Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 11 » Issue 1
Prognostic role of D-dimer for in-hospital and 1-year mortality in exacerbations of COPD
Authors Hu G, Wu Y, Zhou Y, Wu Z, Wei L, Li Y, Peng G , Liang W, Ran P
Received 16 May 2016
Accepted for publication 30 August 2016
Published 31 October 2016 Volume 2016:11(1) Pages 2729—2736
DOI https://doi.org/10.2147/COPD.S112882
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Guoping Hu,1 Yankui Wu,2 Yumin Zhou,3 Zelong Wu,1 Liping Wei,1 Yuqun Li,1 GongYong Peng,3 Weiqiang Liang,1 Pixin Ran3
1Department of Respiratory Medicine, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, 2Department of Respiratory Disease of People’s Hospital of Guangxi Zhuang Autonomous Region, Guangxi Zhuang Autonomous Region, 3Guangzhou Institute of Respiratory Disease, State Key Lab of Respiratory Disease, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, People’s Republic of China
Background and objective: Serum D-dimer is elevated in respiratory disease. The objective of our study was to investigate the effect of D-dimer on in-hospital and 1-year mortality after acute exacerbations of chronic obstructive pulmonary disease (AECOPD).
Methods: Upon admission, we measured 343 AECOPD patients’ serum D-dimer levels and arterial blood gas analysis, and recorded their clinical characteristics. The level of D-dimer that discriminated survivors and non-survivors was determined using a receiver operator curve (ROC). The risk factors for in-hospital mortality were identified through univariate analysis and multiple logistic regression analyses. To evaluate the predictive role of D-dimer for 1-year mortality, univariate and multivariate Cox regression analyses were performed.
Results: In all, 28 patients died, and 315 patients survived in the in-hospital period. The group of dead patients had lower pH levels (7.35±0.11 vs 7.39±0.05, P<0.0001), higher D-dimer, arterial carbon dioxide tension (PaCO2), C-reactive protein (CRP), and blood urea nitrogen (BUN) levels (D-dimer 2,244.9±2,310.7 vs 768.2±1,078.4 µg/L, P<0.0001; PaCO2: 58.8±29.7 vs 46.1±27.0 mmHg, P=0.018; CRP: 81.5±66, P=0.001; BUN: 10.20±6.87 vs 6.15±3.15 mmol/L, P<0.0001), and lower hemoglobin levels (118.6±29.4 vs 128.3±18.2 g/L, P=0.001). The areas under the ROC curves of D-dimer for in-hospital death were 0.748 (95% confidence interval (CI): 0.641–0.854). D-dimer ≥985 ng/L was a risk factor for in-hospital mortality (relative risk =6.51; 95% CI 3.06–13.83). Multivariate logistic regression analysis also showed that D-dimer ≥985 ng/L and heart failure were independent risk factors for in-hospital mortality. Both univariate and multivariate Cox regression analyses showed that D-dimer ≥985 ng/L was an independent risk factor for 1-year death (hazard ratio (HR) 3.48, 95% CI 2.07–5.85 for the univariate analysis; and HR 1.96, 95% CI 1.05–3.65 for the multivariate analysis).
Conclusion: D-dimer was a strong and independent risk factor for in-hospital and 1-year death for AECOPD patients.
Keywords: AECOPD, chronic obstructive pulmonary diseases, D-dimer, mortality, prognosis
Introduction
Acute exacerbation is a common phenomenon for chronic obstructive pulmonary disease (COPD) patients during the course of their disease.1 Acute exacerbations of COPD (AECOPD) impact long-term prognosis and are associated with substantial in-hospital mortality. The most important factors that determine the overall prognosis of COPD are the frequency and severity of exacerbations;2,3 and AECOPD are often companied with respiratory failure.1 The blood of most of AECOPD patients is in a hypercoagulable state for hypoxemia and carbon dioxide retention.4–6 This state causes the formation of small pulmonary thrombosis and leads to an adverse prognosis.7–9 Some clinical evidence shows that hypercoagulable state and thrombosis in the pulmonary vessels can alter the clinical course of patients with COPD, especially during the period of acute exacerbations.10 The D-dimer is a product of fibrinolysis, which may increase during many illnesses and physiological conditions associated with thrombosis and thrombolysis.11 Studies have showed that elevated plasma D-dimer was associated with adverse outcomes, and D-dimer has been recommended as a prognostic factor for these conditions.10,12–17 However, there are few prospective studies that have investigated the role of D-dimer in patients with exacerbations of COPD. We therefore performed a prospective study to investigate the role of serum D-dimer in the prediction of in-hospital mortality and all-cause mortality within 1 year in AECOPD patients.
Methods
Subjects
We screened all the AECOPD patients admitted to the respiratory medicine department of the Third Affiliated Hospital of Guangzhou Medical University (Guangzhou, People’s Republic of China) from November 2012 to November 2014. All subjects had been diagnosed with COPD previously by respiratory doctors. The exclusion criteria were: hospitalization for a reason other than AECOPD, inability or unwillingness to cooperate with the doctors, and not providing spirometry data. We invited all the AECOPD patients to participate in the present study on the first day of admission to the ward. The ethics committee of The Third Affiliated Hospital of Guangzhou Medical University approved the research protocol.
Study design
Patient demographics, including age, sex, the number of hospitalizations for AECOPD in the previous year, smoking habit, and comorbidities, with a special emphasis on cardiovascular disease, were recorded. Clinical data, such as vital signs and arterial blood gases (pH, arterial carbon dioxide tension (PaCO2), arterial oxygen tension (PaO2), and arterial oxygen saturation), were examined on admission. We collected the blood samples from each patient at the time of admission to the department of respiratory disease for D-dimer and standard laboratory measurements (creatinine, blood urea nitrogen (BUN), platelets, hemoglobin, hematocrit, fibrinogen, and C-reactive protein (CRP)). The glomerular filtration rate (GFR) was calculated within 24 hours of admission by the simplified modification of diet in renal disease equation.18 GFR <90 mL/min/1.73 m2 was considered as renal dysfunction. Congestive heart failure was diagnosed on the base of the Chinese guidelines published in 2007 for the diagnosis and management of chronic heart failure.19 Attending physicians not involved in this study made the treatment programs according to the Global initiative for chronic Obstructive Lung Disease (GOLD) guidelines.1 Patients were followed up with telephone interviews every 3 months for 1 year by the study investigators. Patients with at least one hospitalization for AECOPD in the previous year were considered as frequent exacerbators.
Statistical analysis
The primary outcomes were in-hospital and all-cause mortality at 1 year. The secondary outcome was the factors associated with in-hospital death. Categorical variables are presented as n (%), and normally distributed values are presented as mean ± standard deviation. Comparisons between groups were made using chi-squared test (for categorical variables) or analysis of variance (for continuous variables). Receiver operator curve analysis was applied to define the minimal optimal D-dimer level that predicted death. Multivariate logistic regression analysis was applied to determine the independent factors of in-hospital death. To evaluate the influence of D-dimer levels on 1-year mortality, we performed Cox regression univariate and multivariate analyses. Significant confounders, including age, sex, current smoking status, the comorbidities of heart failure and renal dysfunction, pH, PaO2, PaCO2, and GOLD stage, were evaluated in the Cox regression analyses. Kaplan–Meier survival curves and log-rank tests were used to compare the time to death between those with elevated D-dimer levels and those without. The results are presented as hazard ratios (HRs) with 95% confidence interval (CI). We analyzed the data using the Stata statistical software package (Version 7.0, Stata Corporation, College Station, TX, USA) and SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
D-dimer and in-hospital mortality
We evaluated 391 AECOPD patients. However, there were only 343 AECOPD patients included in our study. Figure 1 shows the flow chart of the included participants. Twenty-eight subjects experienced in-hospital mortality. Table 1 shows the differences between survivors and non-survivors in the hospital. There were more patients who suffered from renal dysfunction and congestive heart failure in the non-survivor group. Additionally, the non-survivors were significantly older (80.4±8.1 years old) and more hypercapnic (PaCO2: 58.8±29.7 mmHg) than the survivors (PaCO2: 46.1±27.0 mmHg and 75.8±9.9 years old, respectively). There was no difference in lung function, fibrinogen, platelets, PaO2, or hematocrit between survivors and those who died. The pH was significantly lower in the patients who died (pH: 7.346±0.106) compared to survivors (pH: 7.389±0.054). The plasma levels of D-dimer, CRP, and BUN were higher in non-survivors (D-dimer: 2,244.9±2,310.7 ng/L, CRP 81.5±66.0 mg/L, and BUN 10.2±6.87 mmol/L) than in survivors (D-dimer: 768.2±1,078.4 ng/L, CRP: 42.0±56.2 mg/L, and BUN: 6.15±3.15 mmol/L).
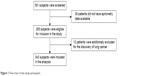  | Figure 1 Flow chart of the study participants. |
Associations between Serum D-dimer levels and clinically relevant outcomes
Figure 2 shows that the area under the curve of serum D-dimer to predict in-hospital death was 0.748 (95% CI 0.641–0.854), and the cutoff point 985 ng/L, with a sensitivity of 0.714 and a specificity of 0.794. According to the serum D-dimer levels, the entire cohort was divided into two groups. There were 85 patients with D-dimer levels ≥985 ng/L and 258 with D-dimer levels <985 ng/L. Table 2 shows non-statistically significant associations of D-dimer levels with sex, PaCO2, pH, fibrinogen, platelets, and PaO2 (P>0.05) and statistically significant associations with age, renal dysfunction, hemoglobin, hematocrit, CRP, and the concentration of creatinine and BUN (P<0.05).
Serum D-dimer levels and in-hospital mortality
Univariate analyses (Table 3) showed that a pH <7.35, PaCO2 ≥50 mmHg, PaO2 <60 mmHg, congestive heart failure, renal dysfunction, and D-dimer ≥985 ng/L were risk factors of in-hospital mortality, while age, GOLD stage, sex, and frequent exacerbators in the past year were not risk factors for in-hospital mortality. However, multivariate logistic regression analysis only showed that congestive heart failure and D-dimer ≥985 ng/L were associated with in-hospital mortality (Table 4).
Serum D-dimer levels and 1-year mortality
Fifty-seven subjects died within 1 year. Figure 3 shows the Kaplan–Meier survival curves, which evaluated the time to death within 1 year based on serum D-dimer levels. Patients with serum D-dimer ≥985 ng/L had an increased risk of 1-year mortality compared to those with serum D-dimer <985 ng/L. Univariate Cox regression analyses showed that D-dimer was a risk factor for 1-year mortality (HR 3.48, 95% CI 2.07–5.85; P=0.001) (Table 5). And multivariate analysis also showed that the serum D-dimer level still was an independent risk factor of 1-year mortality (HR 1.96, 95% CI 1.05–3.65; P=0.035).
  | Figure 3 Kaplan–Meier survival curves evaluating the time to death in days for patients with D-dimer levels above (≥985 ng/L) and below (<985 ng/L) the median value (P=0.000 by log-rank test). |
Univariate analyses (Table 5) showed that age, pH <7.35, PaCO2 ≥50 mmHg, PaO2 <60 mmHg, congestive heart failure, renal dysfunction, CRP, and D-dimer ≥985 ng/L were risk factors of 1-year death, and GOLD stage, sex, and frequent exacerbators in the past year were not associated with 1-year death. Multivariate analysis confirmed that CRP, congestive heart failure, renal dysfunction, and D-dimer ≥985 ng/L were risk factors of 1-year death (Table 5).
Discussion
This study is a comprehensive prospective study reporting the associations between D-dimer levels and in-hospital and 1-year mortality for COPD exacerbation. In the present study, our result showed that the serum D-dimer level was an independent risk factor for in-hospital death (relative risk =6.51, 95% CI: 3.06–13.83) and 1-year mortality (HR =3.48, 95% CI 2.07–5.85; P=0.001 for univariate analysis; and HR =1.96, 95% CI 1.05–3.65; P=0.035 for multivariate analysis) for AECOPD. Many studies have reported that the D-dimer was an independent predictor for cardiovascular and all-cause death among elderly persons.10,13,15,20 Our study results were consistent with the retrospective study reported by Oren Fruchter,10 which showed that D-dimer level examined on admission could be used as a predictive biomarker for short- and long-term mortality for AECOPD.10
Several factors have been previously reported to be risk factors for death, including the frequency of AECOPD.21 Soler-Cataluna et al have reported that frequent exacerbations were a risk factor for mortality.21 However, in the present study, we found that frequent exacerbations were not a risk factor for in-hospital or 1-year mortality. The differing outcomes between studies may be related to the dissimilar definitions of frequent exacerbators. In our study, we could not collect the exact data on the exacerbations in the past year. But, we could collect the previous year’s information of hospitalization due to AECOPD; therefore, frequent exacerbators were defined as patients who had at least one hospitalization for AECOPD in the past year. We did find that AECOPD patients with coexisting congestive heart failure had higher 1-year and in-hospital death, which was consistent with previous studies.22,23
In our study, we found that elevated CRP was an unfavorable factor for both 1-year and in-hospital death for AECOPD. There are some studies that have reported that elevated HsCRP was a risk factor for adverse outcomes of AECOPD.24,25 Of course, there are also studies that showed that elevated HsCRP was not associated with mortality of AECOPD.26 In a previous study from our group, we showed that plasma cystatin C was a risk factor for in-hospital mortality.27 Additionally, in another study from our group, the PSI index was associated with in-hospital death.28 D-dimer was an easy-to-obtain biomarker and an independent risk factor of in-hospital and 1-year mortality, which suggest that D-dimer could be used to identify serious patients who need more intensive treatment. So, serum D-dimer levels could be used to construct a multicomponent score in future studies.
Additionally, we also explored the association between D-dimer levels with laboratory tests and clinical characteristics for AECOPD patients. We found that D-dimer levels were higher in patients with renal dysfunction and congestive heart failure and were associated with CRP, hemoglobin, hematocrit, and old age. Alternatively, D-dimer was not associated with sex, PaCO2, pH, fibrinogen, platelets, and PaO2. Ya-Jun Song et al have reported that the serum D-dimer levels significantly negatively related to PaO2 and positively related to PaCO2 in the patients with AECOPD combined with respiratory failure.29 There were also some studies that showed D-dimer levels in patients with renal dysfunction were elevated.30,31 The study by Jafri et al showed that D-dimer levels in patients with heart failure were higher than patients without heart failure.32
Our study has several limitations. The first is that pulmonary embolism, diagnosed by computed tomography pulmonary angiography (CTPA) or pulmonary angiography, was not excluded, which may generate bias. The second limitation is that the patients were followed up only by telephone and every 3 months, which may generate interview bias. The third limitation is that we could not collect the exacerbation times in the past years. The frequent exacerbators were defined as patients with at least one hospitalization for AECOPD in the previous year.
Conclusion
Conclusively, D-dimer was a risk predictor both for in-hospital and 1-year mortality of AECOPD patients. Additionally, the serum D-dimer is a widely and rapidly examined cheaper biomarker, which means that D-dimer could be used to identify serious AECOPD patients.
Acknowledgments
This study was supported by The National Key Technology R&D Program of the 12th National Five-year Development Plan 2012BAI05B01 (P.R.), the Medical Science and Technology Research Fund of Guangdong Province (A2016265), and the scientific research fund of the health department of Guangxi Zhuang Autonomous Region (Z2012248).
Author contributions
Guoping Hu, Yankui Wu, and Pixin Ran conceived the idea for this report. Guoping Hu, Yankui Wu, and Yumin Zhou wrote the first draft of the article. Guoping Hu, Yankui Wu, Yumin Zhou, GongYong Peng, Zelong Wu, Liping Wei, Yuqun Li, Weiqiang Liang, and Pixin Ran contributed to the final version. Guoping Hu, Zelong Wu, Liping Wei, Yuqun Li, and Weiqiang Liang collected data for the study. Guoping Hu and Yumin Zhou performed the statistical analyses. Guoping Hu and Yankui Wu are co-first authors. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
All authors report that there is no potential conflict of interest with any companies/organizations. Yankui Wu is the wife of Guoping Hu. All the other authors are from a different family.
References
Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4): 347–365. | ||
Miravitlles M, Guerrero T, Mayordomo C, Sanchez-Agudo L, Nicolau F, Segu JL. Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. The EOLO Study Group. Respiration. 2000;67(5):495–501. | ||
Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest. 2003;124(2):459–467. | ||
Macnee W. Pathogenesis of chronic obstructive pulmonary disease. Clin Chest Med. 2007;28(3):479–513, v. | ||
Rizkallah J, Man SF, Sin DD. Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2009;135(3):786–793. | ||
Ashitani J, Mukae H, Arimura Y, Matsukura S. Elevated plasma procoagulant and fibrinolytic markers in patients with chronic obstructive pulmonary disease. Intern Med. 2002;41(3):181–185. | ||
Lim W, Eikelboom JW, Ginsberg JS. Inherited thrombophilia and pregnancy associated venous thromboembolism. BMJ. 2007;334(7607):1318–1321. | ||
Dahl M, Tybjaerg-Hansen A, Vestbo J, Lange P, Nordestgaard BG. Elevated plasma fibrinogen associated with reduced pulmonary function and increased risk of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(6):1008–1011. | ||
Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005; 293(6):715–722. | ||
Fruchter O, Yigla M, Kramer MR. D-dimer as a prognostic biomarker for mortality in chronic obstructive pulmonary disease exacerbation. Am J Med Sci. 2015;349(1):29–35. | ||
Haapaniemi E, Tatlisumak T. Is D-dimer helpful in evaluating stroke patients? A systematic review. Acta Neurol Scand. 2009;119:141–150. | ||
Guo YJ, Chang MH, Chen PL, Lee YS, Chang YC, Liao YC. Predictive value of plasma (D)-dimer levels for cancer-related stroke: a 3-year retrospective study. J Stroke Cerebrovasc Dis. 2014;23:e249–e254. | ||
Hu X, Fang Y, Ye F, et al. Effects of plasma D-dimer levels on early mortality and long-term functional outcome after spontaneous intracerebral hemorrhage. J Clin Neurosci. 2014;21(8):1364–1367. | ||
Rodger MA, Le Gal G, Wells P, et al. Clinical decision rules and D-Dimer in venous thromboembolism: current controversies and future research priorities. Thromb Res. 2014;134(4):763–768. | ||
Turak O, Canpolat U, Ozcan F, et al. D-dimer level predicts in-hospital mortality in patients with infective endocarditis: a prospective single-centre study. Thromb Res. 2014;134(3):587–592. | ||
Keller K, Beule J, Schulz A, Coldewey M, Dippold W, Balzer JO. D-dimer for risk stratification in haemodynamically stable patients with acute pulmonary embolism. Adv Med Sci. 2015;60(2):204–210. | ||
Minami Y, Haruki S, Jujo K, et al. Elevated D-dimer levels predict an adverse outcome in hospitalized patients with acute decompensated heart failure. Int J Cardiol. 2015;204:42–44. | ||
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. | ||
Chinese Society of Cardiology of Chinese Medical A, Editorial Board of Chinese Journal of Cardiology. [Guidelines for the diagnosis and management of chronic heart failure]. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35(12):1076–1095. | ||
Grau E, Tenias JM, Soto MJ, et al. D-dimer levels correlate with mortality in patients with acute pulmonary embolism: Findings from the RIETE registry. Crit Care Med. 2007;35(8):1937–1941. | ||
Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. | ||
Yoshihisa A, Takiguchi M, Shimizu T, et al. Cardiovascular function and prognosis of patients with heart failure coexistent with chronic obstructive pulmonary disease. J Cardiol. 2014;64(4):256–264. | ||
Ahn YH, Lee KS, Park JH, et al. Independent risk factors for mortality in patients with chronic obstructive pulmonary disease who undergo comprehensive cardiac evaluations. Respiration. 2015;90(3):199–205. | ||
Karadeniz G, Polat G, Senol G, Buyuksirin M. C-reactive protein measurements as a marker of the severity of chronic obstructive pulmonary disease exacerbations. Inflammation. 2013;36(4):948–953. | ||
Man SF, Connett JE, Anthonisen NR, Wise RA, Tashkin DP, Sin DD. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax. 2006;61(10):849–853. | ||
Lomholt FK, Laulund AS, Bjarnason NH, Jorgensen HL, Godtfredsen NS. Meta-analysis of routine blood tests as predictors of mortality in COPD. Eur Clin Respir J. 2014;1. | ||
Hu G, Wu Y, Zhou Y, Yu Y, Liang W, Ran P. Cystatin C as a predictor of in-hospital mortality after exacerbation of COPD. Respir Care. 2016;61(7):950–957. | ||
Hu G, Zhou Y, Wu Y, Yu Y, Liang W, Ran P. The pneumonia severity index as a predictor of in-hospital mortality in acute exacerbation of chronic obstructive pulmonary disease. PLos One. 2015;10(7): e0133160. | ||
Song YJ, Zhou ZH, Liu YK, Rao SM, Huang YJ. Prothrombotic state in senile patients with acute exacerbations of chronic obstructive pulmonary disease combined with respiratory failure. Exp Ther Med. 2013;5(4):1184–1188. | ||
Karami-Djurabi R, Klok FA, Kooiman J, Velthuis SI, Nijkeuter M, Huisman MV. D-dimer testing in patients with suspected pulmonary embolism and impaired renal function. Am J Med. 2009;122(11):1050–1053. | ||
Lindner G, Funk GC, Pfortmueller CA, et al. D-dimer to rule out pulmonary embolism in renal insufficiency. Am J Med. 2014;127(4):343–347. | ||
Jafri SM, Ozawa T, Mammen E, Levine TB, Johnson C, Goldstein S. Platelet function, thrombin and fibrinolytic activity in patients with heart failure. Eur Heart J. 1993;14(2):205–212. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






