Back to Journals » OncoTargets and Therapy » Volume 11
Prognostic role of aspartate aminotransferase-lymphocyte ratio index in patients with metastatic colorectal cancer: results from the randomized ITACa trial
Authors Casadei Gardini A , Scarpi E , Orlandi E , Tassinari D, Leo S, Bernardini I, Gelsomino F, Tamberi S, Ruscelli S , Vespignani R , Ronconi S, Frassineti GL, Amadori D, Passardi A
Received 26 February 2018
Accepted for publication 12 May 2018
Published 29 August 2018 Volume 2018:11 Pages 5261—5268
DOI https://doi.org/10.2147/OTT.S166614
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yao Dai
Andrea Casadei Gardini,1,* Emanuela Scarpi,2,* Elena Orlandi,3 Davide Tassinari,4 Silvana Leo,5 Ilaria Bernardini,6 Fabio Gelsomino,7 Stefano Tamberi,8 Silvia Ruscelli,1 Roberto Vespignani,9 Sonia Ronconi,10 Giovanni Luca Frassineti,1 Dino Amadori,1 Alessandro Passardi1
1Department of Medical Oncology, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy; 2Unit of Biostatistics and Clinical Trials, IRST IRCCS, Meldola, Italy; 3Medical Oncology Department, Piacenza Hospital, Piacenza, Italy; 4Department of Oncology, City Hospital, Rimini, Italy; 5Medical Oncology Unit, Vito Fazzi Hospital, Lecce, Italy; 6Medical Oncology Unit, Ramazzini Hospital, Carpi, Italy; 7Department of Oncology and Hematology, Division of Oncology, University Hospital Modena, Italy; 8Oncolgy Unit, Degli Infermi Hospital, Faenza, Italy; 9IT Service, IRST IRCCS, Meldola, Italy; 10Hematology Unit, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy
*These authors contributed equally to this work
Background: The aim of this study was to investigate the role of pre-treatment aspartate aminotransferase-lynphocyte ratio (ALRI) as a predictor of prognosis and treatment efficacy in patients with metastatic colorectal cancer (mCRC) enrolled in the prospective multicenter randomized ITACa (Italian Trial in Advanced Colorectal Cancer) trial to receive first-line chemotherapy (CT) + bevacizumab (B) or CT alone.
Patients and methods: Patients randomly received CT+B or CT alone as first-line therapy. CT consisted of either FOLFOX4 or FOLFIRI at the clinician’s discretion.
Results: Out of the 284 patients enrolled, increased ALRI levels were associated with shorter PFS and OS (p<0.0001). At baseline, median PFS was 10.3 months (95% CI 9.4–12.0) and 8.0 months (95 % CI 6.8–8.9), and median OS was 25.2 months (95 % CI 21.3–30.2) and 18.8 months (95 % CI 16.6–21.7) for patients with low (<14) and high (≥14) ALRI levels, respectively (HR 1.43, 95% CI 1.12–1.82, p=0.004; HR=1.51, 95% CI 1.17–1.96, p<0.001). Interaction tests on ALRI levels and treatment efficacy in the CT+B and the CT groups were statistically significant for PFS (p=0.0003), but not for OS (p=0.228).
Conclusion: Our results indicate that ALRI is a good prognostic and predictive marker for mCRC patients candidate for CT+B.
Keywords: metastatic colorectal cancer, bevacizumab, first line, prognosis, aspartate aminotransferase-lymphocyte ratio index, clinical outcome, rectal cancer
Introduction
Colorectal cancer (CRC) is the third most common cause of death from cancer in Western Europe and North America.1
Literature has shown the relationship between systemic chronic inflammation and various types of cancer, including CRC,2 as the activation of the inflammatory cascade by malignant tumors leads to apoptosis inhibition and angiogenesis promotion, eventually causing tumor genesis, proliferation, and metastasis.3,4
In clinical practice, physicians commonly test the liver enzyme aspartate aminotransferase (AST), without evidence of any correlation with the response to bevacizumab (B).
To our knowledge, no population-based study has yet been conducted on the prognostic value of baseline AST-lymphocyte ratio index (ALRI) in predicting progression-free survival (PFS) in CRC patients treated with B. We think that the ALRI is an important parameter that provides data after combining the inflammatory state with a hepatic index.
We investigated the prognostic and predictive roles of baseline ALRI in metastatic CRC (mCRC) patients treated with first-line chemotherapy (CT)+B or CT alone in the Phase III prospective multicenter randomized ITACa trial (Italian Trial in Advanced Colorectal Cancer; EudraCT no 2007-004539-44 and on ClinicalTrials.gov NCT01878422).5
Patients and methods
The ITACa trial
Patients randomly received CT+B or CT alone as first-line therapy. CT consisted of either FOLFOX4 (a 2-hour infusion of 85 mg/m2 oxaliplatin on day 1 and a 2-hour infusion of 100 mg/m2 leucovorin followed by 400 mg/m2 bolus 5-FU and a 22-hour infusion of 600 mg/m2 5-FU on days 1–2 every 2 weeks) or FOLFIRI (a 2-hour infusion of 100 mg/m2 leucovorin followed by 400 mg/m2 bolus 5-FU and a 22-hour infusion of 600 mg/m2 5-FU on days 1–2 every 2 weeks with the addition of a 90-minute infusion of 180 mg/m2 irinotecan on day 1) at the clinician’s discretion. A 30–90-minute intravenous infusion of 5 mg/kg B was administered on day 1 of each 2-week cycle.
Treatment continued until either progressive disease (PD) or unacceptable toxicity or withdrawal of consent. Tumor assessment was performed before the start of treatment and repeated every 8 weeks until PD. Responses were defined according to the Response Evaluation Criteria in Solid Tumors guidelines (per investigator assessment). The National Cancer Institute Common Toxicity Criteria were used for evaluating adverse events.
The ITACa trial was approved by the local ethics committee (Comitato Etico Area Vasta Romagna) on September 19, 2007 and registered in our National Clinical Trials Observatory (Osservatorio delle Sperimentazioni Cliniche) and in the European Clinical Trials Database (EudraCT no 2007-004539-44) before patient recruitment began. Registration on ClinicalTrials.gov (NCT01878422) was not mandatory but was carried out at a later date (June 7, 2013). All patients provided written informed consent. The study was carried out in accordance with the Declaration of Helsinki under good clinical practice conditions and after full approval of the ethics committees of all participating centers (Comitato Etico Area Vasta Romagna e IRST, Comitato Etico Provinciale di Modena, Comitato Etico AUSL di Piacenza, Comitato Etico Interaziendale AOU “Maggiore della Carità” di Novara, Comitato Etico Interaziendale dell’A.S.O. Santa Croce e Carle di Cuneo, Comitato Etico della Provincia di Modena, Comitato Etico della Provincia di Ferrara, Comitato Etico Unico per la Provincia di Parma, Comitato Etico Indipendente Azienda USL di Bologna, Comitato Etico della ASL LE di Lecce, Comitato Etico Provinciale di Belluno per la Sperimentazione Clinica).
Statistical analysis
PFS and overall survival (OS) were, respectively, the primary and one of the secondary endpoints of the ITACa study. Information on AST and lymphocyte counts from blood tests carried out at baseline (before systemic treatment) was collected subsequently for the purpose of this post hoc analysis. ALRI was calculated as AST/lymphocyte count. The aim of this analysis was to evaluate the association between baseline ALRI levels and PFS and OS in the intent-to-treat population.
PFS was defined as the time from the date of random assignment to the date of either first documentation of PD, or last tumor evaluation, or death from any cause. Patients undergoing curative metastasectomy were censored at the date of surgery. OS was defined as the time from the date of random assignment to the date of death or last follow-up visit. PFS and OS were estimated by Kaplan–Meier method and curves were compared using the log rank test. Cox regression model was used to estimate HRs, their 2-sided 95% CI, and HR adjusted by center, baseline characteristics (gender, age, Eastern Cooperative Oncology Group performance status [ECOG PS], KRAS status, and tumor localization), CT regimen, and ITACa treatment. Cox regression model was also used to evaluate the effect on PFS and OS of the interaction between ALRI levels and treatment, including ALRI levels, treatment, and treatment-by-ALRI levels. X-tile 3.6.1 software (Yale University, New Haven, CT, USA) was used for bioinformatics analysis of baseline data to determine the cutoff value for pretreatment levels of ALRI. ALRI ≥14 was considered as a high level.
Patient characteristics were compared with χ2 test, and median values were compared using nonparametric ranking test (median test). All p-values were based on 2-sided testing. Statistical analyses were performed using SAS statistical software version 9.4 (SAS Inc., Cary, NC, USA).
Results
Patient characteristics
Between November 14, 2007 and March 6, 2012, 284 patients diagnosed with CRC were available for baseline AST and lymphocyte analysis (Figure 1); the study sample included 170 (59.8%) males and 114 (40.2%) females with a median age at diagnosis of 66 years (range 33–83). Median follow-up was 36 months (range 1–65). Overall, median PFS was 9.1 (95% CI 8.4–9.8) and median OS was 21.4 months (95% CI 19.9–24.5). The 2 groups of patients were comparable with regard to age, gender, tumor localization, stage at diagnosis, KRAS status, and ITACa treatment. A considerable proportion of patients with high ALRI had performance status 1–2.
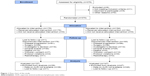  | Figure 1 Flow chart of the study. |
ALRI values and clinical outcome in all patients
Considering ALRI as a continuous variable, we observed that increased ALRI levels were associated with decreased PFS (HR 1.02, 95% CI 1.01–1.02, p<0.0001) and OS (HR 1.02, 95% CI 1.02–1.03, p<0.0001).
The 2 groups of patients were comparable for all the major clinical characteristics investigated, except for EOCG and CT regimen (Table 1).
At baseline, median PFS was 10.3 months (95% CI 9.4–12.0) and 8.0 months (95% CI 6.8–8.9) for patients with low (<14) and high (≥14) ALRI levels, respectively (HR 1.43, 95% CI 1.12–1.82, p=0.004) (Figure 2A). Median OS was 25.5 months (95% CI 21.3–30.2) and 18.8 months (95% CI 16.6–21.7) for patients with low and high baseline ALRI levels, respectively (HR 1.51, 95% CI 1.17–1.96, p<0.001) (Figure 2B). ALRI, AST, and lymphocyte values were associated with different toxicities (Table 2).
  | Figure 2 Kaplan–Meier curves of PFS (A), OS (B). |
  | Table 2 Comparison of median values of ALRI, AST and lymphocytes as a function of toxicity |
Following adjustment for clinical covariates (age, gender, ECOG, tumor localization, CT regimen, KRAS status, ITACa treatment, and LDH level and liver metastasis), multivariate analysis confirmed ALRI as an independent prognostic factor for predicting PFS (HR 1.35, 95% CI 1.02–1.79, p=0.034) and OS (HR 1.35, 95% CI 1.01–1.83 p=0.048) (Table 3).
ALRI values and clinical outcome in patients treated with CT+B
Considering ALRI as a continuous variable, we observed also in patients undergoing CT+ B that increased ALRI levels were associated with decreased PFS (HR 1.01, 95% CI 1.01–1.02, p=0.001) and OS (HR 1.02, 95% CI 1.01–1.03, p<0.0001).
At baseline, median PFS was 11.3 months (95% CI 9.1–13.4) and 9.2 months (95% CI 6.8–11.3) for patients with low and high ALRI levels, respectively (p=0.304; HR 1.20, 95% CI 0.85–1.69, p=0.304) (Figure 3A). On the other hand, median OS was 22.7 and 15.9 months for patients with low and high baseline ALRI, respectively (HR 1.36, 95% CI 0.95–1.96, p=0.095) (Figure 3B).
ALRI values and clinical outcome in patients treated with CT alone
Considering ALRI as a continuous variable, we observed also in patients undergoing CT alone that increased ALRI levels were associated with decreased PFS (HR 1.04, 95% CI 1.03–1.05, p<0.0001) and OS (HR 1.03, 95% CI 1.02–1.04, p<0.0001).
At baseline, median PFS was 10.0 months (95% CI 9.0–11.8) and 7.0 months (95% CI 5.9–8.4) for patients with low and high ALRI levels, respectively (p=0.002; HR 1.71, 95% CI 1.22–2.41, p=0.002) (Figure 3C). Median OS was 27.1 and 20.2 months for patients with low and high baseline ALRI levels, respectively (p=0.007; HR 1.64, 95% CI 1.14–2.36, p=0.007) (Figure 3D).
Interaction tests on ALRI levels and treatment efficacy in the CT+B and CT only groups were statistically significant for PFS (p=0.0003) but not for OS (p=0.228).
Figure 4 shows the Kaplan–Meier curves of PFS of patients treated with CT+B and CT alone with respect to ALRI levels.
AST and lymphocyte values and clinical outcome in all patients
Considering ALRI as a continuous variable, we observed that increased AST levels were associated with decreased PFS (HR 1.01, 95% CI 1.01–1.02, p=0.0002) and OS (HR 1.02, 95% CI 1.01–1.02, p<0.0001). Among basal characteristics, we observed that AST levels were higher in patients with liver disease (Table 4).
  | Table 4 Liver disease and median value of AST |
Considering lymphocyte as a continuous variable, we observed that increased lymphocyte levels were associated with decreased PFS (HR 0.84, 95% CI 0.72–0.99, p=0.036), but not OS (HR 0.91, 95% CI 0.77–1.06, p=0.214).
Discussion
Our study showed that high levels of ALRI at baseline were associated with shorter PFS and OS than low ALRI levels. To our knowledge, our study is the first to have assessed the prognostic value of ALRI in patients with CRC, potentially representing a noninvasive predictor of prognosis for CRC patients. However, the cutoff value of ALRI in our report was different from what was previously reported in other diseases.6,7 ALRI was evaluated in particular in patients with hepatocellular carcinoma, as levels of AST are higher and lymphocyte levels are lower due to cirrhosis.
AST plays a role in the metabolism of amino acids in the liver, and thus its increase becomes a biochemical marker of liver failure.8 Lymphocytes play a role in the antitumor immune response, and their presence relates to a reduced risk of relapse in several situations.9 Increased serum AST may be a result of the progression of liver diseases, as intracellular AST is released when the hepatic parenchymal cells are injured. Conversely, decreased lymphocytes can reduce the antitumor immunity of the patients. For this reason, ALRI may be used for predicting response and OS.
B, a recombinant humanized monoclonal antibody directed against vascular endothelial growth factor, was found to improve survival in both first- and second-line settings when added to CT.10 There is no current validated clinical or biological factor that can predict response to B. A positive result to the interaction test indicates that the ALRI index may be predictive of B efficacy. We hypothesize that B may be capable of contrasting the negative predictive value of high baseline levels of ALRI. In fact, among patients with high ALRI levels, PFS was lower in those treated with CT alone than in those treated with CT+B.
With regard to toxicity, it is noteworthy that high values of ALRI and AST were mostly associated with a higher incidence of fatigue, probably as result of a greater amount of liver disease at baseline, which may induce higher chemo-related toxicity.
In conclusion, pretreatment levels of ALRI may constitute a marker of aggressiveness in CRC patients. Elevated ALRI levels prior to treatment seem to be indicators of poor prognosis.
Author contributions
The study was conceptualized and designed by Andrea Casadei Gardini. All authors assisted with collection and assembly of data. Andrea Casadei Gardini, Emanuela Scarpi, and Alessandro Passardi analyzed and interpreted the data. All authors were involved in manuscript writing. All authors finally approved the manuscript. The corresponding author had the final responsibility to submit for publication. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This trial was partially supported by the Italian Medicines Agency (Agenzia Italiana del Farmaco, AIFA – research grant no FARM6FJJAY). The study sponsor was involved neither in the study design nor in the collection, analysis, and interpretation of data. The study sponsor did not provide writing support for the report. All authors had full access to all the data in the study.
The authors would like to thank Cristiano Verna and Veronica Zanoni for editing the manuscript and Angela Ragazzini, Monia Dall’Agata (Meldola), Luigi Cavanna, Maria Angela Palladino, Camilla Di Nunzio, Claudia Biasini (Piacenza), Britt Rudnas, Barbara Venturini (Rimini), Caterina Accettura (Lecce), Giorgia Razzini (Carpi), Jody Corbelli, Alessandra Piancastelli (Faenza), Bernadette Vertogen, and Federica Zumaglini (Ravenna) for participating in this study.
The abstract of this paper was presented at the ESMO 2017 Conference as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Annals of Oncology: https://academic.oup.com/annonc/article-abstract/28/suppl_6/mdx422.035/4583119?redirectedFrom=fulltext.
Disclosure
The authors report no other conflicts of interest in this work.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. | ||
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. | ||
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related in ammation. Nature. 2008;454:436–444. | ||
Ko E, Jung G. Positive association of long telomeres with the invasive capacity of hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2014;447(2):358–363. | ||
Passardi A, Nanni O, Tassinari D, et al. Effectiveness of bevacizumab added to standard chemotherapy in metastatic colorectal cancer: final results for first-line treatment from the ITACa randomized clinical trial. Ann Oncol. 2015;26(6):1201–1207. | ||
Jin J, Zhu P, Liao Y, Li J, Liao W, He S. Elevated preoperative aspartate aminotransferase to lymphocyte ratio index as an independent prognostic factor for patients with hepatocellular carcinoma after hepatic resection. Oncotarget. 2015;6(22):19217–19227. | ||
Yang Z, Zhang J, Lu Y. Aspartate aminotransferase-lymphocyte ratio index and systemic immune-inflammation index predict overall survival in HBV-related hepatocellular carcinoma patients after transcatheter arterial chemoembolization. Oncotarget. 2015;6(40):43090–43098. | ||
Okuda M, Li K, Beard MR, et al. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122(2):366–375. | ||
Pagès F, Galon J, Dieu-Nosjean MC, Tartour E, Sautès-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–1102. | ||
Hurwitz HI, Tebbutt NC, Kabbinavar F, et al. Efficacy and safety of bevacizumab in metastatic colorectal cancer: pooled analysis from seven randomized controlled trials. Oncologist. 2013;18(9):1004–1012. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




