and contains 22,000 amino acids. It normally cannot enter the blood circulation due to the blocking action of the basement membrane and the connection between cells. However, when the tissue undergoes malignant transformation, intracellular CA125 concentrates on the edge of cells, depolarizes local cell membranes and is released off the cells.
Theoretically, there are two possible reasons for the increase of serum CA125. One is that the production of CA125 is increased during the development and progression of malignant tumors. It is well known that hypoxia accelerates malignant tumors to proliferate and production of MUC16, which cause synthesizing more CA125. The other reason is that more CA125 may be released into the blood circulation due to various tumor behaviors of severe cell damage, angiogenesis, vascular invasion and destruction.25 On the other hand, the liver is the main site for degrading glycoproteins derived from the blood circulation.26 It is proved that serum CA125 levels are higher in HCC patients with liver cirrhosis and hepatitis due to weak metabolic ability in dysfunctional liver.27 Thus, elevated serum CA125 might indicate that the tumor has broken through the capsulation, stayed at an advanced stage and the patients subjected to worse liver function. About 11.9% (409/3440) of enrolled patients in this study had elevated preoperative serum CA125 levels. It is not a high proportion, probably because that the enrolled patients are all resectable and have well liver function.
The results of this study showed that preoperative serum CA125 was significantly associated with many clinicopathological factors in patients with HCC who underwent curative hepatectomy, including larger tumor size, multiple tumor, increased macro- or micro-vascular invasion, absence of tumor capsulation, satellite nodules, more advanced TNM stages and BCLC stages. These indicators all point to more advanced HCC. This correlation may contribute to the fact that CA125 was a valuable tool to predict the prognosis of HCC after curative hepatectomy.
The results of this study showed that the patients with HCC who had elevated preoperative serum CA125 had lower RFS and OS after curative hepatectomy. Moreover, multivariate analysis revealed that preoperative serum CA125 was an independent prognostic factor after curative hepatectomy for hepatocellular carcinoma. The result suggests that, to the patients with HCC who had elevated preoperative serum CA125 levels, more effective anti-recurrence therapy and closer follow-up are required after curative hepatectomy.
The ability of serum CA125 to predict tumor prognosis appears to be superior to many commonly used tumor indicators. For example, although CA19-9 is most commonly used in pancreatic cancer, serum CA125 is approved more efficient than CA19-9 in predicting resectability of pancreatic cancer.28,29 In lung cancer, serum CA125 shows a better performance than CEA in predicting the prognosis.30 Studies have confirmed that preoperative serum CA125 is an independent prognostic factor for overall survival of colon cancer, rather than CEA.31 Serum AFP is the most common tumor marker for HCC, following with elevated serum AFP among 2/3 of HCC patients. A markedly elevated serum AFP level usually indicates a poor prognosis of HCC.32 However, the role of serum CA125 level in the prognosis of HCC has been underestimated for a long time. The stratified results of this study showed that the patients of HCC with elevated preoperative serum CA125 levels suffered from worse prognosis, regardless of whether serum AFP level was normal or elevated, which further demonstrate the powerful ability of CA125 to predict cancer prognosis.
However, the prediction of the prognosis of patients with HCC after curative hepatectomy should not depend on a certain tumor mark or technique. Combining various prediction methods provide wiser options for the clinical prediction.
Conclusion
The current study demonstrated that elevated preoperative serum CA125 level as an ideal HCC biomarker is significantly associated with many malignant factors of HCC, which is also an effective and valuable prognostic factor of OS and RFS in HCC.
Abbreviations
HCC, hepatocellular carcinoma; CA125, carbohydrate antigen 125; OS, overall survival; RFS, recurrence-free survival; N-CA125, normal CA125; H-CA125, high CA125; AFP, alpha-fetoprotein; HBV, hepatitis B virus; HCV, hepatitis C virus; NBNC, non-B non-C; ALBI, albumin–bilirubin; BCLC, Barcelona Clinic Liver Cancer; AJCC, American Joint Committee on Cancer; TNM, tumor, node, metastases; IQR, interquartile range; CI, confidence interval.
Acknowledgments
The authors thank Zhenwei Chen for his help in data collection and thank Yingchao Wang for the advise of English language editing.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Padhya KT, Marrero JA, Singal AG. Recent advances in the treatment of hepatocellular carcinoma. Curr Opin Gastroenterol. 2013;29(3):285–292. doi:10.1097/MOG.0b013e32835ff1cf
2. Morise Z, Kawabe N, Tomishige H, et al. Recent advances in the surgical treatment of hepatocellular carcinoma. World J Gastroenterol. 2014;20(39):14381–14392. doi:10.3748/wjg.v20.i39.14381
3. Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099–2108. doi:10.1002/hep.27406
4. Mokdad AA, Lopez AD, Shahraz S, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12:145.
5. Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of hepatocellular carcinoma in the Asia-Pacific region. Gut Liver. 2016;10(3):332–339. doi:10.5009/gnl15257
6. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907–1917.
7. Ercolani G, Grazi GL, Ravaioli M, et al. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg. 2003;237(4):536–543.
8. Lu X, Zhao H, Yang H, et al. A prospective clinical study on early recurrence of hepatocellular carcinoma after hepatectomy. J Surg Oncol. 2009;100(6):488–493.
9. Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4(2):439–474.
10. Luo T, Chen W, Wang L, Zhao H. CA125 is a potential biomarker to predict surgically incurable gastric and cardia cancer: a retrospective study. Medicine (Baltimore). 2016;95(51):e5297. doi:10.1097/MD.0000000000005297
11. Liu L, Xu HX, Wang WQ, et al. Serum CA125 is a novel predictive marker for pancreatic cancer metastasis and correlates with the metastasis-associated burden. Oncotarget. 2016;7(5):5943–5956. doi:10.18632/oncotarget.6819
12. Zhong W, Yu Z, Zhan J, et al. Association of serum levels of CEA, CA199, CA125, CYFRA21-1 and CA72-4 and disease characteristics in colorectal cancer. Pathol Oncol Res. 2015;21(1):83–95. doi:10.1007/s12253-014-9791-9
13. Patsner B, Yim GW. Predictive value of preoperative serum CA-125 levels in patients with uterine cancer: the Asian experience 2000 to 2012. Obstet Gynecol Sci. 2013;56(5):281–288. doi:10.5468/ogs.2013.56.5.281
14. Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100(10):698–711. doi:10.1093/jnci/djn134
15. Wang K, Liu J, Yan ZL, et al. Overexpression of aspartyl-(asparaginyl)-beta-hydroxylase in hepatocellular carcinoma is associated with worse surgical outcome. Hepatology. 2010;52(1):164–173. doi:10.1002/hep.23650
16. Guo S, Booten SL, Aghajan M, et al. Antisense oligonucleotide treatment ameliorates alpha-1 antitrypsin-related liver disease in mice. J Clin Invest. 2013;124(1):251–61.
17. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of liver diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
18. European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
19. Niloff JM, Klug TL, Schaetzl E, Zurawski VR, Knapp RC, Bast RC. Elevation of serum CA125 in carcinomas of the fallopian tube, endometrium, and endocervix. Am J Obstet Gynecol. 1984;148(8):1057–1058. doi:10.1016/S0002-9378(84)90444-7
20. Pelissier A, Bonneau C, Chereau E, et al. CA125 kinetic parameters predict optimal cytoreduction in patients with advanced epithelial ovarian cancer treated with neoadjuvant chemotherapy. Gynecol Oncol. 2014;135(3):542–546. doi:10.1016/j.ygyno.2014.09.005
21. Fang C, Cao Y, Liu X, Zeng XT, Li Y. Serum CA125 is a predictive marker for breast cancer outcomes and correlates with molecular subtypes. Oncotarget. 2017;8(38):63963–63970. doi:10.18632/oncotarget.19246
22. Mou W, Liu Z, Luo Y, et al. Development and cross-validation of prognostic models to assess the treatment effect of cisplatin/pemetrexed chemotherapy in lung adenocarcinoma patients. Med Oncol. 2014;31(9):59. doi:10.1007/s12032-014-0059-8
23. Hwang GI, Yoo CH, Sohn BH, et al. Predictive value of preoperative serum CEA, CA19-9 and CA125 levels for peritoneal metastasis in patients with gastric carcinoma. Cancer Res Treat. 2004;36(3):178–181. doi:10.4143/crt.2004.36.3.178
24. Kim DH, Yun HY, Ryu DH, et al. Preoperative CA 125 is significant indicator of curative resection in gastric cancer patients. World J Gastroenterol. 2015;21(4):1216–1221. doi:10.3748/wjg.v21.i4.1216
25. Zhang M, Zhang Y, Fu J, Zhang L. Serum CA125 levels are decreased in rectal cancer but increased in fibrosis-associated diseases and in most types of cancers. Prog Mol Biol Transl Sci. 2019;162:241–252. doi:10.1016/bs.pmbts.2018.12.012
26. Park EI, Manzella SM, Baenziger JU. Rapid clearance of sialylated glycoproteins by the asialoglycoprotein receptor. J Biol Chem. 2003;278(7):4597–4602. doi:10.1074/jbc.M210612200
27. Sanchez Vega JF, Murillo Bacilio MDR, Vintimilla Condoy AS, Palta Gonzalez AM, Crespo Astudillo JA, Mora-Bravo FG. Predictive equation of metastasis in patients with malignant ovarian epithelial tumors with the Ca-125 marker. BMC Cancer. 2018;18(1):587. doi:10.1186/s12885-018-4242-8
28. Showalter TN, Winter KA, Berger AC, et al. The influence of total nodes examined, number of positive nodes, and lymph node ratio on survival after surgical resection and adjuvant chemoradiation for pancreatic cancer: a secondary analysis of RTOG 9704. Int J Radiat Oncol Biol Phys. 2011;81(5):1328–1335. doi:10.1016/j.ijrobp.2010.07.1993
29. Liu L, Xu H, Wang W, et al. A preoperative serum signature of CEA+/CA125+/CA19-9 >/= 1000 U/mL indicates poor outcome to pancreatectomy for pancreatic cancer. Int J Cancer. 2015;136(9):2216–2227. doi:10.1002/ijc.29242
30. Ying L, Wu J, Zhang D, et al. Preoperative serum CA125 is an independent predictor for prognosis in operable patients with non-small cell lung cancer. Neoplasma. 2015;62(4):602–609. doi:10.4149/neo_2015_072
31. Yang XQ, Li Y, Chen C, Peng CW, Liu SP, Liu Y. Preoperative serum carbohydrate antigen 125 level is an independent negative prognostic marker for overall survival in colorectal cancer. Med Oncol. 2011;28(3):789–795. doi:10.1007/s12032-010-9518-z
32. Tranchart H, Chirica M, Sepulveda A, et al. Long-term outcomes following aggressive management of recurrent hepatocellular carcinoma after upfront liver resection. World J Surg. 2012;36(11):2684–2691. doi:10.1007/s00268-012-1723-9
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


