Back to Journals » OncoTargets and Therapy » Volume 12
Prognostic implications of MYBL2 in resected Chinese gastric adenocarcinoma patients
Authors Jia YX, Gao YP, Li J, Chang ZW , Yan J, Qin YR
Received 26 September 2018
Accepted for publication 21 December 2018
Published 11 February 2019 Volume 2019:12 Pages 1129—1135
DOI https://doi.org/10.2147/OTT.S188820
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr William C. Cho
Yongxu Jia,* Yaping Gao,* Jing Li,* Zhiwei Chang, Jie Yan, Yanru Qin
Department of Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, People’s Republic of China
*These authors contributed equally to this work
Background and aim: Gastric cancer (GC), a malignant tumor worldwide, is mostly diagnosed at an advanced stage. We selected the oncogene encoding transcription factors MYBL2 to investigate the connection between MYBL2 expression and GC prognosis.
Materials and methods: MYBL2 mRNA and protein expression were measured by real-time PCR and immunohistochemistry, respectively. The relationship between MYBL2 protein expression and survival time was estimated by the Kaplan–Meier analysis. Cox proportional hazards model was used to evaluate the prognostic impact of MYBL2 expression.
Results: The overexpression of MYBL2 was related to tumor cell differentiation, Lauren type, and metastasis of lymph nodes (P<0.05). In the MYBL2 overexpression group, the median disease free survival was even poorer (P=0.000) and it comes to median overall survival (P=0.000). The study showed that MYBL2 expression was an independent hazard for disease free survival (P=0.004).
Conclusion: The results of this study suggest that MYBL2 could indicate a promisingly prognostic biomarker for GC patients.
Keywords: MYBL2, gastric cancer, prognosis, upregulation, survival analysis
Introduction
Globally, gastric cancer (GC) ranks fifth for incidence and third for mortality.1 In 2015, an estimated 679,100 people were diagnosed with GC, among whom there were ~498,000 deaths in People’s Republic of China.2 Results so far inspire little optimism that 5-year survival rate of GC retains ~15%. As an intricate disease, GC is promoted by biological processes of multigene and multistep synergy, including the amplification of oncogene or the deletion of mutated tumor suppressor genes or the instability of the microsatellite. miRNAs also participate in tumorigenesis and development by regulating the expression of oncogenes and tumor suppressor genes. In this, it is indispensable to search for valuable biomarkers for diagnosis, effective treatments, and prognosis.
The MYB transcription family consists of three members: MYBL1, MYBL2, and MYBL3. Studies have shown that the MYB transcription factor family is widely involved in cell cycle regulation to maintain genomic integrity, DNA replication, cell differentiation, apoptosis, and other physiological functions. By extension, MYBL2 is widely expressed and closely related to the degree of cell proliferation.3 Recently, MYBL2 has been found overexpressed in a variety of malignant tumors, such as acute myeloid leukemia,4 breast cancer,5 and colorectal cancer,6 which indicates that MYBL2 plays a critical role in process of cell apoptosis, cell senescence, and carcinogenesis and progression regulated by gene expression. The relationship between MYBL2 expression and GC development and progression have not yet been studied in depth.
The level of MYBL2 expression in mRNA and protein in resected gastric adenocarcinoma and matched adjoining tissues was detected in this paper, meanwhile. Its clinicopathological implication and prognostic significance in GC were explored in depth.
Materials and methods
Clinical samples
A total of 45 paired tumor and adjacent tissues with R0 resection were selected from the First Affiliated Hospital of Zhengzhou University (Henan, People’s Republic of China) from October 2015 to March 2016. Those were used for RNA extraction. In addition, 74 cases of paraffin samples from patients with GC R0 resection in the same hospital from January 2014 to December 2014 were used for immunohistochemical analysis. Written informed consent was obtained from all guardians for the use of their tissue samples. The sample acquisition and its subsequent use were approved by the Ethics Committee of Zhengzhou University, which were guided by international and national ethical requirements concerning biomedical research. In addition to this, the study was conducted in compliance with the Declaration of Helsinki.
The standard inclusion criteria were as follow: 1) histopathologic diagnosis of gastric adenocarcinoma and 2) no previous neoadjuvant chemoradiotherapy before surgery. Clinical information and pathological data were obtained from archives, and tumors were staged as stipulated according to the seventh edition of TNM American Joint Committee on Cancer staging criteria for cases of staging.
cDNA synthesis and quantitative real-time PCR
TRIzol (Thermo Fisher Scientific, Waltham, MA, USA) was used for extracting total RNA and we applied Reverse Transcription for PCR Kit (Takara, Kusatsu, Japan) to acquire the same amount of cDNA, according to the manufacturer’s specification. We used quantitative real-time PCR SYBR Green PCR Kit (Thermo Fisher Scientific) to determine the level of MYBL2 and GAPDH mRNA with the assistance of the ABI7900HT Fast Real-Time PCR system (Thermo Fisher Scientific). The primers of MYBL2 and GAPDH are shown in Table 1. The 2−ΔΔCT method was applied to calculate the relative expression of MYBL2 mRNA with GAPDH as an internal reference.
  | Table 1 Primer sequences of MYBL2 and GAPDH |
GC tissue microarray and immunohistochemical staining
The paraffin-embedded tissues were cut into sections each 4 μm thick. The streptavidin–biotin complex method was used to investigate the expression of MYBL2. In short, slices were treated with deparaffinage and dehydration at the certain concentration gradient after being baked. The antigen repair should be accomplished by 0.1 mol/L citrate buffer solution (pH 6.0) in a microwave oven for about 15 minutes. After adding the normal goat serum to eliminate nonspecific binding to the greatest extent possible, the slices were hatched by embedding in the primary anti-MYBL2 (Abcam, Cambridge, UK), diluted with 1:150, and incubated overnight at 4°C in humidity. The slices were then incubated into the second antibody for 30 minutes at the room temperature, followed by diaminobenzidine for 1 minute and hematoxylin counterstaining for 2 minutes. According to the existing literature, MYBL2 protein is found in both the nucleus and cytoplasm.
In this report, immunohistochemical results were obtained by two pathologists. The intensity of tissue staining was divided into four levels: negative, no staining, 0 point; weak positive, pale yellow, 1 point; middle positive, with medium strength staining, 2 points; and strong positive, brown and tan, 3 points. The percentage of positive cells in the total number of tumor cells/normal mucosal epithelial cells was recorded, and the percentage of positive cells was <5%, 0 point; 5%–25%, 1 point; 26%–50%, 2 points; 51%–75%, 3 points; and >75%, 4 points. The staining scores were evaluated according intensity and percentage score. Receiver operating characteristic curve analysis was conducted to determine the cutoff score for MYBL2 overexpression and low expression.
Statistical analysis
SPSS17.0 software was chosen for the data processing and statistical analysis. Paired McNemar test was used to compare the expression of MYBL2 protein in cancer tissues and adjacent tissues. In addition, the relationship between MYBL2 protein expression and the clinical features was measured with chi-squared test, and the survival curve was drawn with the Kaplan–Meier method. The prognostic value of MYBL2 was calculated with the Cox-proportional hazard model. A P-value <0.05 was considered to be statistically significant.
Results
MYBL2 is dramatically overexpressed in resected GC
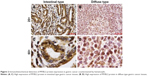
The level of MYBL2 mRNA expression in gastric adenocarcinoma tissues is significantly higher than in the tissues adjacent to carcinoma (P=0.0008) (Figure 1). The overexpression of MYBL2 protein was observed in 85.1% (63/74) in cancerous tissues and 43.2% (32/74) in corresponding normal tissues, which showed statistically significant (P=0.000; Figure 2).
Correlation of MYBL2 protein expression with clinicopathological variables
To further explore connection of MYBL2 protein expression and the clinical pathological features, we got the result as shown in Table 2. In those parameters, the overexpression of MYBL2 protein was proved to be statistically significant in diffuse type of Lauren classification (P=0.028), poor differentiation (P=0.006), and lymph node metastasis (P=0.0367).
  | Table 2 Relationship between MYBL2 protein expression in gastric cancer tissue and its clinical pathological parameters |
Association between MYBL2 expression and patient survival
The study indicated that pTNM stage, lymph node metastasis, and MYBL2 protein expression levels have prognostic implications on OS and disease free survival (PFS) (Table 3). Then in multivariate analysis, as a result, TNM stage and MYBL2 protein expression were independent factors influencing the prognosis of gastric adenocarcinoma in median disease free survival (mPFS) (Table 4). The over-MYBL2 expression group showed dramatically shorter time than that with normal MYBL2 expression group in the median overall survival (27 vs 53 months; Figure 3A) and the mPFS (25 vs 46 months; Figure 3B). There was also a statistically significant difference between MYBL2 expression quantity in patients with stage I–II (P=0.033) and in those with stage III–IV (P=0.008) in OS (Figure 3C and D).
  | Table 3 Univariate Cox regression analysis for OS and PFS in GC |
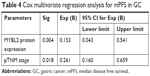  | Table 4 Cox multivariate regression analysis for mPFS in GC |
Discussion
The mainstream tumorigenesis processes involved in GC are characterized by phenotypic, multistep progression cascades.7 Unfortunately, even if a large amount of progress has been made in the diagnosis and comprehensive treatment, patients’ prognosis remains poor.
MYBL2 could bind to the promoter regions of DNA topoisomerase II, cdc2, cyclinB1, cyclinA2, and other genes to promote the transcription of these genes, so that cells can enter the G2/M phase smoothly.8–10 One study11 showed that MYBL2 can regulate the expression of transcription factor snail to promote the transformation of mammary gland epithelial cells to mesenchymal cells. Several studies have shown that the activity of MYBL2 is essential to the cell cycle progression.12,13 According to the results of DNA microarray analysis on breast cancer, high expression of MYBL2 is closely associated with p53 mutations.14 Some studies have also shown that DT40 cells lacking MYBL2 expression are more sensitive to DNA damage.15
MYBL2 can inhibit cell apoptosis by inducing the expression of antiapoptosis genes such as Bcl-2, Survivin, and ApoJ/clusterin.3,16–18 A number of foreign studies have indicated that the MYBL2 gene amplified in a variety of tumor tissues, including human non-small-cell lung cancer, liver cancer, and skin T-cell lymphoma.19,20 As such, when inhibiting the expression of MYBL2, the proliferation of a number of cells, such as lymphocyte, glioblastoma cells, fibroblasts cells, neuroblastoma cells, was found to be suppressed.21,22 Studies have shown that MYBL2 expression is significantly increased and associated with poor prognosis in neuroblastoma, prostate cancer, and basal cell breast cancer.3,23,24 Ren et al’s study6 showed that MYBL2 overexpression was an independent prognostic factor in colorectal cancer development and progression.
In our study, significantly more overexpression of MYBL2 mRNA and protein was observed in resected GC than in paracancerous tissues. The intratumoral and intertumoral heterogeneous expression of MYBL2 protein exists in GC tissue, which exhibits different expression level and localization of MYBL2 protein between different sites of the same tumor and between different tumors. A meaningful correlation was found between the overexpression of MYBL2 protein and clinical factors, specifically Lauren classification, differentiation, and lymph node metastasis. There are many differently pathological and clinical characteristics between the diffuse type and intestinal type GC, including histogenesis, carcinogenesis, and prognosis. In our study, the MYBL2 protein expression level in diffuse type GC is higher than the intestinal type GC, which may indicate a molecular mechanism of diffuse type GC. In addition, the rate of MYBL2 protein overexpression in GC with poor differentiation and lymph node metastasis is much higher. The finding may support that MYBL2 relate to the malignant biological behavior of GC. We then found that patients with MYBL2 overexpression had a significantly shorter survival time. Results demonstrated that MYBL2 overexpression might be considered a negative prognostic factor for mPFS. We drew the conclusion that with respect to pTNM stage, overexpression of MYBL2 had poorly prognostic significance in OS, as indicated by further stratified analysis.
Recently, MYBL2 may have been associated with the chemotherapy drug resistance. In non-small-cell lung cancer, the cetuximab drug-resistant clone has a higher MYBL2 mRNA and protein levels than nonresistant clones do.25 As a colon cancer cell line, DLD-1, resistant to 5-FU, had MYBL2 mRNA accumulation behavior, which, according to researchers, indicated that the cell cycle-related mRNA could be one of 5-FU’s resistance mechanisms.26
In a word, the present work shows that resected GC patients with MYBL2 overexpression had poorer survival and predicted MYBL2 may be potentially used as an important biomarker for early diagnosis, prognosis, and clinical therapy for GC.
Acknowledgment
This work was supported by grants from the National Natural Science Foundation of China (grant number 81472605).
Disclosure
The authors report no conflicts of interest in this work.
References
Venerito M, Link A, Rokkas T, Malfertheiner P. Gastric cancer – clinical and epidemiological aspects. Helicobacter. 2016;21(Suppl 1):39–44. | ||
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. | ||
Sala A, B-Myb SA. B-Myb, a transcription factor implicated in regulating cell cycle, apoptosis and cancer. Eur J Cancer. 2005;41(16):2479–2484. | ||
Fuster O, Llop M, Dolz S, et al. Adverse prognostic value of MYBL2 overexpression and association with microRNA-30 family in acute myeloid leukemia patients. Leuk Res. 2013;37(12):1690–1696. | ||
Sotiriou C, Neo S-Y, McShane LM, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003;100(18):10393–10398. | ||
Ren F, Wang L, Shen X. MYBL2 is an independent prognostic marker that has tumor-promoting functions in colorectal cancer. Am J Can Res. 2015;5(4):1542–1552. | ||
Fassan M, Baffa R, Kiss A. Advanced precancerous lesions within the GI tract: the molecular background. Best Pract Res Clin Gastroenterol. 2013;27(2):159–169. | ||
Gualdrini F, Corvetta D, Cantilena S. Addiction of MYCN amplified tumours to B-MYB underscores a reciprocal regulatory loop. Oncotarget. 2010;1(4):278–288. | ||
Brandt TL, Fraser DJ, Leal S, Halandras PM, Kroll AR, Kroll DJ. c-Myb trans-activates the human DNA topoisomerase IIalpha gene promoter. J Biol Chem. 1997;272(10):6278–6284. | ||
Zhu W, Giangrande PH, Nevins JR. E2Fs link the control of G1/S and G2/M transcription. EMBO J. 2004;23(23):4615–4626. | ||
Tao D, Pan Y, Jiang G, et al. B-Myb regulates snail expression to promote epithelial-to-mesenchymal transition and invasion of breast cancer cell. Med Oncol. 2015;32(1):412. | ||
Lewis PW, Beall EL, Fleischer TC, Georlette D, Link AJ, Botchan MR. Identification of a Drosophila Myb-E2F2/RBF transcriptional repressor complex. Genes Dev. 2004;18(23):2929–2940. | ||
Korenjak M, Taylor-Harding B, Binné UK, et al. Native E2F/RBF complexes contain Myb-interacting proteins and repress transcription of developmentally controlled E2F target genes. Cell. 2004;119(2):181–193. | ||
Mannefeld M, Klassen E, Gaubatz S. B-Myb is required for recovery from the DNA damage-induced G2 checkpoint in p53 mutant cells. Cancer Res. 2009;69(9):4073–4080. | ||
Ahlbory D, Appl H, Lang D, Klempnauer KH. Disruption of B-Myb in DT40 cells reveals novel function for B-Myb in the response to DNA-damage. Oncogene. 2005;24(48):7127–7134. | ||
Knight AS, Notaridou M, Watson RJ. A Lin-9 complex is recruited by B-Myb to activate transcription of G2/M genes in undifferentiated embryonal carcinoma cells. Oncogene. 2009;28(15):1737–1747. | ||
Cervellera M, Raschella G, Santilli G, et al. Direct transactivation of the anti-apoptotic gene apolipoprotein J (clusterin) by B-Myb. J Biol Chem. 2000;275(28):21055–21060. | ||
Lang G, Gombert WM, Gould HJ. A transcriptional regulatory element in the coding sequence of the human bcl-2 gene. Immunology. 2005;114(1):25–36. | ||
Mao X, Orchard G, Lillington DM, Russell-Jones R, Young BD, Whittaker SJ. Amplification and overexpression of JunB is associated with primary cutaneous T-cell lymphomas. Blood. 2003;101(4):1513–1519. | ||
Zondervan PE, Wink J, Alers JC, et al. Molecular cytogenetic evaluation of virus-associated and non-viral hepatocellular carcinoma: analysis of 26 carcinomas and 12 concurrent dysplasias. J Pathol. 2000;192(2):207–215. | ||
Arsura M, Introna M, Passerini F, Mantovani A, Golay J. B-Myb antisense oligonucleotides inhibit proliferation of human hematopoietic cell lines. Blood. 1992;79(10):2708–2716. | ||
Raschellà G, Negroni A, Sala A, Pucci S, Romeo A, Calabretta B. Requirement of b-myb function for survival and differentiative potential of human neuroblastoma cells. J Biol Chem. 1995;270(15):8540–8545. | ||
Bar-Shira A, Pinthus JH, Rozovsky U, et al. Multiple genes in human 20q13 chromosomal region are involved in an advanced prostate cancer xenograft. Cancer Res. 2002;62(23):6803–6807. | ||
Thorner AR, Hoadley KA, Parker JS, Winkel S, Millikan RC, Perou CM. In vitro and in vivo analysis of B-Myb in basal-like breast cancer. Oncogene. 2009;28(5):742–751. | ||
Iida M, Brand TM, Campbell DA, Li C, Wheeler DL. Yes and Lyn play a role in nuclear translocation of the epidermal growth factor receptor. Oncogene. 2013;32(6):759–767. | ||
Kurokawa K, Tanahashi T, Iima T, et al. Role of miR-19b and its target mRNAs in 5-fluorouracil resistance in colon cancer cells. J Gastroenterol. 2012;47(8):883–895. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.