Back to Journals » Cancer Management and Research » Volume 11
Prognostic significance of a novel indicator (PSApostd3/PSApre) for PSA recurrence in patients after radical prostatectomy
Authors Zhou Z, Xu Y , Li Q, Yan W, Zhou Y, Zheng Z, Li H, Ji Z
Received 8 December 2018
Accepted for publication 24 May 2019
Published 25 June 2019 Volume 2019:11 Pages 5777—5783
DOI https://doi.org/10.2147/CMAR.S197521
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Zhien Zhou,1,* Yinyan Xu,2,* Qianyue Li,3 Weigang Yan,1 Yi Zhou,1 Zhibo Zheng,1 Hanzhong Li,1 Zhigang Ji1
1Department of Urology, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 2Clinical Medicine School, Peking Union Medical College, Beijing, People’s Republic of China; 3Department of Urology, General Hospital of Xinjiang Production and Construction Corps, Xinjiang, People’s Republic of China
*These authors contributed equally to this work
Purpose: Radical prostatectomy (RP) is a common treatment for prostate cancer, but a fraction of patients may experience PSA recurrence after surgery, manifesting as an elevation in prostate specific antigen (PSA). Vast literature has reported different prognostic factors for PSA recurrence without reaching a consensus. This retrospective study investigated the efficacy of a new indicator in predicting PSA recurrence in patients after RP.
Patients and methods: From October 2000 to December 2015, 102 PCa patients who underwent laparoscopic prostatectomy in the Urology Department of Peking Union Medical College Hospital were analyzed. We calculated PSApostd3/PSApre, defined as the ratio of the PSA on day 3 postop as the numerator and the pre-operative PSA as the denominator, in these patients to represent PSA decrement after surgery, and investigated its relationship with PSA recurrence during follow-up.
Results: The receiver operating characteristic (ROC) curve of PSApostd3/PSApre derived a cut-off at 0.453 (sensitivity=0.704, specificity=0.853, P<0.0001), suggesting an increased risk of PSA recurrence in patients whose PSA on day 3 postop did not decrease to approximately half of their preoperative levels. Among several factors, PSApostd3/PSApre (P<0.0001), pathological T stage (P=0.042) and Gleason Grade (P=0.021) were determined to be significantly associated with PSA recurrence by Fisher’s exact test, while only PSApostd3/PSApre (P<0.001) was significantly related to PSA recurrence-free survival (PRFS) by multivariate logistic regression analysis.
Conclusion: These results imply that PSApostd3/PSApre could provide substantial information for PSA recurrence prediction in patients after RP.
Keywords: prognosis, prostate cancer, prostate-specific antigen
Introduction
Radical prostatectomy (RP) is one of the most common and effective methods to treat prostate cancer (PCa). However, 15–33% of patients develop PSA recurrence, ie, a prostate-specific antigen (PSA) increase to 0.2 ng/mL or greater in 8–8.8 years after surgery.1,2 According to the previous literature, elevation of PSA after RP mainly originates from remnant active tumour cells, and the retained benign prostate tissue contributes little to PSA increases.3,4 Hence, a postoperative PSA elevation indicates recurrence of PCa theoretically, and PSA recurrence is a common outcome surrogate in current studies. In addition, much of the literature has reported that postoperative PSA is associated with PSA recurrence, although most studies have focused on the absolute value of PSA, measured from 1 month to 3 years after RP.5–7 Other studies have implied that the kinetics of PSA can predict prognosis more accurately.8
A high level of postoperative PSA might suggest the existence of remnant tumour cells,3 which could lead to PSA recurrence and even clinical recurrence (CLR). As the half-life period of PSA is 2–3 days,9,10 we hypothesize that patients with a >50% drop in PSA on day 3 postop compared with preoperative PSA are less likely to develop PSA recurrence. We defined PSApostd3/PSApre as the ratio of the PSA on day 3 postop as the numerator and the pre-operative PSA as the denominator. This study aimed to investigate the efficacy of PSApostd3/PSApre in PSA recurrence prediction.
Materials and methods
Study population and data collection
The present study was a retrospective study approved by the Institutional Review Board (IRB) of Peking Union Medical College Hospital (PUMCH). According to the National Comprehensive Cancer Network (NCCN) Guidelines on prostate cancer, a total of 102 patients diagnosed with PCa received laparoscopic prostatectomy in the Urology Department of PUMCH from Oct. 2000 to Dec. 2015. The inclusion criteria are listed as follows: 1) patients who were diagnosed with localized PCa and received laparoscopic RP in PUMCH; 2) patients with complete preoperative clinical data and records; 3) patients with available PSA data in a week before surgery and on day 3 postop; 4) patients who had been followed up for at least two years with a complete follow-up record; and 5) patients who did not receive endocrine therapy or radiotherapy before or after surgery. Written informed consent was obtained from all of the participants.
The following information was evaluated for all patients: medical history, physical examination, digital rectal examination and serum PSA. Clinical staging was based on digital rectal examination and, when clinically indicated, chest radiography, bone scintigraphy, CT-scan and/or magnetic resonance imaging (MRI) of the pelvis.
All RP surgeries were performed by experienced surgeons in the Department of Urology of PUMCH. Surgery was performed by a pure laparoscopic prostatectomy, with the extent of pelvic lymph node dissection based upon the risk category of the patient. Serum total PSA measurements were analysed by the Clinical Laboratory of PUMCH, adopting the chemiluminescent microparticle immunoassay method with the ARCHITECT PSA-T kit (Abbott, Lake Bluff, Illinois, United States). The prostatic specimens were evaluated by the pathologists at PUMCH, who also reported Gleason score and surgical margin status. Serum PSA was continuously monitored by follow-up clinics at PUMCH, ie, every 3 months in the first 2 years after RP and every 6 months afterward. The primary outcome of the present study was PSA recurrence, which is defined as PSA >0.2 ng/mL. All patients with PSA recurrence were confirmed by more than two PSA tests.
Statistical analysis
Independent samples t-test was used to compare the differences of PSApostd3/PSApre between PSA recurrence-free group and PSA recurrence group. A receiver operating characteristic (ROC) curve of PSApostd3/PSApre was plotted, and the area under the curve (AUC) was calculated. The cut-off value of PSApostd3/PSApre was derived when the Youden index was maximal (0.557). The correlation of preoperative PSA with PSA on day 3 postop was examined by Spearman’s rank correlation test. The Fisher’s exact test was applied to analyse the PSA recurrence rate in patients with different PSApostd3/PSApre value, pT stage, postoperative Gleason Grade, age grouping and surgical margin status. Kaplan-Meier curves of each investigated factor were plotted, and the Log Rank test was adopted to examine significance in PRFS. Multivariate analysis of PSA recurrence predictors was performed using a logistic regression analyses. The alpha level for all tests is 0.05. A P-value <0.05 was considered to be significant. Statistical analyses were performed using SPSS software 21 (IBM Inc., Armonk, New York, United States) and GraphPad Prism 8 (GraphPad Software, La Jolla, California, United States).
Results
Baseline data
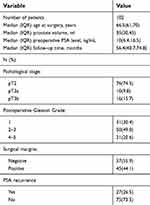
Clinical and pathological characteristics are shown in Table 1. A total of 102 patients diagnosed with PCa were analysed after RP. The median (interquartile range, IQR) age of the patients was 66.5 years old (61–70). The median (IQR) prostate volume was 35 mL (30–45). The median (IQR) preoperative PSA was 10.0 ng/mL (6.4–16.5). After a median (IQR) follow-up time of 56.4 months (40.7–74.8), 27 (26.5%) patients developed PSA recurrence, and the median (IQR) time from surgery to PSA recurrence was 22 months (12–33). One patient died of lung cancer during follow up. None was discovered to develop metastasis from PCa by the end of this study.
 | Table 1 Clinical and pathological characteristic of the population |
Based on the NCCN Guidelines on prostate cancer,11 the pathological T stage of these patients ranged from T2a to T3b. 76 (74.5%) of them were in stage T2 and 26 (25.5%) were in stage T3. The occurrence rates of PSA recurrence were 21.1% and 42.3% in T2 and T3 patients, respectively. Pathological analysis showed that 71 (69.6%) patients had a Gleason Grade ≥2, and 45 (44.1%) had a positive surgical margin (PSM).
The predictive ability of PSApostd3/PSApre for PSA recurrence
As the half-life of PSA is 2–3 days, we analysed the relationship between PSApostd3/PSApre and PSA recurrence. PSApostd3/PSApre was not associated with the absolute value of preoperative PSA (P>0.05).
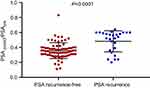
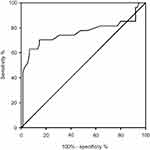
The PSApostd3/PSApre was significantly higher in PSA recurrence patients compared with PSA recurrence-free patients (P<0.0001) (Figure 1). The ROC curve of PSApostd3/PSApre is shown in Figure 2 with an area under the curve (AUC) of 0.760. The cut-off of PSApostd3/PSApre was derived at 0.453 (sensitivity=0.704, specificity=0.853, positive predictive value=0.633, negative predictive value=0.889, P<0.0001). This outcome indicated that the risk of PSA recurrence increased in patients with a PSApostd3/PSApre>0.453.
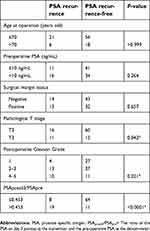
It is noteworthy that the value of PSApostd3/PSApre has a great overlap between PSA recurrent patients and patients without PSA recurrence (Figure 1). Among 27 patients with PSA recurrence, there were 8 patients with PSApostd3/PSApre ≤0.453, while among 75 patients without PSA recurrence there were 11 patients with PSApostd3/PSApre >0.453 (Table 2). We divided these patients into four subgroups according to whether they had PSA recurrence and whether PSApostd3/PSApre was greater than 0.453. The Fisher’s exact test was conducted to reveal the difference of the clinical and pathological characteristic of these patients, including their age at operation, pathological T stage, surgical margins, preoperative PSA and postoperative Gleason Grade. The result was only significant for pT staging (P=0.035), with an interesting finding that all 11 patients with PSA recurrence-free and PSApostd3/PSApre >0.453 belonged to pT2. This supported the important influence of pT on PSA recurrence outcome, which the multivariate analysis did not reveal (see below), probably due to a small sample size.
 | Table 2 Fisher’s exact test results of different factors (n=102) |
The association between PSA recurrence and multiple factors
Previous studies have suggested that PSA recurrence is associated with many preoperative and postoperative factors (see discussion). In this study, based on the accessible data, the relationships of PSA recurrence and the following indicators were investigated: age at operation (>70 or ≤70 years old), preoperative PSA (>10 or ≤10 ng/mL), surgical margin status (positive or negative), pathological T stage (pT stage, ie T2 or T3), postoperative Gleason Grade (1, 2–3 or 4–5) and PSApostd3/PSApre (>0.453 or ≤0.453). Fisher’s exact test was applied to all of the indicators, and the P-values were presented in Table 2. Patients with different PSApostd3/PSApre (P<0.0001), pT stage (P=0.042) and Gleason Grade (P=0.021) had significantly different PSA recurrence rates.
A multivariable logistic regression model was established to identify factors associated with PSA recurrence rates, and the results were shown in Table 3. On multivariate analysis of the entire cohort, only PSApostd3/PSApre (P<0.001) was significantly related to PRFS. Figure 3 shows the probability of PRFS over time in patients with different values of PSApostd3/PSApre. The probability of PRFS in patients with PSApostd3/PSApre≤0.453 was significantly higher than in patients with PSApostd3/PSApre>0.453.
 | Table 3 Multivariable logistic regression analyses for developing PSA recurrence |
Discussion
Many studies have explored the prognostic value of different preoperative and postoperative factors for PSA recurrence, but no consensus has been reached. Commonly investigated indicators include the following. 1) Preoperative PSA: Most studies have concluded that a high level of preoperative PSA was associated with PSA recurrence,6,7,12–15 although Takeuchi16 and Aguilera17 reported insignificant results. Preoperative PSA doubling time (PSADT) has been a rising indicator in recent years, especially early PSADT and ultrasensitive PSADT, but their relationships with PSA recurrence are controversial.8 2) Pathological Gleason score: Much evidence has supported that a higher Gleason score increases the risk of PSA recurrence,7,13–18 while a few studies have also reported insignificant results.12,19 3) Pathological T stage: Generally, a higher T stage suggests a greater probability of developing PSA recurrence.7,12,14–16 4) Age: According to Wang et al, patients older than 70 years old were less likely to undergo PSA recurrence than those who were younger,12 although most other studies have reported an insignificant relationship between age and PSA recurrence.7,14,16,20,21 5) Positive surgical margin: The prognostic value of PSM has been controversial.7,14,15,17,19,20 Yossepowitch et al concluded in a review in 2014 that positive margins were associated with a twofold increased hazard of biochemical relapse.22 Other previously investigated pathological indicators include capsule invasion,20 extracapsular extension,13,20 lymph node invasion,15,19 and seminal vesicle invasion.18,20 In our study, several possible prognostic factors besides postoperative PSA were also investigated. According to our results, patients with different PSApostd3/PSApre (p<0.0001), pT stage (P=0.042) and Gleason Grade (P=0.021) had significantly different PSA recurrence rates by Fisher’s exact test, while on multivariate analysis of the entire cohort, only PSApostd3/PSApre (P<0.001) was significantly related to PRFS.
Postoperative PSA after RP is a frequent factor included in various clinical outcome (including PSA recurrence) prediction tools in PCa patients.23 Previous research usually focused on the relationship between the absolute value of postoperative PSA and PSA recurrence.5–7,23–26 For example, Vesely held that PSA on day 30 postop was significantly associated with PSA recurrence, but such an association was not observed with PSA on day 14 postop.23 Furthermore, Skove et al concluded that men with a detectable PSA nadir after RP had an increased risk of PSA recurrence, and a shorter time to nadir suggested a higher risk of PSA recurrence.26 Little research has been implemented to reveal the relationship between PSA recurrence and postoperative PSA at as early as the first week after surgery. Only one study investigated the ratio of PSA on the 5th day after salvage radiotherapy (PSA5) and pre-treatment PSA (PSART) and found that PSA5/PSART <1 was a significant predictor of PSA recurrence.27 This study provided some information about the relationship of early postoperative PSA decline with biochemical failure in patients receiving RP and salvage radiotherapy.
Based on the half-life of PSA, the present study discovered that PSApostd3/PSApre was an efficacious predictor for PSA recurrence. This indicator has the following advantages: 1) the specificity and negative predictive value of PSApostd3/PSApre were high, which could prevent over-treatment as a screening method; 2) it allowed for early recognition for possible PSA recurrence during the first week after RP; and 3) it was an easily measurable indicator. Because preoperative PSA is often high, ultrasensitive PSA equipment is not necessary to detect an approximately 50% decline. However, PSApostd3/PSApre has some shortcomings: 1) its sensitivity is not very high, thus long term follow up with PSA tests is indispensable, even in patients with a low PSApostd3/PSApre; and 2) because PSA is metabolized by the liver and kidneys mainly,28 insufficiency of these organs might influence PSA kinetics and thus impair the predictive value of PSApostd3/PSApre.
The present study has several limitations.
- The sample size of PSA on day 3 postop was relatively small, future study should expand the sample size to reduce error. In addition, further studies may complete PSA measurements on day 1–7 postop to compare the AUC of the PSA ratio on different days and to identify the optimal time to measure postoperative PSA during the first week.
- Though commonly considered an outcome surrogate for PCa patients, PSA recurrence remains controversial for its clinical significance and interpretation. Some research has shown that PSA recurrence had limited significance in disease progression and mortality in PCa patients after RP. 66.7–74% of PSA recurrence patients did not develop CLR or metastasis,1,2,29 and the overall survival rates in PSA recurrence and PSA recurrence-free patients were not significantly different (88% vs 93%, P=0.94).29 Similar conclusions were drawn regarding cancer-specific mortality (P=0.23).15 Therefore, interpretation of this study should be cautious.
- The follow-up was relatively short in this study, with a median follow-up of 56.4 months. However, a previous study reported that the mean time to PSA recurrence was 38.4 months,30 and 77% of cases of PSA recurrence occurred within 2 years after RP,31 so the majority of PSA recurrence events should have been observed. The patients in the present study would be continuously followed up to observe other outcomes, such as CLR, metastasis and death.
Conclusion
In conclusion, the present study identified a new indicator, PSApostd3/PSApre, for predicting PSA recurrence in patients after RP. Patients with a PSApostd3/PSApre >0.453 were more likely to develop PSA recurrence than those with a lower PSApostd3/PSApre, with an increased risk of 9–20 times. By a convenient test of preoperative PSA and PSA on day 3 postop and simple calculation, PSApostd3/PSApre might serve as an early indicator for PSA recurrence in the future, which may facilitate recognition of potential relapse and could translate into more intense follow-up and even efficacious salvage therapy in selected patients.
Abbreviation list
AUC, area under the curve; CLR, clinical recurrence; IQR, interquartile range; IRB, institutional review board; NCCN, national comprehensive cancer network; PCa, prostate cancer; PRFS PSA, recurrence-free survival; PSA, prostate-specific antigen; PSADT, prostate-specific antigen doubling time; PSApostd3/PSApre, the ratio of the PSA on day 3 postop as the numerator and the pre-operative PSA as the denominator; PSM, positive surgical margin; PUMCH, Peking Union Medical College Hospital; ROC, receiver operating characteristic; RP, radical prostatectomy.
Ethics approval and informed consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board (IRB) of Peking Union Medical College Hospital (PUMCH) and with the 1975 Declaration of Helsinki and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included in the study.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ward JF, Blute ML, Slezak J, Bergstralh EJ, Zincke H. The long-term clinical impact of biochemical recurrence of prostate cancer 5 or more years after radical prostatectomy. J Urol. 2003;170(5):1872–1876. doi:10.1097/01.ju.0000091876.13656.2e
2. Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. Jama. 1999;281(17):1591–1597.
3. Odisho AY, Washington SL
4. Godoy G, Tareen BU, Lepor H. Does benign prostatic tissue contribute to measurable PSA levels after radical prostatectomy? Urology. 2009;74(1):167–170. doi:10.1016/j.urology.2008.07.067
5. Inagaki T, Kohjimoto Y, Nishizawa S, et al. PSA at postoperative three months can predict biochemical recurrence in patients with pathological T3 prostate cancer following radical prostatectomy. Int J Urol. 2009;16(12):941–946. doi:10.1111/j.1442-2042.2009.02401.x
6. Vollmer RT, Humphrey PA. The relative importance of anatomic and PSA factors to outcomes after radical prostatectomy for prostate cancer. Am J Clin Pathol. 2001;116(6):864–870. doi:10.1309/7MQ7-MWAR-4W8A-R75F
7. Eisenberg ML, Davies BJ, Cooperberg MR, Cowan JE, Carroll PR. Prognostic implications of an undetectable ultrasensitive prostate-specific antigen level after radical prostatectomy. Eur Urol. 2010;57(4):622–629. doi:10.1016/j.eururo.2009.03.077
8. Tilki D, Kim SI, Hu B, Dall’Era MA, Evans CP. Ultrasensitive prostate specific antigen and its role after radical prostatectomy: a systematic review. J Urol. 2015;193(5):1525–1531. doi:10.1016/j.juro.2014.10.087
9. Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317(15):909–916. doi:10.1056/NEJM198710083171501
10. Zattoni F, Piazza R, Vianello R, Garbeglio A, Pagano F. Preoperative and postoperative evaluation of prostate-specific antigen in localized prostatic cancer treated by radical prostatectomy. Eur Urol. 1992;21(Suppl 1):99–101.
11.
12. Wang H, Gao X, Fang Z, et al. The older the better: the characteristic of localized prostate cancer in Chinese men. Asian J Urol. 2015;2(3):129–132. doi:10.1016/j.ajur.2015.07.001
13. Turk H, Celik O, Un S, et al. Predictive factors for biochemical recurrence in radical prostatectomy patients. Cent European J Urol. 2015;68(4):404–409. doi:10.5173/ceju.2015.606
14. Sachdeva A, Veeratterapillay R, Voysey A, et al. Positive surgical margins and biochemical recurrence following minimally-invasive radical prostatectomy - An analysis of outcomes from a UK tertiary referral centre. BMC Urol. 2017;17(1):91. doi:10.1186/s12894-017-0262-y
15. Garcia-Barreras S, Rozet F, Nunes-Silva I, et al. Predictive factors and the important role of detectable prostate-specific antigen for detection of clinical recurrence and cancer-specific mortality following robot-assisted radical prostatectomy. Clin Transl Oncol. 2018;20(8):1004–1010. doi:10.1007/s12094-017-1812-1.
16. Takeuchi H, Ohori M, Tachibana M. Clinical significance of the prostate-specific antigen doubling time prior to and following radical prostatectomy to predict the outcome of prostate cancer. Mol Clin Oncol. 2017;6(2):249–254. doi:10.3892/mco.2016.1116
17. Aguilera A, Banuelos B, Diez J, Alonso-Dorrego JM, Cisneros J, Pena J. Biochemical recurrence risk factors in surgically treated high and very high-risk prostate tumors. Cent European J Urol. 2015;68(3):302–307. doi:10.5173/ceju.2015.485
18. Zhang YD, Wu CJ, Bao ML, et al. MR-based prognostic nomogram for prostate cancer after radical prostatectomy. J Magn Reson Imaging. 2017;45(2):586–596. doi:10.1002/jmri.25441
19. Chalieopanyarwong V, Attawettayanon W, Kanchanawanichkul W, Pripatnanont C. The prognostic factors of biochemical recurrence-free survival following radical prostatectomy. Asian Pac J Cancer Prev. 2017;18(9):2555–2559. doi:10.22034/APJCP.2017.18.9.2555
20. Un S, Turk H, Koca O, Divrik RT, Zorlu F. Factors determining biochemical recurrence in low-risk prostate cancer patients who underwent radical prostatectomy. Turk J Urol. 2015;41(2):61–66. doi:10.5152/tud.2015.65624
21. Da Cruz JAS, Passerotti CC, Dos Reis ST, et al. Is age an independent factor for prostate cancer? A paired analysis. Curr Urol. 2017;9(4):183–187. doi:10.1159/000447138
22. Yossepowitch O, Briganti A, Eastham JA, et al. Positive surgical margins after radical prostatectomy: a systematic review and contemporary update. Eur Urol. 2014;65(2):303–313. doi:10.1016/j.eururo.2013.07.039
23. Vesely S, Jarolim L, Duskova K, Schmidt M, Dusek P, Babjuk M. The use of early postoperative prostate-specific antigen to stratify risk in patients with positive surgical margins after radical prostatectomy. BMC Urol. 2014;14:79. doi:10.1186/1471-2490-14-79
24. Audenet F, Seringe E, Drouin SJ, et al. Persistently elevated prostate-specific antigen at six weeks after radical prostatectomy helps in early identification of patients who are likely to recur. World J Urol. 2012;30(2):239–244. doi:10.1007/s00345-011-0707-y
25. Malik RD, Goldberg JD, Hochman T, Lepor H. Three-year postoperative ultrasensitive prostate-specific antigen following open radical retropubic prostatectomy is a predictor for delayed biochemical recurrence. Eur Urol. 2011;60(3):548–553. doi:10.1016/j.eururo.2011.05.036
26. Skove SL, Howard LE, Aronson WJ, et al. Timing of prostate-specific antigen nadir after radical prostatectomy and risk of biochemical recurrence. Urology. 2017;108:129–134. doi:10.1016/j.urology.2017.07.009
27. Blanchard P, Bakkour M, De Crevoisier R, et al. Early PSA level decline is an independent predictor of biochemical and clinical control for salvage postprostatectomy radiotherapy. Urol Oncol. 2015;33(3):
28. Kilic S, Yalcinkaya S, Guntekin E, Kukul E, Deger N, Sevuk M. Determination of the site of metabolism of total, free, and complexed prostate-specific antigen. Urology. 1998;52(3):470–473.
29. Jhaveri FM, Zippe CD, Klein EA, Kupelian PA. Biochemical failure does not predict overall survival after radical prostatectomy for localized prostate cancer: 10-year results. Urology. 1999;54(5):884–890.
30. Vassilikos EJ, Yu H, Trachtenberg J, et al. Relapse and cure rates of prostate cancer patients after radical prostatectomy and 5 years of follow-up. Clin Biochem. 2000;33(2):115–123.
31. Dillioglugil O, Leibman BD, Kattan MW, Seale-Hawkins C, Wheeler TM, Scardino PT. Hazard rates for progression after radical prostatectomy for clinically localized prostate cancer. Urology. 1997;50(1):93–99. doi:10.1016/S0090-4295(97)00106-4
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.