Back to Journals » OncoTargets and Therapy » Volume 11
Prognostic impact of the number of lymph nodes examined in different stages of colorectal mucinous adenocarcinoma
Authors Ma Y, Luo YQ, Lin N, Lv YZ, Zhou Y, Li B, Han KN, Jiang S, Gao JJ
Received 19 January 2018
Accepted for publication 25 April 2018
Published 25 June 2018 Volume 2018:11 Pages 3659—3670
DOI https://doi.org/10.2147/OTT.S163076
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Carlos E Vigil
Yong Ma,* Yiqian Luo,* Nan Lin, Yongzhu Lv, Yang Zhou, Bing Li, Kunna Han, Song Jiang, Jianjun Gao
Department of General Surgery, 210 Hospital of Chinese People’s Liberation Army, Dalian 116000, Liaoning, China
*These authors contributed equally to this work
Background: Mucinous adenocarcinoma (MC) is a special kind of colorectal adenocarcinoma that occurs more frequently in young patients and females, but the prognostic effect of lymph nodes in MC patients is unclear. This population-based study was conducted to analyze the prognostic value of the number of lymph nodes examined in different stages of colorectal MC.
Methods: We included 17,001 MC patients from the Surveillance, Epidemiology, and End Results program database between 2003 and 2013, of which 12,812 (75%) had >12 lymph nodes examined.
Results: Compared to the group with insufficient lymph nodes examined, patients with more lymph nodes (>12) examined tended to come from east and central America, were more frequently female and young, were diagnosed after 2008, had larger-sized tumors of less differentiated grade and in later stages, had not received radiation therapy and had more positive nodal status. Patients with more lymph nodes (>12) examined demonstrated significantly better survival than those with insufficient lymph nodes examined only in stages II and III (stage II: overall, P<0.001; cancer-specific, P<0.001; stage III: overall, P=0.093; cancer-specific, P=0.032), even though the overall (P<0.001) and cancer-specific survival (P<0.001) showed significant differences between the two groups. Both univariate (overall, HR=0.739, 95% CI=0.703–0.777, P<0.001; cancer-specific, HR=0.742, 95% CI=0.698–0.788, P<0.001) and multivariate (overall, HR=0.601, 95% CI=0.537–0.673, P<0.001; cancer-specific, HR=0.582, 95% CI=0.511–0.664, P<0.001) Cox proportional hazards models verified the association between >12 lymph nodes examined and better survival.
Conclusion: More number of lymph nodes >12) examined significantly increased the survival probability of MC patients in stages II and III, but had no significant influence on patients in stages I and IV, indicating the effect of number of lymph nodes examined was a stage-dependent prognostic factor in clinical utility.
Keywords: number of lymph nodes examined, stages, mucinous carcinoma
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality in the USA.1,2 Different histological subtypes have been reported to demonstrate distinct survival probabilities, clinical characteristics and response to clinical therapies.3,4 According to the World Health Organization (WHO), mucinous adenocarcinoma (MC) is a special histological type of CRC with >50% of extracellular mucin within the tumor and is found in 1.6%–25.4% of CRC cases.5,6 Compared to the non-mucinous adenocarcinoma (NMC), MC was reported to occur more frequently in younger and female patients.7,8
Besides, the survival probability of MC patients was believed to be worse than NMC patients considering the later-stage presentations of this kind of disease.9–11
The number of metastatic lymph nodes is an important factor in staging criteria worldwide, one of which is the most popularly used TNM staging system.12 Therefore, the number of lymph nodes examined has played an indispensable role in classifying the tumor stages, and different histological stages were often connected with distinct survival probabilities and treatment options.13–15 However, the findings about the prognostic power of the number of lymph nodes examined were constantly inconsistent; some studies reported the number of lymph nodes examined as a good prognostic factor in CRC,16–18 while others showed contradictory results.19–21 Several studies had insufficient number of observed cases, and some were not taking stages into consideration. Furthermore, the prognostic effect of the number of lymph nodes examined in MC has not been well established.
Therefore, this population-based study was conducted to investigate the prognostic impact of the number of lymph nodes examined in different stages of MC.
Methods
Clinical dataset
The Surveillance, Epidemiology, and End Results (SEER)22 is the largest cancer database in the USA, representing about 30% of the population. We included CRC cases diagnosed between 2003 and 2013 from 18 population-based cancer registries where the number of lymph nodes examined at the time of primary surgical resection was known. MC was defined according to the codes 8480 and 8481 of the International Classification of Diseases for Oncology, third edition. Characteristics of patients including age at diagnosis, geographical location, sex, race, year of diagnosis, tumor numbers, tumor size, tumor grade, American Joint Committee on Cancer (AJCC) stages, receipt of radiation therapy, nodal status and lymphadenectomy were used in the analysis. The lymph node ratio (LNR) was calculated as the number of positive lymph nodes divided by the total number of lymph nodes dissected. Patients with LNR higher than the median value of 0.17 were classified as “high” lymph node group, and the other patients were classified as “low” lymph node group. Patients were grouped into the following age categories: <50 years old, 50–65 years and >65 years old. The 18 registries were divided into three classes according to the geographical location as central (Metropolitan Detroit, Iowa, Kentucky, Utah and Louisiana), west (Alaska, Greater California, Hawaii, Los Angeles, New Mexico, San Francisco-Oakland SMSA, San Jose-Monterey and Seattle) and east (New Jersey, Metropolitan Atlanta, Rural Georgia and Greater Georgia). Patients were classified into four groups based on race as white, black, Asian or Pacific Islander and American Indian/Alaska Native (AI/AN). Tumor size was divided into two categories by cut-off of 5 cm. Tumor grade was characterized as well differentiated (G1), moderately differentiated, poorly differentiated and undifferentiated. TNM stages were reclassified into stage I, stage II, stage III and stage IV based on the criteria of the AJCC Staging Manual, 7th edition (2010). Patients with unknown ages, no available survival status, unknown follow-up survival times or undefined treatments were excluded from the analysis. The final analytic set consisted of 17,001 patients, for whom all the survival information was available.
Survival analysis
Survival information included vital status, cause of death and survival time in years. Patients with unavailable survival information were excluded from the analysis. Overall survival and cancer-specific survival were both calculated. Patients who had died from causes other than CRC were marked as “dead” in the overall survival analysis, but “censored” in the cancer-specific survival analysis. The Kaplan–Meier method was used to generate survival curves in the study, and the log-rank test was applied to calculate the differences between the curves. HRs and their 95% CIs were estimated for each variate by univariate and multivariate Cox proportional hazards models with the R package “survival”.
Statistical analysis
R version 3.3.2 (http://www.R-project.org/) was used to conduct all the statistical analyses in this work. The differences in clinicopathological characteristics between the group with <12 and the group with >12 lymph nodes examined were analyzed using Chi-square test. All tests conducted were two-sided, and the significant difference was considered at P<0.05.
Data availability
Data included in this analysis were downloaded from the SEER website (https://seer.cancer.gov/data/, SEER Incidence Data, 1973–2013). The data are freely available upon request from SEER by signing the “SEER Data-Use Agreement form”. Our research does not contain any identifiable private information of the patients, so it is not within the scope of the Institutional Review Board review.
Results
Clinical and demographic characteristics of CRC patients
In this study, we included 17,001 patients with MC, of which three-quarter cases had met the criteria of 12 examined lymph nodes. The clinicopathological characteristics of these patients are shown in Table 1. Cases from the western registries comprised nearly half of the population in both groups, while patients from eastern registries were more likely to have >12 lymph nodes examined (P=0.003). The numbers of female patients and male patients were basically equivalent to each other in the group with <12 lymph nodes examined, and in the group meeting the standard, females constituted more than males (P=0.02). More than half of the CRC patients were diagnosed at ages >65 years old, and patients >65 years old tended to have less lymph nodes examined than young people (P<0.001). More than 80% of patients were white, while only <1% were AI/AN in both groups. Cases with <12 lymph nodes examined were more likely diagnosed at the former half of the studied time interval (P<0.001). The proportion of tumors with sizes <5 cm in group with >12 lymph nodes examined was significantly higher than that of the other group (P<0.001). Well-differentiated tumors comprised 13.8% in the less examined group, but only 10% in the well-examined group (P<0.001), and the cases in the well-examined group were more likely to be in stage II or later of the pathological processes (P<0.001). Patients treated with radiation were less in number in the well-examined group than the other group (P<0.001), but most patients met the standard of 12 lymph nodes examined. More patients had positive nodal status in the well-examined group than in the less examined group in our dataset (P<0.001). Not surprisingly, patients with >12 lymph nodes examined had lower LNR than patients with inadequate examined lymph nodes (P<0.001). To reduce the bias that might be caused by the imbalance of sample sizes in the two groups, we repeated the analysis by randomly sampling equivalent numbers of patients in the two conditions. We found all the covariates were significantly different between the well-examined (≥12 lymph nodes) group and the group with inadequate (<12) retrieved lymph nodes (Table S1).
  | Table 1 Demographic and clinical characteristics of MC patients |
Insufficient lymph nodes examined as a poor prognostic factor in stage II and III patients
The group with <12 lymph nodes examined had both significantly worse overall (P<0.001) and cancer-specific survival probability (P<0.001) in our dataset (Figure 1). Among patients in different tumor stages, insufficient lymph nodes examined indicated worse overall and cancer-specific survival rates in stage II patients (overall, P<0.001; cancer-specific, P<0.001), and worse cancer-specific survival rate in stage III patients (overall, P=0.093; cancer-specific, P=0.032), while the number of lymph nodes examined had no significant influence on survival of patients with stage I and IV MC (stage I: overall, P=0.196; cancer-specific, P=0.796; stage IV: overall, P=0.917; cancer-specific, P=0.798). Specific information is listed in Table 2 and Figure 2.
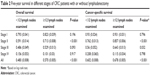  | Table 2 Five-year survival in different stages of CRC patients with or without lymphadenectomy |
Univariate and multivariate analysis
Univariate Cox proportional hazards analyses were conducted on all the clinical factors to explore their effect on the overall and cancer-specific survival. Both the overall survival and cancer-specific survival showed that the group with more lymph nodes (≥12) examined demonstrated better survival than the group with insufficient lymph nodes examined (HR=0.739, 95% CI=0.703–0.777, P<0.001; HR=0.742, 95% CI=0.698–0.788, P<0.001). In our analysis, age at diagnosis, race, tumor numbers, tumor size, tumor grade, AJCC stages, radiation therapy, nodal status and LNR all showed significant prognostic value for both overall and cancer-specific survival, while lymphadenectomy only demonstrated significant prognostic value for cancer-specific survival (Table 3). Including all these clinical characteristics with prognostic value from the univariate analysis into a multivariate Cox proportional hazards model, we found that the number of no less than 12 lymph nodes examined was an independent predictor of both better overall (HR=0.601, 95% CI=0.537–0.673, P<0.001) and cancer-specific survival (HR=0.582, 95% CI=0.511–0.664, P<0.001). Higher ages (>65 years old), tumor grades including moderately differentiated, poorly differentiated and undifferentiated, stages II and IV and positive nodal status were all significantly associated with worse overall and cancer-specific survival (P<0.001, respectively). On the other hand, Asian ethnicity (overall, HR=0.848, 95% CI=0.730–0.984, P=0.03; cancer-specific, HR=0.844, 95% CI=0.707–1.006, P=0.059), higher tumor numbers (overall, HR=0.883, 95% CI=0.795–0.981, P=0.021; cancer-specific, HR=0.683, 95% CI=0.590–0.790, P<0.001) and lower LNR (overall, HR=0.834, 95% CI=0.759–0.916, P<0.001; cancer-specific, HR=0.743, 95% CI=0.668–0.825, P<0.001) were associated with better survival probabilities. The detailed results of multivariate analysis are shown in Table 4.
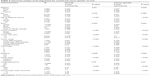  | Table 3 Univariate analysis of the population for overall and cancer-specific survival |
  | Table 4 Cox proportional hazards model of the population for overall and cancer-specific survival |
Discussion
In this population-based study, we analyzed 17,001 MC patients with available information on the number of lymph nodes examined from SEER. Of all the tumors, 9.4% were MC, similar to the proportion reported in previous literature irrespective of the histological stages.5,6 In our study, the number of lymph nodes examined (≥12) was associated independently with better overall and cancer-specific survival in MC in both the univariate and multivariate Cox proportional hazards models in a stage-dependent manner, where it acted as a good prognostic factor for stage II and III MC patients, while not for those in the other two stages.
MC is a distinct subtype of colorectal adenocarcinoma that requires special attention despite its low occurrence. MC has a propensity to exhibit a worse-differentiated grade and a higher likelihood of lymph node metastasis according to previous studies.7,23–25 The production of mucus under pressure allows the cancers to separate tissue planes in the bowel wall and more frequently gain access to the regional lymph.26 Therefore, lymph node retrieval is important in this subtype of CRC.
Positive lymph node assessment is critical for staging and to determine the need for adjuvant chemotherapy for patients with colon cancer.27,28 Thus, an adequate number of lymph nodes needs to be examined. The evaluation of at least 12 lymph nodes was first recommended in the 1990 Working Party Report to the World Congresses of Gastroenterology, and then reiterated by a National Cancer Institute-sponsored panel of experts to ensure adequate sampling.29–32
Our findings were consistent with several previous studies that an increased number of lymph nodes evaluated was associated with improved survival among patients with stage II colon cancer.18,27,33 While for stage III diseases, our results showed more number of lymph nodes examined significantly increased the cancer-specific survival, but the other studies found this prognostic value only existed in node-positive group.17,34–36 This might be caused by the different cut-offs of lymph nodes examined in previous studies. For example, Chang et al evaluated the prognostic effect of lymph nodes examined with the cut-off of 7,32 and Gumus et al separated the population with 9 lymph nodes examined.36 Moreover, in our dataset, three-quarter patients had been evaluated for >12 lymph nodes, which was much higher than the proportion reported earlier, where a population-based study suggested that only 37% of colon cancer patients had adequate lymph node evaluation (at least 12 nodes examined),27 indicating the increased retrieval of lymph nodes in recent years. Multivariate analysis confirmed the prognostic value of the number of lymph nodes retrieved (Table 4), while the overall survival showed that stage III disease was associated with a slightly better survival than stage II disease; this might probably have been due to the fact that there were cases of death from other causes rather than CRC, since for the cancer-specific survival, stage III patients had significantly larger HR than the stage II group.
The examination of lymph nodes also correlated closely with the surgical procedures and the quality of surgeons in clinical practice.27 Relatively little is known about the factors that influence the adequacy of lymph node evaluation. Our data indicated that the number of lymph nodes examined was affected by many clinical characteristics, such as the geographic location – patients from eastern America were more likely to have sufficient lymph nodes examined than the west or central registries. Besides, females with MC were examined for more lymph nodes than males, and more lymph nodes were evaluated in younger patients (≤65 years old). Patients diagnosed after 2008 evidently had increased number of lymph nodes examined, suggesting the improvement in health care and pathological practice, which agreed with the previous report that lymph node retrieval was correlated with surgeon factors like procedure volume.22,37 Tumor factors (tumor size, grade, stage) also had great influence on the number of lymph nodes examined based on our analysis. We observed that more lymph nodes were evaluated in MC patients with not well-differentiated tumors and stage II and III diseases. This phenomenon was also reported in several other studies, but the reason for the association was unclear.38,39 It is possible that with the increased number of lymph nodes examined, the probability to retrieve positive lymph nodes will increase, as the positive lymph nodes play an important role in tumor staging. However, the LNR in the well-examined group was significantly lower than that of the patients with inadequate lymph nodes retrieved, and LNR also served as an independent prognostic factor in our analysis, suggesting the necessity of consideration of LNR in staging and diagnosis in clinical practice. Radiation therapy was also associated with the number of lymph nodes examined based on our data – patients receiving the radiation therapy were less likely to have sufficient lymph nodes examined, while radiation itself was not an independent prognostic factor, indicating that the number of lymph nodes should be taken into consideration when considering the radiation therapy in clinical utility.
To our knowledge, the large number of patients from national population-based data in our study avoided the biases from single-institution experiences or limited sample sizes. However, we noticed that several limitations still need further comment. First, considering the retrospective nonrandomized nature of SEER, individual pathological diagnosis was not feasible to review in a large population size, so the variations caused by different pathologists may lead to misclassification of patients. Second, the different criteria used by registries or surgical methods used for the lymph nodes evaluation may slightly affect the results of our analysis. Furthermore, despite that we included as many potential clinical cofactors in our analysis as feasible, there were limited information on surgical and treatment options such as the procedure strategy, specimen adequacy and chemotherapy dose or duration, which may lead us to overlook the influences of these factors in prognosis besides the number of lymph nodes examined. Further randomized large-scale trial in the Chinese population is needed to obtain more definitive conclusion and give more clues for the treatment of Chinese patients. In summary, lymph nodes retrieval was associated with geographic location, and more number of lymph nodes was examined in female patients, who were at younger ages, diagnosed after 2008, had larger-sized tumors of less-differentiated grade and in later stages, less likely to have radiation therapy, more positive lymph nodes and lower LNR. The increased number of lymph nodes examined (≥12) significantly improved the survival probability of MC patients in a stage-dependent manner. Although the number of lymph nodes examined was not associated with better survival for stage I and IV patients, it remained an independently good prognostic factor for MC patients in stages II and III.
Author contributions
YM, YQL and JJG conceived and designed the project. YM and YQL collected and analyzed data. YM and YQL wrote the manuscript. NL, YZL, YZ, BL, KNH, SJ revised the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work. All the authors approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. | ||
Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. | ||
Ling CR, Wang R, Wang MJ, Ping J, Zhuang W. Prognosis and value of preoperative radiotherapy in locally advanced rectal signet-ring cell carcinoma. Sci Rep. 2017;7:45334. | ||
Simkens GA, van de Velde CJ, van Krieken JH, et al. Histological subtype and systemic metastases strongly influence treatment and survival in patients with synchronous colorectal peritoneal metastases. Eur J Surg Oncol. 2016;42:794–800. | ||
Hermanek P. Colorectal carcinoma: histopathological diagnosis and staging. Baillieres Clin Gastroenterol. 1989;3:511–529. | ||
Hugen N, van Beek JJ, de Wilt JH, Nagtegaal ID. Insight into mucinous colorectal carcinoma: clues from etiology. Ann Surg Oncol. 2014;21:2963–2970. | ||
Hyngstrom JR, Hu CY, Xing Y, et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann Surg Oncol. 2012;19:2814–2821. | ||
Kanemitsu Y, Kato T, Hirai T, et al. Survival after curative resection for mucinous adenocarcinoma of the colorectum. Dis Colon Rectum. 2003;46:160–167. | ||
Kang H, O’Connell JB, Maggard MA, Sack J, Ko CY. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum. 2005;48:1161–1168. | ||
Catalano V, Loupakis F, Graziano F, et al. Prognosis of mucinous histology for patients with radically resected stage II and III colon cancer. Ann Oncol. 2012;23:135–141. | ||
Wang MJ, Ping J, Li Y, et al. Prognostic significance and molecular features of colorectal mucinous adenocarcinomas: a strobe-compliant study. Medicine (Baltimore). 2015;94:e2350. | ||
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. | ||
Jessmon P, Boulanger T, Zhou W, Patwardhan P. Epidemiology and treatment patterns of epithelial ovarian cancer. Expert Rev Anticancer Ther. 2017;17:427–437. | ||
Jorge S, Jones NL, Chen L, et al. Characteristics, treatment and outcomes of women with immature ovarian teratoma, 1998–2012. Gynecol Oncol. 2016;142:261–266. | ||
Chung HH, Kim JW, Park NH, Song YS, Cheon GJ. Prognostic importance of peritoneal lesion-to-primary tumour standardized uptake value ratio in advanced serous epithelial ovarian cancer. Eur Radiol. 2017;27:4510–4515. | ||
Tsai CJ, Crane CH, Skibber, et al. Number of lymph nodes examined and prognosis among pathologically lymph node-negative patients after preoperative chemoradiation therapy for rectal adenocarcinoma. Cancer. 2011;117:3713–3722. | ||
Le Voyer TE, Sigurdson ER, Hanlon AL, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003;21:2912–2919. | ||
Sarli L, Bader G, Iusco D, et al. Number of lymph nodes examined and prognosis of TNM stage II colorectal cancer. Eur J Cancer. 2005;41:272–279. | ||
Wong SL. Lymph node counts and survival rates after resection for colon and rectal cancer. Gastrointest Cancer Res. 2009;3:S33–S35. | ||
Tepper JE, O’Connell MJ, Niedzwiecki D, et al. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol. 2001;19:157–163. | ||
Guan X, Chen W, Li S, et al. Alterations of lymph nodes evaluation after colon cancer resection: patient and tumor heterogeneity should be taken into consideration. Oncotarget. 2016;7:62664–62675. | ||
Wright FC, Law CH, Last L, et al. Lymph node retrieval and assessment in stage II colorectal cancer: a population-based study. Ann Surg Oncol. 2003;10:903–909. | ||
Chew MH, Yeo SA, Ng ZP, et al. Critical analysis of mucin and signet ring cell as prognostic factors in an Asian population of 2,764 sporadic colorectal cancers. Int J Colorectal Dis. 2010;25:1221–1229. | ||
Nitsche U, Zimmermann A, Späth C, et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann Surg. 2013;258:775–782; discussion 782–773. | ||
Du W, Mah JT, Lee J, et al. Incidence and survival of mucinous adenocarcinoma of the colorectum: a population-based study from an Asian country. Dis Colon Rectum. 2004;47:78–85. | ||
Sugarbaker PH. Mucinous colorectal carcinoma. J Surg Oncol. 2001;77:282–283. | ||
Baxter NN, Virnig DJ, Rothenberger DA, et al. Lymph node evaluation in colorectal cancer patients: a population-based study. J Natl Cancer Inst. 2005;97:219–225. | ||
Chau I, Cunningham D. Adjuvant therapy in colon cancer: current status and future directions. Cancer Treat Rev. 2002;28:223–236. | ||
Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583–596. | ||
Fielding LP, Newland C, Arsenault P, et al. Clinicopathological staging for colorectal cancer: an International Documentation System (IDS) and an International Comprehensive Anatomical Terminology (ICAT). J Gastroenterol Hepatol. 1991;6:325–344. | ||
Otchy D, Hyman NH, Simmang C, et al. Practice parameters for colon cancer. Dis Colon Rectum. 2004;47:1269–1284. | ||
Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99:433–441. | ||
Goldstein NS. Lymph node recoveries from 2427 pT3 colorectal resection specimens spanning 45 years: recommendations for a minimum number of recovered lymph nodes based on predictive probabilities. Am J Surg Pathol. 2002;26:179–189. | ||
Jestin P, Pahlman L, Glimelius B, Gunnarsson U. Cancer staging and survival in colon cancer is dependent on the quality of the pathologists’ specimen examination. Eur J Cancer. 2005;41:2071–2078. | ||
Ratto C, Sofo L, Ippoliti M, et al. Accurate lymph-node detection in colorectal specimens resected for cancer is of prognostic significance. Dis Colon Rectum. 1999;42:143–154; discussion 154–158. | ||
Gumus M, Yumuk PF, Atalay G, et al. What is the optimal number of lymph nodes to be dissected in colorectal cancer surgery? Tumori. 2005;91:168–172. | ||
Johnson PM, Malatjalian D, Porter GA. Adequacy of nodal harvest in colorectal cancer: a consecutive cohort study. J Gastrointest Surg. 2002;6:883–888; discussion 889–890. | ||
Wong JH, Severino R, Honnebier MB, Tom P, Namiki TS. Number of nodes examined and staging accuracy in colorectal carcinoma. J Clin Oncol. 1999;17:2896–2900. | ||
Monig SP, Baldus SE, Zirbes TK, et al. Lymph node size and metastatic infiltration in colon cancer. Ann Surg Oncol. 1999;6:579–581. |
Supplementary material
  | Table S1 Demographic and clinical characteristics of MC patients with balanced sample sizes |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


