Back to Journals » International Journal of General Medicine » Volume 15
Prognostic Role of Fasting Remnant Cholesterol with In-Stent Restenosis After Drug-Eluting Stent Implantation
Authors Luo Y, Cui S, Zhang C, Huang R, Zhao J, Su K, Luo D, Li Y
Received 19 November 2021
Accepted for publication 28 January 2022
Published 18 February 2022 Volume 2022:15 Pages 1733—1742
DOI https://doi.org/10.2147/IJGM.S348148
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yinhua Luo,1,* Shengyu Cui,2,* Changjiang Zhang,2 Rui Huang,2 Jinbo Zhao,3 Ke Su,3 Dan Luo,3 Yuanhong Li3
1Department of Central Hospital of Tujia and Miao Autonomous Prefecture, Hubei University of Medicine, Shiyan, People’s Republic of China; 2Department of Cardiology, Renmin Hospital of Wuhan University, Wuhan, People’s Republic of China; 3Cardiovascular Disease Center, Central Hospital of Tujia and Miao Autonomous Prefecture, Hubei University of Medicine, Enshi Prefecture, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuanhong Li, Cardiovascular Disease Center, Central Hospital of Tujia and Miao Autonomous Prefecture, Hubei University of Medicine, Enshi Prefecture, People’s Republic of China, Email [email protected]
Objective: In-stent restenosis (ISR) is regarded as a critical limiting factor in stenting for coronary heart disease (CHD). Recent research has shown that fasting residual cholesterol (RC) has been shown to have a substantial impact on coronary heart disease. Unfortunately, there have not been much data to bear out the relationship between RC and ISR. Then, the predictive value of RC for in-stent restenosis in patients with coronary heart disease was analyzed.
Patients and Methods: Aiming to explore the relationship between RC and ISR, we designed a retrospective study of patients with CHD after drug-eluting stent (DES) implantation, combining the data from a public database and selecting the best-fitting model by comparing the optical subset with least absolute shrinkage and selection operator (LASSO) regression.
Results: Analysis of the abovementioned two models showed that the optical subset optimal subset model, which was based on RC, creatine, history of diabetes, smoking, multi-vessel lesions (2 vessels or more lesions), peripheral vascular lesions (PAD), and blood uric acid, had a better fit (AUC = 0.68), and that RC was an independent risk factor for ISR in the abovementioned two models. Notwithstanding its limitation, this study does suggest that RC has good predictive value for ISR.
Conclusion: Remnant cholesterol is an independent risk factor for in-stent restenosis after percutaneous coronary intervention (PCI) and is a reliable predictor of ISR.
Keywords: in-stent restenosis, ISR, percutaneous coronary intervention, PCI, remnant cholesterol, RC, drug-eluting stents, DES, least absolute shrinkage and selection operator, LASSO
Introduction
It is a truth universally acknowledged that Cardiovascular disease is a significant killer. In terms of coronary artery disease treatment, PCI has significantly improved symptomatic relief and quality of life in patients with coronary artery disease, and it is now firmly established as an effective treatment.1 However, the use of stents has always been limited by the occurrence of ISR.2 ISR and target lesion revascularization are significantly reduced with DES compared to bare-metal stents (BMS), but they are not completely eliminated. ISR after DES implantation, which occurs in 3–20% of cases, is a common clinical condition that should not be overlooked.3–5 What is more, the occurrence of ISR has a significant impact on the quality of life and long-term prognosis of patients.
An increasing number of studies have shown that dyslipidemia and ISR are indissolubly linked.6 To the best of our knowledge, the majority of the previous related literature7,8 has concentrated on investigating the impact of low-density lipoprotein cholesterol (LDL-C) levels on ISR. However, a growing number of studies have demonstrated that patients are still at considerable risk of developing cardiovascular disease even after lowering LDL-C to recommended concentrations and controlling other risk factors (eg, hypertension and diabetes). Nonetheless, an extensive protocol demonstrated that RC reflects more of the state of lipid metabolism than LDL-C, and an increasing number of scholars have noted that high levels of remnant cholesterol may also be an important pathogenic risk factor for the development of cardiovascular events.9–14
Unfortunately, there have not been much data to bear out the relationship between RC and ISR. What is more, most of the published studies8,10,11,15 are retrospective with small sample sizes and use multi-factor logistic regression statistics. It is well known that retrospective studies have many confounding factors and that multifactor logistic regression may suffer from overfitting and covariance, so we attempted to address this by reducing the dimensionality16 (using LASSO regression). Furthermore, we selected the best-fitting model by comparing the optical subset with LASSO regression, and exploring the relationship between RC and ISR.
Hence, we designed a retrospective study of patients with CAD after DES implantation, combining the data from a public database to explore the relationship between ISR and RC. And it was found that fasting RC levels were significantly and positively linked with the development of in-stent restenosis, indicating that RC has a strong prognostic value for ISR.
Patients and Methods
Study Population
Our Supplementary data consist of two parts: one from public databases and one from data collected retrospectively at the Enshi Tujia and Miao Autonomous Prefectures. The public data was obtained from the “DATADRY AD” database, a public, non-profit computerized database established by the Rich Healthcare Group in China (www.Datadryad.org). We downloaded the raw data shared by Yao et al29 from: long-term follow-up results in patients undergoing percutaneous coronary intervention (PCI) with drug-eluting stents: results from a single high-volume PCI Center. Furthermore, the raw data is freely accessible to the public. The original study enrolled 2533 participants who underwent PCI between July 2009 and August 2011, at a single high-volume PCI center in China. The study, covered by the data from the public database, was conducted in accordance with the Declaration of Helsinki and did not require patient consent, referring to the original research articles.
For our data, we retrospectively collected data from Enshi Hospital on patients who underwent percutaneous coronary drug-eluting stent implantation between 2017 and 2020 (research approval was obtained from Ethical Committees of Central Hospital of Enshi Tujia and Miao Autonomous Prefecture). We combined the data from a public database with our data to retrospectively analyze the relationship between RC and ISR.
Finally, 668 subjects were included in our study for analysis (Figure 1).
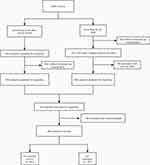 |
Figure 1 The selective procession of the participants. |
Variable Extraction from Public Databases
For public data, variables were extracted as follows: general information about the patient (age, gender, smoking status, history of diabetes, history of heart failure, history of atrial fibrillation, history of peripheral vascular disease, history of hypertension), laboratory data including total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), serum creatinine (SCR), uric acid (UA), and total bilirubin (TBIL).
Laboratory Analysis for Our Data
Fasting blood samples were collected prior to coronary procedures for baseline PCI and follow-up angiography. Baseline indicators include age, gender, smoking status, underlying diseases (including hypertension, diabetes, stroke), and coronary artery disease at the time of the first drug-eluting stent implantation. The RC level was calculated as TC minus LDL-C and HDL-C, according to the relevant literature.9
Coronary Angiography and Evaluation of ISR for Our Data
All the patients mentioned in our data underwent DES implantation at our catheterization center. Patients were divided into ISR and non-ISR groups based on angiographic follow-up results. The main outcome measure was ISR, which is defined as ≥50% luminal narrowing at follow-up angiography.
Statistic Analysis
Differences in baseline information between the two groups were compared. Continuous variables were expressed as mean (standard deviation, SD) in a normal distribution with chi-square, and differences in continuous variables between the ISR and non-ISR groups were tested using a t-test. When the distribution was skewed, data was expressed as medians (25th and 75th percentiles: P25, P75) and compared using the Mann–Whitney U-test. Categorical variables were presented as numbers and percentages and compared using the Wilcoxon test.
The data was subjected to internal validation of the data according to a ratio of 3:7. Optimal subset + multi-factor logistic regression analysis (Model 1) was used to investigate the relationship between each variable and ISR. Considering the large number of covariates affecting ISR, we used Lasso logistic regression, a regularization method for creating parsimonious models to identify the critical determinants of in-stent restenosis and compare the strengths and weaknesses of the models. The LASSO model applied to high-dimensional data reduction was used to select the best predictive features among risk factors for ISR patients. Features with nonzero coefficients in the LASSO regression model were selected. The feature selected in the LASSO regression model (Model 2) was then incorporated into the predictive model using multivariable logistic regression analysis. The characteristics were evaluated using an odds ratio (OR) with a 95% confidence interval (CI) and a P-value. Variables with a P value of 0.05 were included in the model, while those associated with disease and treatment characteristics were considered in the model. We used receiver operating characteristic curve (ROC) analysis to assess the fit of the two models. A 2-tailed P < 0.05 was considered significant.
Results
Baseline Clinical Characteristics
Of the 668 patients who met the inclusion criteria, in-stent restenosis was observed in 192 of the 678 (40.3%) patients. Baseline characteristics were compared between 192 patients with ISR and 478 patients without ISR (Table 1).
 |
Table 1 The Baseline Clinical Characteristics |
According to Table 1, the general conditions, such as age, sex, and history of hypertension, diabetes (DM), heart failure (HF), atrial fibrillation (AF), as well as stroke in both groups, were not statistically significant. However, the proportion of smokers in the ISR group was significantly higher than in the non-ISR group, as well as the proportion of patients with a history of peripheral vascular disease.
Regarding the PCI-related content, the proportion of patients with multiple lesions (2 vessels or more lesions) was significantly higher in the in-stent restenosis group compared with the control group, with a statistically significant difference [102 (53.1%) vs 153 (32.1%), p < 0.001]. Compared to the control group, the ISR group had a higher proportion of the target left anterior descending artery (LAD), [173 (90.1%) vs 388 (81.5%)], p = 0.009], while the vessel-right coronary artery (RCA) showed the opposite trend in the non-ISR group (p = 0.013). There was no significant difference in the number of stents and the number of diseased vessels between the two groups.
Regarding postoperative treatment, there were no differences between the groups.
In terms of liver and kidney function and thyroid function, creatinine levels were significantly higher in the ISR group than in the control group, and the differences were statistically significant [69 vs 74.13, p = 0.002], while there were no statistically significant differences between groups in indicators, such as blood uric acid (UA) and indirect bilirubin (BIL) (all p >0.05).
When talking to lipids, the levels of RC and TG in the ISR group were higher than those in the control group, and the differences were statistically significant (P < 0.05). The levels of HDL-C in the ISR group were significantly lower than in the non-ISR group. In contrast, the differences between LDL and TC did not differ between the two groups.
Comparison Between Logistic Regression Optimal Subset Model and LASSO Regression Model
For the selection of the optimal subset model (Model 1), all variables from the baseline information were included in the initial logistic regression model, and a multi-factor logistic regression was performed using the backward method to select the optimal subset based on the principle of choosing the model with the lowest AIC value as the optimal model. As shown in Table 2, we found that RC, creatine, history of diabetes, smoking, multi-vessel lesions (2 vessels or more lesions), peripheral vascular lesions (PAD), and blood uric acid were independent predictors for ISR after implantation of drug-eluting stents (AIC =−780.31).
 |
Table 2 The Optimal Subset Regression Analysis of Predictive for ISR |
In terms of feature selection for the predictive model for the risk of developing in-stent restenosis in patients after PCI, based on the patients in the cohort (3:1 ratio), 22 features were reduced to 10 potential predictors and had nonzero coefficients in the LASSO regression model (Model 2, Figure 2). These factors included RC, age, PAD, history of hypertension, smoking, multi-vessel lesions, left main stem lesion, left anterior descending branch lesion, creatine, and HDL-C.
The area under the ROC curve for the optimal subset based on the training set is 0.68 (Figure 3), which is slightly higher than the area under the ROC curve for the LASSO model (Figure 4). Therefore, we used the optimal subset to assess the predictive value of RC for ISR. And we could get the conclusion that RC played an important role in ISR and was also an independent risk factor for ISR.
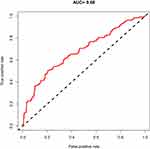 |
Figure 3 The area under the ROC curve for the optimal subset. |
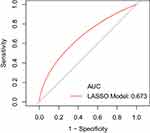 |
Figure 4 The area under the ROC curve for the LASSO model. |
Discussion
Dyslipidemia is a major pathogenic risk factor for atherosclerosis, and numerous studies6,9–12,17 have confirmed that dyslipidemia is not only an independent risk factor for CHD but also closely related to ISR. For the postoperative lipid management of PCI patients, lipid-lowering therapy is recommended for the secondary prevention of PCI patients according to the guidelines for PCI.18 The clinical benefits of lipid-lowering therapy should be long term.19–21 For patients following PCI, the known risk factors for in-stent restenosis include patient factors, biomarkers, coronary artery lesion factors, stent-related factors, and operative factors,22,23 but a significant number of studies9,12,13,19,23 have confirmed that lipid management is vital among these factors. It is suggested that monitoring and managing lipids should prioritize the treatment and prevention of CHD patients following drug-eluting stent (DES) implanted.
LDL-C is currently the leading indicator used for lipid monitoring in lipid management, but it is found that the incidence of coronary heart disease and ISR is still high, although it targets LDL-C levels in patients at high risk for coronary heart disease or post-PCI.9 Furthermore, several studies have shown that elevated RC significantly increases the risk of cardiovascular events.9,10
Although there have been many clinical studies9,10,24 on lipid control in patients with in-stent restenosis, most have focused on LDL and few on remnant cholesterol. What is more, current articles are limited by small sample sizes and differing methodological approaches. This study is a retrospective analysis of the relationship between fasting remnant cholesterol levels and the occurrence of restenosis after PCI in Chinese patients with coronary artery disease after DES implantation, combining the data from a public database aimed at standardizing lipid-lowering medications. Fasting remnant cholesterol level was found to be closely related to ISR occurrence and to be an independent risk factor for ISR. This finding is consistent with previous studies that found that elevated fasting remnant cholesterol levels increased the degree of coronary atherosclerotic stenosis. Furthermore, in this study, a retrospective analysis of patients with ISR revealed that RC, creatine, history of diabetes, smoking, multi-vessel lesions (2 vessels or more lesions), peripheral vascular lesions, and blood uric acid were all independent risk factors for restenosis.
Regarding remnant cholesterol as an independent risk factor for ISR and as a strong predictor of ISR, the following mechanisms are currently documented: the pathogenic mechanisms of RC are currently considered to include oxidative stress as well as inflammatory mechanisms. Anette Varbo proposed15,25–27 that RC is similar to LDL-C in terms of atherogenicity. The mechanism is similar in that macrophages and smooth muscle cells capture RC and turn into foam cells, which eventually lead to atherosclerotic plaque formation. Due to its large size, RC enters the arterial wall at a slightly slower rate, but its ability to bind to macrophages and smooth muscle cells is ten times greater than that of LDL-C, which is the mechanism of oxidative stress that makes RC pathogenic.28 Regarding the inflammatory mechanism, on the one hand, RC entering the arterial wall can exert inflammatory effects, and the triacylglycerol component of RC entering the arterial wall can trigger local and even systemic low-grade inflammation.28 It was found28 that remnant cholesterol lipoproteins (RLP) can stimulate inflammatory factors in endothelial cells and promote monocyte adhesion. On the other hand, elevated TC in the circulating blood can also lead to systemic inflammation. In summary, when abnormal lipid metabolism leads to an increased concentration of circulating RC, more and more RC enters the arterial wall, contributing to foam cell formation, which also triggers a systemic inflammatory response, ultimately leading to cardiovascular events.
Conclusion
Fasting remnant cholesterol levels were significantly and positively correlated with the occurrence of in-stent restenosis in our post-PCI population. Therefore, remnant cholesterol is an essential predictor of ISR after PCI and an essential guide for different lipid-lowering therapies in this population. The risk assessment model (model1) based on RC, creatine, history of diabetes, smoking, multi-vessel lesions (2 vessels or more lesions), PAD, and blood uric acid after PCI had good predictive value as well as goodness of fit for in-stent restenosis. Nevertheless, the research still has several limitations. First of all, its validity is limited by the retrospective design with an inadequate level of evidence. Secondly, this is an observational single-center registry and may have an inherent bias common to this type of study.
Abbreviation
ISR, In-stent restenosis; CAD, Coronary artery atherosclerotic heart disease; RC, remnant cholesterol; DES¸ drug-eluting stents; LASSO, least absolute shrinkage and selection operator; PAD, peripheral vascular lesions; Cr, Creatine; UA, Uric acid; DM, Diabetes; HF, heart failure; AF, Atrial fibrillation; PCI, Percutaneous coronary intervention; LDL-C, Low-density lipoprotein cholesterol; TC, Total cholesterol; TG, Triglyceride; HDL-C, High-density lipoprotein cholesterol; TBIL¸ Total Bilirubin; RLP, Remnant cholesterol lipoproteins; ACEI, Angiotensin-converting enzyme inhibitor; CCB, Calcium channel antagonist.
Data Sharing Statement
All relevant data supporting the conclusions of this article are included within the article.
Ethical Approval
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this study and any accompanying images. The study was approved by the Ethical Committees of the Central Hospital of Enshi Tujia and Miao Autonomous Prefecture. The data are anonymous, and the requirement for informed consent was therefore waived.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval for the version to be published; and agreed to be accountable for all aspects of the work.
Funding
This work was supported in part by the National Natural Science Foundation of China (82160072) and the Science and Technology Support Project of Enshi Science and Technology Bureau (D20210024). The funding bodies played no role in the design of the study, collection, analysis, and interpretation of the data and in writing the manuscript.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Khattab MH, Sherry AD, Barker CM. The birth, decline, and contemporary re-emergence of endovascular brachytherapy for prevention of in-stent restenosis. Brachytherapy. 2021;20(2):485–493. doi:10.1016/j.brachy.2020.09.012
2. Singh AD, Singal AK, Mian A, et al. Recurrent drug-eluting stent in-stent restenosis: a state-of-the-art review of pathophysiology, diagnosis, and management. Cardiovasc Revasc Med. 2020;21(9):1157–1163. doi:10.1016/j.carrev.2020.01.005
3. Dangas GD, Claessen BE, Caixeta A, et al. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56(23):1897–1907. doi:10.1016/j.jacc.2010.07.028
4. Stolker JM, Cohen DJ, Kennedy KF, et al. Repeat revascularization after contemporary percutaneous coronary intervention: an evaluation of staged, target lesion, and other unplanned revascularization procedures during the first year. Circ Cardiovasc Interv. 2012;5(6):772–782. doi:10.1161/CIRCINTERVENTIONS.111.967802
5. Nakano M, Otsuka F, Yahagi K, et al. Human autopsy study of drug-eluting stents restenosis: histomorphological predictors and neointimal characteristics. Eur Heart J. 2013;34(42):3304–3313. doi:10.1093/eurheartj/eht241
6. Duran EK, Pradhan AD. Triglyceride-rich lipoprotein remnants and cardiovascular disease. Clin Chem. 2021;67(1):183–196. doi:10.1093/clinchem/hvaa296
7. Díaz Rodríguez Á, Mantilla Morató T. LDL as a therapeutic objective. Clin Investig Arterioscler. 2019;31 Suppl 2:1–15. doi:10.1016/j.arteri.2019.10.004
8. Gai M-T, Zhu B, Chen X-C, et al. A prediction model based on platelet parameters, lipid levels, and angiographic characteristics to predict in-stent restenosis in coronary artery disease patients implanted with drug-eluting stents. Lipids Health Dis. 2021;20(1):118. doi:10.1186/s12944-021-01553-2
9. Castañer O, Pintó X, Subirana I, et al. Remnant cholesterol, not LDL cholesterol, is associated with incident cardiovascular disease. J Am Coll Cardiol. 2020;76(23):2712–2724. doi:10.1016/j.jacc.2020.10.008
10. Elshazly MB, Mani P, Nissen S, et al. Remnant cholesterol, coronary atheroma progression and clinical events in statin-treated patients with coronary artery disease. Eur J Prev Cardiol. 2020;27(10):1091–1100. doi:10.1177/2047487319887578
11. Chait A, Ginsberg HN, Vaisar T, et al. Remnants of the triglyceride-rich lipoproteins, diabetes, and cardiovascular disease. Diabetes. 2020;69(4):508–516. doi:10.2337/dbi19-0007
12. Burnett JR, Hooper AJ, Hegele RA. Remnant cholesterol and atherosclerotic cardiovascular disease risk. J Am Coll Cardiol. 2020;76(23):2736–2739. doi:10.1016/j.jacc.2020.10.029
13. Bonfiglio C, Leone CM, Silveira LVA, et al. Remnant cholesterol as a risk factor for cardiovascular, cancer or other causes mortality: a competing risks analysis. Nutr Metab Cardiovasc Dis. 2020;30(11):2093–2102. doi:10.1016/j.numecd.2020.07.002
14. Qin Z, Zhou K, Li Y-P, et al. Remnant lipoproteins play an important role of in-stent restenosis in type 2 diabetes undergoing percutaneous coronary intervention: a single-centre observational cohort study. Cardiovasc Diabetol. 2019;18(1):11. doi:10.1186/s12933-019-0819-z
15. Varbo A, Benn M, Smith GD, et al. Remnant cholesterol, low-density lipoprotein cholesterol, and blood pressure as mediators from obesity to ischemic heart disease. Circ Res. 2015;116(4):665–673. doi:10.1161/CIRCRESAHA.116.304846
16. Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med. 2007;26(30):5512–5528. doi:10.1002/sim.3148
17. Hari P, Khandelwal P, Smoyer WE. Dyslipidemia and cardiovascular health in childhood nephrotic syndrome. Pediatr Nephrol. 2020;35(9):1601–1619. doi:10.1007/s00467-019-04301-y
18. Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol. 2016;67(10):1235–1250. doi:10.1016/j.jacc.2015.10.005
19. ESC Committee for Practice Guidelines (CPG). 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140–205. doi:10.1016/j.atherosclerosis.2019.08.014
20. Mach F, Baigent C, Catapano AL. Erratum to “2019 ESC/EAS guidelines for the management of dyslipidemias: lipid modification to reduce cardiovascular risk” [Atherosclerosis 290 (2019) 140-205]. Atherosclerosis. 2020;292(p):160–162. doi:10.1016/j.atherosclerosis.2019.11.020
21. Ray KK, Kastelein JJP, Matthijs Boekholdt S, et al. The ACC/AHA 2013 guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: the good the bad and the uncertain: a comparison with ESC/EAS guidelines for the management of dyslipidaemias 2011. Eur Heart J. 2014;35(15):960–968. doi:10.1093/eurheartj/ehu107
22. He W, Xu C, Wang X, et al. Development and validation of a risk prediction nomogram for in-stent restenosis in patients undergoing percutaneous coronary intervention. BMC Cardiovasc Disord. 2021;21(1):435. doi:10.1186/s12872-021-02255-4
23. Kim C, Kim B-K, Lee S-Y, et al. Incidence, clinical presentation, and predictors of early neoatherosclerosis after drug-eluting stent implantation. Am Heart J. 2015;170(3):591–597. doi:10.1016/j.ahj.2015.06.005
24. Salinas CAA, Chapman MJ. Remnant lipoproteins: are they equal to or more atherogenic than LDL? Curr Opin Lipidol. 2020;31(3):132–139. doi:10.1097/MOL.0000000000000682
25. Varbo A, Freiberg JJ, Nordestgaard BG. Extreme nonfasting remnant cholesterol vs extreme LDL cholesterol as contributors to cardiovascular disease and all-cause mortality in 90000 individuals from the general population. Clin Chem. 2015;61(3):533–543. doi:10.1373/clinchem.2014.234146
26. Varbo A, Langsted A, Nordestgaard BG. Commentary: nonfasting remnant cholesterol simplifies triglyceride-rich lipoproteins for clinical use, and metabolomic phenotyping ignites scientific curiosity. Int J Epidemiol. 2016;45(5):1379–1385. doi:10.1093/ije/dyw215
27. Varbo A, Nordestgaard BG. Remnant cholesterol and risk of ischemic stroke in 112,512 individuals from the general population. Ann Neurol. 2019;85(4):550–559. doi:10.1002/ana.25432
28. Tada H, Nohara A, Inazu A, et al. Remnant lipoproteins and atherosclerotic cardiovascular disease. Clin Chim Acta. 2019;490:1–5. doi:10.1016/j.cca.2018.12.014
29. Yao HM, Wan YD, Zhang XJ, et al. Long-term follow-up results in patients undergoing percutaneous coronary intervention (PCI) with drug-eluting stents: results from a single high-volume PCI centre. BMJ Open. 2014;4(8):e004892. doi:10.1136/bmjopen-2014-004892
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

