Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Prognostic Role of NT-proBNP for in-Hospital and 1-Year Mortality in Patients with Acute Exacerbations of COPD
Authors Li H , Zeng Z , Cheng J , Hu G, Li Y, Wei L, Zhou Y, Ran P
Received 20 September 2019
Accepted for publication 10 December 2019
Published 8 January 2020 Volume 2020:15 Pages 57—67
DOI https://doi.org/10.2147/COPD.S231808
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chunxue Bai
Haiqing Li, 1 Zixiong Zeng, 1 Juan Cheng, 1 Guoping Hu, 1 Yuqun Li, 1 Liping Wei, 1 Yumin Zhou, 2 Pixin Ran 2
1Department of Respiratory Medicine, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, People’s Republic of China; 2Guangzhou Institute of Respiratory Disease, State Key Laboratory of Respiratory Disease, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, People’s Republic of China
Correspondence: Guoping Hu
Department of Respiratory Medicine, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, People’s Republic of China
Tel +86 20-81292146
Email [email protected]
Pixin Ran
Guangzhou Institute of Respiratory Diseases, The First Affiliated Hospital, Guangzhou Medical University, 151 Yanjiang Road, Guangzhou, Guangdong 510120, People’s Republic of China
Tel/Fax +86 20-81340482
Email [email protected]
Background and objective: The association between N-terminal pro B-type natriuretic peptide (NT-proBNP) concentrations and in-hospital and 1-year mortality in acute exacerbations of chronic obstructive pulmonary disease (AECOPD) patients is largely unknown. Our objective was to explore the usefulness of NT-proBNP concentrations in AECOPD patients as a prognostic marker for in-hospital and 1-year mortality.
Methods: NT-proBNP levels were measured in patients upon admission and laboratory and clinical data were also recorded. The cut-point for the NT-proBNP concentration level for in-hospital death was obtained using the receiver operating characteristic (ROC) curve. Univariate and multivariate logistic regression and Cox regression were used in the analyses of factors of in-hospital and 1-year mortality.
Results: A total of 429 patients were enrolled. Twenty-nine patients died during hospitalization and 59 patients died during the 1-year follow-up. Patients who died in-hospital compared with those in-hospital survivors were older (80.14± 6.56 vs 75.93± 9.45 years, p=0.003), had a higher percentage of congestive heart failure (65.52% vs 33.75%, p< 0.001), had higher NT-proBNP levels (5767.00 (1372.50– 12,887.00) vs 236.25 (80.03– 1074.75) ng/L, p< 0.001), higher neutrophil counts (10.52± 5.82 vs 7.70± 4.31, p=0.016), higher D-dimer levels (1231.62± 1921.29 vs 490.11± 830.19, p=0.048), higher blood urea nitrogen levels (9.91± 6.33 vs 6.51± 4.01 mmol/L, p=0.001), a lower body mass index (19.49± 3.57 vs 22.19± 4.76, p=0.003), and higher hemoglobin levels (122.34± 25.36 vs 130.57± 19.63, p=0.034). The area under the ROC curve (AUC) for NT-proBNP concentration was 0.88 (95% confidence interval [CI], 0.84– 0.93). NT-proBNP concentrations ≥ 551.35 ng/L were an independent prognostic factor for both in-hospital and 1-year mortality after adjustment for relative risk (RR) (RR=29.54, 95% CI 3.04– 286.63, p=0.004 for the multivariate logistic regression analysis) and hazard ratio (HR) (HR=4.47, 95% CI, 2.38– 8.41, p < 0.001 for the multivariate cox regression analysis).
Conclusion: NT-proBNP was a strong and independent predictor of in-hospital and 1-year mortality in AECOPD patients.
Keywords: AECOPD, NT-proBNP, mortality, prognosis
Corrigendum for this paper has been published
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of disability and mortality worldwide.1,2 An increasing rate of COPD severity has been associated with decreasing cardiac function.3 Cardiovascular disease is a frequent and important comorbidity in COPD patients. Congestive heart failure (CHF) affects 20%–70% of the COPD patient population.4,5 N-terminal pro B-type natriuretic peptide (NT-proBNP), as a biomarker of cardiac function, is recommended for heart failure diagnosis in guidelines from many countries.6–8
There have been some studies on the correlation between the concentration of NT-proBNP and prognosis of COPD patients.9–11 The study of Chang et al showed that elevated levels of NT-proBNP were only associated with 30-day mortality but not with long-term mortality.10 They found that compared with patients with normal NT-proBNP and troponin T, patients with elevated NT-proBNP levels had higher 1-year mortality rates; however, elevated NT-proBNP levels not predicted deaths between 30 days and 1 year of follow-up.. In their cohort, patients with a malignancy were not excluded, which might have influenced the results. Another study from Pervez et al also found that elevated NT-proBNP levels did not predict long-term mortality in COPD patients;12 however, there were only 84 COPD patients in their study. A retrospective study showed that elevated levels of NT-proBNP were not associated with in-hospital mortality.13 In their study, they could only analyze data from patients with available data on NT-proBNP levels, which introduced sample bias into their cohort. Two studies showed that elevated levels of NT-proBNP were associated with long-term mortality;9,11 however, their studies had relatively few patients. Therefore, the association between the concentration of NT-proBNP and in-hospital and 1-year mortality in COPD patients is largely unknown. To ascertain the role of NT-proBNP as a prognostic marker for in-hospital and 1-year mortality in acute exacerbations of chronic obstructive pulmonary disease (AECOPD) patients, we performed a prospective study.
Method
Study Design and Patients
We recruited consecutive patients who were hospitalized for AECOPD in the Third Affiliated Hospital of Guangzhou Medical University (Guangzhou, People’s Republic of China) from January 01, 2013 to June 30, 2018. For patients admitted more than once during the study period, only the first admission was included in the analysis. Patients had been clinically diagnosed with COPD, defined according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria14 (a ratio of forced expiratory volume in one second (FEV1) to forced vital capacity (FVC) of <70% after bronchodilator use). The primary diagnosis of patients at admission was AECOPD. Patients included in the study had no signs and/or symptoms that would cause their attending physicians to alter their primary diagnosis to pneumonia or other diseases. Patients with active tuberculosis, pulmonary fibrosis, or a pulmonary embolism were not included in this study. Patients with asthma, obstructive sleep apnea-hypopnea syndrome, or bronchiectasis were not excluded from this study. We excluded patients who did not have the ability or willingness to cooperate with the study physicians as well as those who had a malignancy. All patients were recruited on the first day of hospitalization. After discharge, patients were followed up every three months for 1 year by telephone. The first day of 1-year follow-up was defined as the day of discharge. The ethics committee of The Third Affiliated Hospital of Guangzhou Medical University approved the research protocol. All patients provided written informed consent. This study was conducted in accordance with the Declaration of Helsinki.
Data Collection and Definitions
Demographic data for patients were recorded at admission. We also recorded clinical data, such as the number of exacerbations in the previous year, vital signs, and comorbidities (specifically cardiovascular disease). Blood samples of patients were collected on the day of hospitalization and before patients received any medications to determine NT-proBNP concentration, neutrophil count, hemoglobin level, D-dimer level, creatinine level, blood urea level, nitrogen levels, and blood gases. The concentration of NT-proBNP was estimated using the Elecsys proBNP assay (Roche Diagnostics). All lab tests were performed by the hospital laboratory. Exacerbations were defined as an increase in the severity of respiratory symptoms (cough, sputum, wheezing, dyspnea, or chest tightness), requiring hospitalization or an emergency department visit (leading to systemic corticosteroids, antibiotics, or both). The diagnoses of CHF and renal dysfunction have been previously described.15 Patients were managed by their attending physicians, who did not participate in the present study, according to the GOLD guidelines.
Statistical Analysis
The primary outcome was in-hospital mortality and 1-year all-cause mortality. The secondary outcome was the factors associated with in-hospital mortality and 1-year mortality. Continuous variables with normal distribution were presented as means ±standard deviation (SD). Non-normally distributed variables were presented as medians (interquartile range, IQR). Categorical variables were presented as percentages. Comparisons between groups were performed with the use of the analysis of variance, the Mann–Whitney U-test (continuous variables), or a nonparametric Chi-Square test (categorical variables). The threshold concentration of NT-proBNP was determined with the use of the receiver operating characteristic (ROC) curve. Logistic regression was used for analyses of the factors of in-hospital mortality. Cox regression was used for analyses of the factors of 1-year mortality. All variables that were detected in the univariate analyses (with a p-value of ≤0.2) were included in the multivariate analyses,16 with controlling for relevant covariates (age, sex, smoking status, body mass index (BMI), GOLD stage, heart failure, and renal dysfunction). Comparison of mortality between groups with and without elevated NT-proBNP concentrations was performed using the Kaplan–Meier estimator. Survival curves were compared using the log rank test. In subgroup analyses, the homogeneity of the hazard ratio was performed using the Breslow-Day tests. All analyses were two-sided, and a p-value of <0.05 was considered statistically significant. All analyses were performed using SPSS software, version 16 (SPSS Inc., Chicago, IL, USA).
Results
Patient Characteristics
A total of 449 patients hospitalized for AECOPD were recruited for the study, and 429 patients were enrolled and followed up (Figure 1). Of the patients, 66.43% were male. The mean age of patients was 76.21±9.34 years. Table 1 shows the demographic and baseline clinical characteristics of the patients between the survivor group and non-survivor group. Twenty-nine patients died during hospitalization. The non-survivor group had a lower BMI (19.49±3.57 vs 22.19±4.76, p=0.003) and a lower hemoglobin level (122.34±25.36 vs 130.57±19.63 0.034, p=0.034) compared with the survivor group. The non-survivor group was also older than the survivor group (80.14±6.56 vs 75.93±9.45 years old, p=0.003) and had a higher NT-proBNP concentration (5767.00 (1372.50–12,887.00) vs 236.25 (80.03–1074.75) ng/L, p<0.001), a higher neutrophil count (10.52±5.82 vs 7.70±4.31, p=0.016), a higher D-dimer level (1231.62±1921.29 vs 490.11±830.19, p=0.048), and a higher blood urea nitrogen (BUN) level (9.91±6.33 vs 6.51±4.01 mmol/L, p=0.001) compared with the survivor group. Compared with the survivor group, CHF comorbidity was more common in the non-survivor group (65.52% vs 33.75%, p<0.001). There were no significant differences between the two groups regarding hospital stay, sex, smoking status, exacerbations during the preceding year, smoking history (pack-years), renal dysfunction comorbidity, pH, partial pressure of CO2 (PaCO2), partial pressure of O2 (PaO2), and creatinine concentration.
 |
Table 1 Patient Characteristics According to in-Hospital Mortality |
 |
Figure 1 Flow chart of study patients. |
Association Between NT-proBNP Concentrations and Clinically Relevant Outcomes
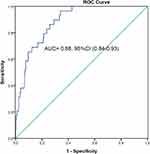
The sensitivity and specificity of NT-proBNP concentrations for in-hospital mortality were explored in this study with the use of the ROC curve. Figure 2 shows that the area under the ROC curve (AUC) for NT-proBNP concentrations was 0.88 (95% confidence interval [CI], 0.84–0.93) and the cut-point for NT-proBNP concentration was 551.35 ng/L with a sensitivity of 0.97 and a specificity of 0.66. The patients in the entire cohort were divided into two groups based on the cut-point concentration of NT-proBNP. There were 164 patients with an NT-proBNP concentration ≥551.35 ng/L and 265 patients with an NT-proBNP concentration <551.35 ng/L. Table 2 shows the demographic and baseline clinical characteristics of the patients in the two groups. There were significant differences between the two groups regarding in-hospital mortality, hospital stay, age, BMI, COPD stage, CHF comorbidity, renal dysfunction, neutrophil count, hemoglobin, D-dimer, and BUN concentration. Conversely, there were no significant differences between the two groups regarding sex, smoking status, smoking history, the number of exacerbations during the preceding year, pH, PaO2, PaCO2, and creatinine concentration.
 |
Table 2 Patient Characteristics Stratified by NT-proBNP Concentration |
Factors Associated with Hospitalization Mortality
In univariate logistic regression analysis, we used all available baseline variables in the entire cohort to assess factors associated with in-hospital mortality. Table 3 showed that age, a history of CHF, an NT-proBNP concentration ≥551.35 ng/L, an increased neutrophil count, an increased D-dimer level, an increased creatinine level, an increased BUN level, pH <7.35, pH >7.45, and PaO2 <60 mmHg were risk factors of in-hospital mortality. An increased BMI, COPD stage (per increase to next stage), and increased hemoglobin levels were protection factors of in-hospital mortality. The best predictor of in-hospital mortality was an NT-proBNP concentration ≥551.35 ng/L (relative risk (RR), 54.35; 95% CI, 7.32–403.77; P<0.001). A multinomial logistic regression analysis showed that BMI, a history of CHF, an NT-proBNP concentration ≥551.35 ng/L, neutrophil count, and pH >7.45 were independent factors associated with in-hospital mortality (Table 3) while controlling for relevant covariates (age, sex, smoking status, BMI, GOLD stage, CHF, and renal dysfunction). The concentration of NT-proBNP ≥551.35 ng/L remained a strong predictor of in-hospital mortality after RR adjustment (RR, 29.54; 95% CI, 3.04–286.63; P=0.004).
 |
Table 3 Univariate and Multivariate Associations with in-Hospital Mortality |
NT-proBNP Concentration and 1-Year Mortality
In the entire cohort, 29 patients died during hospitalization. Two patients did not have follow-up data. A total of 400 patients were included in the analysis. Fifty-nine patients died within 1-year. Figure 3 shows the difference in 1-year mortality between the NT-proBNP concentration ≥551.35 ng/L group and the NT-proBNP concentration <551.35 ng/L group using the Kaplan–Meier estimator. Mean survival times for patients in the two groups were 296.62 days for the NT-proBNP concentration ≥551.35 ng/L group and 356.62 days for the NT-proBNP concentration <551.35 ng/L group. The log rank test revealed that there was a significant difference between the two survival curves, and the survival rate of the NT-proBNP concentration <551.35 ng/L group was higher than of the NT-proBNP concentration ≥551.35 ng/L group (survival rate, 0.939 vs 0.684, p <0.001). The predictive value of NT-proBNP ≥551.35 ng/L on 1-year mortality was maintained after adjustment (HR, 4.47; 95% CI, 2.38–8.41; P<0.001). CHF had an important impact on AECOPD patient mortality. To eliminate the impact of CHF on the prognostic role of NT-proBNP levels for 1-year mortality, we performed a subgroup analysis (Figure 4) on patients with and without CHF. In subgroup analysis, the difference in 1-year mortality was significant between patients with CHF (p <0.001) and without CHF (p <0.001). The association between NT-proBNP ≥551.35 ng/L and 1-year mortality persisted in patients with (HR, 8.92; 95% CI, 3.13–25.12) and without (HR, 3.39; 95% CI, 1.46–7.88) a history of CHF, with controlling for relevant covariates (age, sex, GOLD stage, exacerbations during the preceding year, renal dysfunction, pH, PaCO2, and PaO2).
 |
Figure 4 Subgroup analyses. |
Factors Associated with 1-Year Mortality
In univariate Cox regression analysis (Table 4), we found that p-values associated with age, COPD stage, a history of CHF, exacerbations during the preceding year, NT-proBNP concentration ≥551.35 ng/L, hemoglobin level, D-dimer level, BUN level, pH <7.35, PaO2 <60, and PaCO2 ≥50 were less than 0.2. The variable most strongly associated with 1-year mortality was the concentration of NT-proBNP ≥551.35 ng/L (HR, 6.14; 95% CI, 3.46–10.90; p<0.001). Factors that were independently associated with 1-year mortality, on the basis of a multinomial Cox regression analysis, are shown in Table 4. The relationship between NT-proBNP concentration ≥551.35 ng/L and 1-year mortality remained significant after adjustment (HR, 4.47; 95% CI, 2.38–8.41; P<0.001).
 |
Table 4 Univariate and Multivariate Associations with 1-Year Mortality |
Discussion
Compared with the patients who had a concentration of NT-proBNP <551.35 ng/L, patients who had a concentration of NT-proBNP ≥551.35 ng/L had an increased risk of in-hospital mortality and 1-year mortality. A meta-analysis also found that elevated NT-proBNP levels were associated with both in-hospital mortality and long-term mortality in COPD patients.17 In our study, we also found that CHF was an independent risk factor for in-hospital mortality (RR, 4.78; 95% CI, 1.55–14.72; P=0.006). This was consistent with previous findings in which CHF was a significant and independent risk factor of all-cause mortality in COPD and had a significant impact on COPD course.4 Chang et al10 previously found that elevated NT-proBNP levels (>220 pmol/L) were associated with increased 30-day mortality. However, they did not find that elevated NT-proBNP levels predicted deaths between 30 days and 1 year of follow-up. They considered that elevated NT-proBNP was an indicator of acute pathological damage. Another study from Stolz et al18 showed that BNP can be used as a predictor of whether a patient will need to be placed in the ICU. However, they did not find that BNP was associated with short-term or long-term mortality in AECOPD patients. In Stolz’s study, only 15% of patients had CHF. In our research, we had not done ECG and echocardiography to excluded patients with heart failure in our study. There might be some undiagnosed CHF patients included in our study. In our cohort, there were 35.9% of patients with a history of CHF. This might be the main reason for the different results. The meta-analysis performed by Pavasini et al17 found that elevated NT-proBNP levels were related to all-cause mortality in COPD patients with or without exacerbations. This meta-analysis also suggested that having a history of CHF did not influence the relationship between elevated NT-proBNP levels and long-term mortality. In our study, NT-proBNP ≥551.35 ng/L was still a predictor of 1-year mortality even after patients with a history of CHF were removed from the cohort. Thus, we believe that elevated NT-proBNP levels reflect not only the acute pathology of a severe exacerbation but also a state of long-term cardiac dysfunction in COPD patients, therefore indicating that elevated NT-proBNP levels were associated with long-term prognosis. In stable COPD patients, a study by Chi et al found that the severity of COPD was positively correlated with NT-proBNP levels.19 Another study in vascular surgery patients without a history of CHF showed that those with elevated NT-proBNP levels had an increased risk of mortality, and that patients with COPD had higher NT-proBNP levels and an even greater risk of mortality.20 These studies suggested that patients with stable COPD also had impaired cardiac function, and the higher the NT-proBNP levels, the worse the patient’s prognosis. Given that CHF was one of the most common comorbidities in COPD patients, and given the harmful impact of cardiac dysfunction on the prognosis of COPD patients,21 we recommend that NT-proBNP concentrations should be routinely assessed in AECOPD patients.
We evaluated the effect of the cut-point for 1-year mortality (with a cut-point of 801.55 ng/L, AUC=0.79, 95% CI, 0.73–0.85; sensitivity=0.695, specificity=0.77) on in-hospital mortality. The NT-proBNP ≥801.55 ng/L was a risk factor of in-hospital mortality (multivariate analysis, RR=10.19; 95% CI, 2.38 to 43.64) and 1-year mortality (multivariate analysis, HR=4.95; 95% CI, 2.63 to 9.34). We also evaluated the effect of varying the cut-point in our study using cut-points taken from other studies on in-hospital mortality and 1-year mortality. Cut-points for NT-proBNP taken from van Gestel et al20 (with a cut-point of 500 ng/L), Medina et al22 (with a cut-point of 587.9 ng/L), Chang et al10 (with a cut-point of 1695 ng/L), and Hoiseth et al11 (with a cut-point of 969 ng/L) all showed a statistically significant difference in-hospital mortality (p<0.001) and 1-year mortality (p<0.001) between the two groups (<cut-point value vs ≥cut-point value).
The pathophysiological mechanism behind the association between elevated NT-proBNP levels and COPD prognosis might be attributed to the following elements. Poor oxygen supply was a common problem in COPD patients, especially those suffering from acute exacerbations. First, hypoxia caused pulmonary vasoconstriction,23 which formed pulmonary hypertension, resulting in a right ventricular pressure overload. A study found that NT-proBNP was positively associated with pulmonary arterial pressure.24 Second, hypoxia caused secondary polycythemia and increased blood viscosity. Our data suggested that increased D-dimer levels were associated with both in-hospital mortality (RR, 1.48; 95% CI, 1.18–1.86; p=0.001) and 1-year mortality (HR, 1.39; 95% CI, 1.20–1.60; p<0.001) using univariate analysis. In a previous study from our group, we showed that plasma D-dimer levels were associated with in-hospital and 1-year mortality in AECOPD patients.15 Third, increased oxygen consumption and poor oxygen supply of cardiomyocytes caused acidosis in cells, leading to a decrease in myocardial contractility, which increased the burden of the heart. Exacerbations of COPD worsened the heart condition, causing increased secretion of NT-proBNP by cardiomyocytes. In a study of COPD patients without a history of cardiovascular disease, Lee et al found that exacerbations of COPD had an effect on biventricular dysfunction rather than on isolated right ventricular compromise.25 However, to the best of our knowledge, there have been no studies that have continuously monitored changes in NT-proBNP concentrations. We were unable to determine which factors influenced the secretion of NT-proBNP in COPD patients as well as the relationship between the cause of elevated NT-proBNP levels and prognosis of COPD patients.
Our study had some limitations. Although our study was a single-center trial, it was a prospective study of unselected patients, which was closer to real-world clinical practice, and the results of our study were consistent with previous studies. However, a multicenter trial will still be needed to further confirm the relationship between NT-proBNP levels and prognosis in COPD patients, given that all current studies were single-center trials. Another limitation of this study was that patients lacked an echocardiogram at the time of admission. We were unable to identify those patients who had unrecognized CHF, which might have increased the risk of mortality in the multivariate analysis model. Previous data suggested a parallel relationship between cardiac dysfunction and NT-proBNP levels. However, because patients did not undergo an echocardiogram, we were unable to conduct a comprehensive analysis of the causes of elevated NT-proBNP concentrations. We hypothesized that the mechanism that caused the elevated NT-proBNP levels in COPD was mainly because of hypoxia. Although our data suggested that hypoxia was associated with in-hospital mortality, the correlation between PaO2 levels and NT-proBNP concentrations was weak (Spearman rho=−0.107, p=0.026). We could not rule out the possibility that PaO2 levels and NT-proBNP concentrations were correlated because data on blood gases taken on admission might have been affected because patients already received oxygen therapy as outpatients.
Conclusion
In this prospective study, we found that the concentration of NT-proBNP ≥551.35 ng/L was an independent and strong risk factor of in-hospital mortality and 1-year mortality in unselected patients hospitalized for exacerbations of COPD.
Acknowledgments
This study was supported by The National Natural Science Foundation of China (81670042), The Natural Science Foundation of Guangdong Province (2017A030313887), and The Science and Technology Program of Guangzhou (201707010232, 201504010018).
Author Contributions
Guoping Hu and Pixin Ran conceived the idea for this report. Guoping Hu, Haiqing Li, Zixiong Zeng, Juan Cheng, and Yuqun Li collected data for the study. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Arch Bronconeumol. 2017;53(3):128–149. doi:10.1016/j.arbres.2017.02.001
2. Soriano JB, Abajobir AA, Abate KH, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. doi:10.1016/S2213-2600(17)30293-X
3. Watz H, Waschki B, Meyer T, et al. Decreasing cardiac chamber sizes and associated heart dysfunction in COPD: role of hyperinflation. Chest. 2010;138(1):32–38. doi:10.1378/chest.09-2810
4. Bhatt SP, Dransfield MT. Chronic obstructive pulmonary disease and cardiovascular disease. Transl Res. 2013;162(4):237–251. doi:10.1016/j.trsl.2013.05.001
5. Fabbri LM, Luppi F, Beghe B, Rabe KF. Complex chronic comorbidities of COPD. Eur Respir J. 2008;31(1):204–212. doi:10.1183/09031936.00114307
6. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev Esp Cardiol (Engl Ed). 2016;69(12):1167.
7. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776–803. doi:10.1016/j.jacc.2017.04.025
8. Hildebrandt P, Collinson PO. Amino-terminal Pro–B-type natriuretic peptide testing to assist the diagnostic evaluation of heart failure in symptomatic primary care patients. Am J Cardiol. 2008;101(3, Supplement):S25–S28. doi:10.1016/j.amjcard.2007.11.016
9. Marcun R, Sustic A, Brguljan PM, et al. Cardiac biomarkers predict outcome after hospitalisation for an acute exacerbation of chronic obstructive pulmonary disease. Int J Cardiol. 2012;161(3):156–159. doi:10.1016/j.ijcard.2012.05.044
10. Chang CL, Robinson SC, Mills GD, et al. Biochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPD. Thorax. 2011;66(9):764–768. doi:10.1136/thx.2010.155333
11. Hoiseth AD, Omland T, Hagve TA, Brekke PH, Soyseth V. NT-proBNP independently predicts long term mortality after acute exacerbation of COPD - a prospective cohort study. Respir Res. 2012;13(1):97. doi:10.1186/1465-9921-13-97
12. Pervez MO, Winther JA, Brynildsen J, et al. Prognostic and diagnostic significance of mid-regional pro-atrial natriuretic peptide in acute exacerbation of chronic obstructive pulmonary disease and acute heart failure: data from the ACE 2 Study. Biomarkers. 2018;23(7):654–663. doi:10.1080/1354750X.2018.1474258
13. Adrish M, Nannaka V, Cano E, Bajantri B, Diaz-Fuentes G. Significance of NT-pro-BNP in acute exacerbation of COPD patients without underlying left ventricular dysfunction. Int J Chron Obstruct Pulmon Dis. 2017;12:1183–1189. doi:10.2147/COPD
14. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi:10.1164/rccm.201204-0596PP
15. Hu G, Wu Y, Zhou Y, et al. Prognostic role of D-dimer for in-hospital and 1-year mortality in exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11(1):2729–2736. doi:10.2147/COPD
16. Mould R, Herbert D. Introductory medical statistics (Third Edition). Med Phys. 1999;26:664–665. doi:10.1118/1.598568
17. Pavasini R, Tavazzi G, Biscaglia S, et al. Amino terminal pro brain natriuretic peptide predicts all-cause mortality in patients with chronic obstructive pulmonary disease: systematic review and meta-analysis. Chron Respir Dis. 2017;14(2):117–126. doi:10.1177/1479972316674393
18. Stolz D, Breidthardt T, Christ-Crain M, et al. Use of B-type natriuretic peptide in the risk stratification of acute exacerbations of COPD. Chest. 2008;133(5):1088–1094. doi:10.1378/chest.07-1959
19. Chi SY, Kim EY, Ban HJ, et al. Plasma N-terminal pro-brain natriuretic peptide: a prognostic marker in patients with chronic obstructive pulmonary disease. Lung. 2012;190(3):271–276. doi:10.1007/s00408-011-9363-7
20. van Gestel YR, Goei D, Hoeks SE, et al. Predictive value of NT-proBNP in vascular surgery patients with COPD and normal left ventricular systolic function. COPD. 2010;7(1):70–75. doi:10.3109/15412550903499472
21. Macchia A, Rodriguez MJ, Kleinert M, et al. Unrecognised ventricular dysfunction in COPD. Eur Respir J. 2012;39(1):51–58. doi:10.1183/09031936.00044411
22. Medina AM, Marteles MS, Saiz EB, et al. Prognostic utility of NT-proBNP in acute exacerbations of chronic pulmonary diseases. Eur J Intern Med. 2011;22(2):167–171. doi:10.1016/j.ejim.2010.12.002
23. Dunham-Snary KJ, Wu D, Sykes EA, et al. Hypoxic pulmonary vasoconstriction: from molecular mechanisms to medicine. Chest. 2017;151(1):181–192. doi:10.1016/j.chest.2016.09.001
24. Ozdemirel TS, Ulasli SS, Yetis B, Karacaglar E, Bayraktar N, Ulubay G. Effects of right ventricular dysfunction on exercise capacity and quality of life and associations with serum NT-proBNP levels in COPD: an observational study. Anadolu Kardiyol Derg. 2014;14(4):370–377. doi:10.5152/akd.014
25. Lee MH, Chang CL, Davies AR, Davis M, Hancox RJ. Cardiac dysfunction and N-terminal pro-B-type natriuretic peptide in exacerbations of chronic obstructive pulmonary disease. Intern Med J. 2013;43(5):595–598. doi:10.1111/imj.12112
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.